Abstract
Background
Standard surgical procedures used for the treatment of morbid obesity constitute optional treatments for type 2 diabetes mellitus (T2DM). The aim of the present study was to evaluate the short- and mid-term effects of laparoscopic sleeve gastrectomy (SG) with ileal interposition (II) in T2DM patients (n = 30).
Methods
The variables investigated were the feasibility of the procedure, remission/alleviation of the disease, morbidity, mortality, and weight loss. Patients were followed during a period of 6–18 months after surgery.
Results
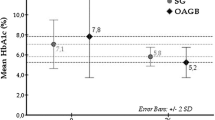
The average time required for the surgical procedure was 181.47 ± 53.23 min, and the mean duration of postoperative hospital stay was 3.17 ± 0.79 days. There were no intraoperative complications, and none of the patients required conversion to open surgery. Postoperatively, all patients experienced a significant weight loss: i.e., the mean body mass index (BMI) values prior to and following surgery were significantly different (P = 0.0001). Postoperative levels of glycosylated hemoglobin, fasting glucose, and fructosamine were significantly reduced (P = 0.0001, 0.0001, and 0.0004, respectively) from those detected prior to surgery. Remission of T2DM was observed in 80% of the patients over the follow-up period, and these subjects no longer required treatment with hypoglycemic drugs or diet. The remaining 20% of patients presented significant improvement in their condition but needed an oral hypoglycemic medication.
Conclusions
Adequate glycemic control, adjustable weight loss, and absence of nutritional deficiencies were the main benefits offered by the surgical intervention. The results indicate that SG/II treatment could be a promising alternative for patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a devastating disease with high morbidity and mortality rates and damaging consequences that include cardiovascular problems, visual deterioration, renal insufficiency, and amputation. The current treatment of T2DM includes diet, control of blood glucose levels, exercise, and the use of medication. However, as the disease progresses, pancreatic beta cell function tends to deteriorate, and the need for drug combinations to control the glycemia increases.
Standard surgical procedures employed in the treatment of morbid obesity are considered a safe optional treatment for T2DM, even in individuals who are not overweight [1]. In a recent review, Roux-en-Y gastric bypass (RYGB) and biliopancreatic derivation (BPD) were reported to induce remission of T2DM in 83.7 and 98.9% of cases, respectively, whereas purely restrictive bariatric surgery (gastric banding) had success rates in only 47.9% of cases [2].
As glycemic control can be observed earlier than weight loss, it has been suggested that T2DM could be regulated by mechanisms involving a group of gastrointestinal hormones known as incretins, which include gastric inhibitory polypeptide (GIP), peptide YY (PYY), and glucagon-like peptide-1 (GLP-1) [3–5]. These hormones, in conjunction with the central nervous system, influence glucose metabolism by (1) increasing insulin secretion, (2) suppressing postprandial glucagon secretion, (3) reducing gastric emptying and diminishing food intake, (4) preserving and/or promoting the hypertrophy of pancreatic beta cells, (5) stimulating the differentiation of pancreatic duct cells into insulin-secreting beta cells, (6) reducing peripheral insulin resistance, and (7) limiting beta cell apoptosis [6–11].
Mediated by GIP and GLP-1, the incretin effect accounts for 50–70% of the insulin response, which is essential for glucose homeostasis. It is also responsible for the deposition of two-thirds of the glucose ingested. The serum concentration of GIP is practically normal in early T2DM patients but tends to decrease as the disease progresses. On the other hand, GLP-1 secretion is reduced. The effect of GIP is severely impaired, although the GLP-1 effect is preserved. Continuous infusion of GLP-1 produced lower fasting and average plasma glucose concentrations, decreased glycosylated hemoglobin (HbA1c) levels, and improved insulin sensitivity [11].
Surgical procedures that increase hindgut (ileum) delivery of nutrients stimulate the release of incretins and could promote control of T2DM. Nevertheless, the more extensive the intestinal bypass, the greater are the chances of nutritional and metabolic complications [2, 12–15].
Recent reports have suggested that the same metabolic benefits (e.g., improved glucose tolerance) could be reproduced without restricting gastric size or intestinal resection through a procedure termed ileal interposition (II). II introduces a segment of terminal ileum into the proximal jejunum and allows premature exposure of nutrients to the interposed ileum, thus stimulating GLP-1- and PYY-producing L cells without disrupting intestinal transit or absorption [16]. The raised level of the anorectic peptides and the delay in gastric emptying reduces hunger and provides a longer sensation of satiety, both of which contribute to weight loss [17, 18]. A study involving experimental animals demonstrated that application of the II procedure does not result in problems relating to malnutrition or nutrient absorption [16].
The identification of ghrelin, an orexigenic hormone (appetite stimulant), signaled the importance of mechanisms involved in the decision to eat. Serum levels of ghrelin are elevated before meals and decrease rapidly after food ingestion. It was shown in patients undergoing RYGB that ghrelin levels did not rise before meals [19]. Ghrelin also stimulates the secretion of counterregulatory hyperglycemic hormones (e.g., glucagon, catecholamines, cortisol, growth hormone) and suppresses secretion of the insulin-sensitizing hormone adiponectin by fat cells, consequently blocking insulin signaling in the liver and inhibiting insulin secretion [20, 21]. In contrast to RYGB, sleeve gastrectomy (SG) foregoes intestinal rerouting but involves removing the fundus, thereby eliminating the diabetic effects of ghrelin.
DePaula et al. [18, 22, 23] have proposed SG + II as a surgical alternative to improve remission of T2DM and its co-morbidities. Following this procedure, the presence of the terminal ileum near the gastric drain would stimulate premature production of incretin hormones, whereas gastric resection would reduce ghrelin levels and accelerate stomach emptying [16, 22]. Initial results are highly encouraging. The aim of the present study was to evaluate the short- and mid-term effects of laparoscopic SG/II in T2DM patients. The variables investigated were the feasibility of the procedure, remission/alleviation of the disease, morbidity, mortality, and weight loss.
Patients and methods
The São José do Avaí Hospital Ethics Committee approved the study registered on the National Information System on Ethics in Human Research (CAAE—0023.0.316.000-10). Informed written consent was obtained from all subjects prior to their inclusion in the study.
Patients
The nonrandomized prospective 1-year study involved 30 T2DM patients who had undergone an SG/II procedure during the period March 2009–March 2010. The time span between the procedure and inclusion of the patient in the study varied from 6 to 18 months (average of 13.0 ± 3.3 months).
Criteria for inclusion/exclusion in the study
The inclusion criteria were as follows: (1) patients diagnosed with T2DM for at least 3 years according to the standards adopted by the American Diabetes Association (ADA) [24]—i.e., fasting glucose ≥126 mg/dl, glycosylated hemoglobin (HbA1c) ≥6.5%, fasting plasma glucose ≥200 mg/dl, and 2-h glucose ≥200 mg/dl in the 75-g oral glucose tolerance test; (2) evidence of stable treatment with oral hypoglycemic drugs and/or insulin for more than 12 months; (3) age between 18 and 70 years; (4) stable weight (defined by an alteration of <3.0% during the last 3 months prior to the study); (5) possibility of clinical follow-up for 24 months; (6) serum peptide C >0.5 ng/ml.
The exclusion criteria were (1) positive anti-glutamic acid decarboxylase (anti-GAD) antibodies; (2) type 1 diabetes; (3) active hepatic disease; (4) serum levels of liver enzymes threefold higher than normal; (5) alcoholism or addiction to illicit drugs; (6) coexisting malignant diseases or occurrence thereof during the last 4 years; (7) glomerular filtration rate <30 ml/h; or (7) American Society of Anesthesiologists (ASA) classification > III.
Surgical intervention
Patient was placed on the right side in a 30° reverse Trendelenburg position. Surgeon and assistant camera stayed at the right side and the assistant on the left side. Devascularization of the large gastric curvature was done with ultrasonic scissors, starting 5 cm from the pylorus, moving in the cranial direction, and up to the His angle.
A 32F Fouchet catheter was introduced. Gastric sectioning was performed with a blue cartridge linear stapler beginning in the antrum–body transition, about 5–10 cm from the pylorus, according to the patient’s body mass index (BMI). In patients with BMI <30 kg/m2, the first gastric sectioning was done 10 cm from the pylorus in the cranial direction. In patients with BMI >30 kg/m2, the starting point was 5 cm from the pylorus. Gastric resection was accomplished with a sharp linear blue load stapler of 45 or 60 mm, until reaching the His angle. A running suture, across the staple line, was done with 3-0 polypropylene thread.
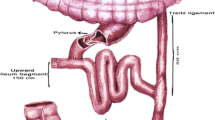
For the II procedure the ligament of Treitz was identified, and the jejunum was divided 30 cm distally. After the cecum was identified, the distal ileum was transected 30 cm proximal to the ileocecal papilla. A total of 170 cm of ileum was measured proximally and transected (Fig. 1). This segment of ileum was interposed in an isoperistaltic way into the proximal jejunum, previously divided. Next, we performed a three side-to-side enteroanastomosis. The first one was the ileoileostomy, followed by the jejunoileostomy, and finally the ileojejunostomy. All three mesenteric defects were closed with interrupted sutures (Fig. 2).
Evaluation of surgical outcome
Surgical outcomes were evaluated according to ADA proposed criteria [25]. Patients were divided into two groups on the basis of their postsurgical status: (1) those in remission of T2DM, defined by HbA1c <6.5% without oral hypoglycemic drugs or insulin; and (2) those with alleviation of their T2DM, defined as a reduction of at least 25% in the fasting plasma glucose level and of at least 1% in the HbA1c level with hypoglycemic drug treatment.
Results
Characteristics of the patients
The study population (n = 30) comprised 10 women (33.3%) and 20 men (66.7%) with an average age of 49.7 ± 8.9 years (median 49 years, range 33–68 years). The average BMI of the population was 30.8 ± 5.1 kg/m2 (median 31.1 kg/m2; range 19.9–40.1 kg/m2), and mean time from T2DM diagnosis was 9.9 ± 4.4 years (range 4–20 years).
Surgical outcome
The mean operating time was 181.47 ± 53.23 min, and the mean postoperative hospital stay was 3.17 ± 0.79 days. There were no intraoperative complications, and none of the patients required conversion to open surgery. There were no deaths.
Postsurgical complications included metabolic ketoacidosis in one patient (3.3%), urinary infection in one (3.3%), and diarrhea in two (6.6%). Three months after the operation, one patient (3.3%) presented with cholecystolithiasis and another (3.3%) with bowel obstruction caused by adhesions. Both problems were resolved by laparoscopy.
Patients were followed for a period of 6–18 months (average 13 ± 3.3 months) after surgery. All 30 patients evolved with weight loss: i.e., the BMI values of the patients prior to and after surgery were significantly different (P = 0.0001) as determined by paired t tests (Table 1). Additionally, the mean postsurgical blood levels of HbA1c, fasting glucose, and fructosamine were significantly lower (P = 0.0001, 0.0001, and 0.0004, respectively) than those determined prior to surgery (Table 2). The distribution of patients according to postsurgical evolution of T2DM is shown in Table 3. All patients evidenced improvement in their T2DM over the months that followed surgery, and remission was observed in 80% of patients such that these subjects no longer required treatment with antidiabetes drugs. Presurgical BMI was not a determinant factor for the postsurgical remission of T2DM. There were no significant differences between patients in the two outcome groups with respect the presurgical duration of T2DM. In addition, there were no significant differences between the three groups of patients regarding duration of insulin usage prior to surgery.
Discussion
The aim of the present study was to evaluate the feasibility of SG/II in 30 T2DM patients and determine the postsurgical outcomes in terms of morbidity, mortality, weight loss, and disease alleviation or remission.
The effects associated with the increased levels of GLP-1 induced by procedures involving intestinal derivation could be the basis of metabolic surgery as this hormone inhibits acid secretion by the stomach, increases the sensation of satiety, and reduces appetite and gastric motility [26–28]. Additionally, augmentation of GLP-1 leads to increased secretion of insulin and postprandial suppression of glucagon secretion, together with preservation, and possible hypertrophy, of the beta cell mass. Moreover, it is believed that GLP-1 is involved in the differentiation of progenitor duct cells into beta cells, thus limiting apoptosis of these cells [11, 29, 30].
Studies based on meta-analyses, such as those reported by Buchwald et al. [2, 31], reveal a high rate of resolution (78.1%) among diabetic patients who had undergone bariatric surgery. Indeed, the rate of T2DM remission following BPD operations can be as high as 95.0% as indicated in a number of reports [32–34].
Glycemic control following RYGB is well documented [34–36], although some studies have questioned the real efficacy of this procedure in the control of T2DM. For instance, DiGiorgi et al. [37], following evaluation of 42 obese diabetic patients who had undergone RYGB, observed remission of the disease in only 27 individuals (67%). Furthermore, the disease relapsed in 26% of the patients exhibiting T2DM remission; that is, only 41% of the patients achieved glycemic control over the short and mid term. Recurrence of the disease was associated with low preoperative BMI, indicating that surgical treatment of T2DM may not have the same results in nonobese patients. These authors concluded that additional investigations would be needed before it would be possible to recommend RYGB as a general alternative treatment for T2DM.
Chikunguwo et al. [38] examined 177 obese diabetic patients for an average of 8.6 years following an RYGB procedure and observed disease remission in 89% of the individuals. Within this group, disease recurrence was observed in 43% of the patients; thus, long-term glycemic control was observed in only 46% of the patients. These authors concluded that durable remission of T2DM correlated most closely with an early disease stage at surgery.
The rate of glycemic control in obese patients who undergo RYGB is apparently low over the mid and long term [37, 38], and the search for more efficient procedures for the treatment of T2DM in nonobese individuals was stimulated by results obtained from morbidly obese diabetic patients who underwent bariatric operations [34, 39–41]. A key outcome of these investigations was the development of the SG, a procedure that has been increasingly employed in the treatment of morbid obesity and T2DM. Among 23 obese diabetic patients who underwent SG, 16 (69.6%) had remission of T2DM within a follow-up period of 36 months [42]. Similarly, T2DM was reportedly resolved in 63.0% of cases treated by SG and followed for a period of 6 months [43].
DePaula et al. [17, 18, 23] demonstrated that SG coupled with II is also effective in the control of T2DM and associated diseases along with their complications in nonobese patients (BMI <30 kg/m2). Under these circumstances, the short- and mid-term resolution of T2DM was successful in as many as 95.7% of the cases. These authors also reported that SG/II induced an elevation in serum levels of GLP-1, GIP, and PYY and a reduction in serum ghrelin. The fundamentals of the control of the metabolic syndrome following SG/II have been stated as [17]: (1) premature increase in GLP-1 levels resulting from the response of L cells (from the interposed ileum) to the presence of nondigested food, thus normalizing first-phase insulin secretion (up to 30 min); and (2) GIP-mediated correction of the defect relating to the amplification of second-phase insulin secretion (from 20 to 120 min) in response to glucose caused by the diminution or absence of stimuli on duodenal K cells. SG can also control the obesity exhibited by up to 60% of T2DM patients by caloric restriction and adjusted weight loss.
In the present study, 80% of T2DM patients who underwent SG/II exhibited remission during the 6- to 18-month follow-up period. Thus, during the follow-up period they no longer required antidiabetes medication. Furthermore, 20% of the T2DM patients exhibited considerable improvement after surgery, although medication was still required in such cases. These findings are similar to those reported by DePaula et al. [44], who also used the ADA evaluation criteria for T2DM [24]. Their study involved 72 patients with a mean T2DM duration of 24.5 months and an average BMI that was slightly high (27 kg/m2). In all, 50% of the patients exhibited total remission, 36.1% showed partial remission, and 13.9% experienced alleviation of their condition.
On the basis of the available evidence, improved status of treated patients could be occasioned by a combination of factors including hormonal alterations, caloric restriction, and weight loss. The identification of a predominant factor is likely to be extremely complex owing to the multifaceted physiology of T2DM. Some studies have demonstrated that the duration of T2DM and the use of insulin are determinant factors for control of the disease after RYGB [34–36]. Our results did not find an association of the duration of T2DM and the possibility of disease control after surgery. The average duration of the disease among the studied subjects was 9.9 ± 4.4 years, a value that was similar to that presented by patients showing alleviation of the T2DM (10.7 ± 5.5 years) and to those exhibiting remission (10.0 ± 4.4 years). Similarly, the presurgical use of insulin was not a determinant factor for diabetes control after SG/II as, prior to surgery, the average HbA1c values were high (9.5 ± 1.75%) indicating inadequate control of the disease even though some of the patients had probably required the use of insulin before surgical intervention. It is possible that a more expressive casuistry and a longer follow-up period would allow a more consistent relation to be established.
Even though the differences in BMI values before and after surgery were statistically significant (Table 1), the procedure did not induce the excessive weight loss that has been reported in previous studies [17, 22, 23]. Presurgical BMI values (i.e., >30 or <30 kg/m2) were not a determining factor for the postsurgical remission of T2DM.
The SG/II procedure employed in the present study was performed using a laparoscopic procedure. As emphasized by other researchers [17, 18, 22, 23, 45], weight control and amelioration of the metabolic syndrome are the foremost benefits of this type of surgery. However, it is essential to consider the technical difficulties and potential complications before opting for this approach. The average operating time (181.5 ± 53.2 min) was similar to that required for RYGB and BPD procedures [46, 47]; and considering the clinical conditions of the patients and the magnitude and complexity of the procedure, it was well within acceptable limits. Similar considerations can be applied to the relatively short hospital stay (3.2 ± 0.8 days).
Although the present study involved a limited number of patients and a short- to mid-term follow-up, the findings presented herein are valuable in that they suggest excellent possibilities for the development of metabolic surgery, and they confirm the results obtained from similar operations performed on rats, dogs, and humans [48–55]. Despite the weight of evidence in favor of SG/II, however, two key points must be considered: Will the long-term (>8 years) outcome be comparable with the optimistic results shown in the short to mid term? Is glycemic control similar to that observed after bariatric surgery (RYGB) in which some recent results show a recurrence rate close to 50% [37, 38]? It is evident that longer postsurgical follow-up of patients is essential to answer these critical issues.
Conclusions
The SG/II procedure can produce adequate glycemic control and satisfactory weight loss with no clinical signs of nutritional deficiencies. Such findings indicate that this operation, which interferes with glucose metabolism, is a promising alternative for patients with T2DM. Laparoscopic SG/II is an effective, safe, reproducible technique that produces few short- or mid-term complications. However, a much longer postsurgical follow-up is essential to answer the critical issues relating to the maintenance of glycemic control in treated patients.
References
Pinkey J (2010) Bariatric surgery for diabetes: gastric banding is simple and safe. Br J Diabetes Vasc Dis 10:139–142
Buchwald H, Estok R, Fahrbach K et al (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122:248–256
Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG et al (2003) Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52:1098–1103
Gautier JF, Fetita S, Sobngwi E et al (2005) Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab 31:233–242
Atkinson RL, Brent EL, Wagner BS et al (1983) Energy balance and regulation of body weight after intestinal bypass surgery in rats. Am J Physiol 245:R658–R663
Kjems LL, Holst JJ, Volund A et al (2003) The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52:380–386
Drucker DJ (2003) Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 26:2929–2940
Irwin N, Gault VA, Green BD et al (2004) Effects of short-term chemical ablation of the GIP receptor on insulin secretion, islet morphology and glucose homeostasis in mice. Biol Chem 385:845–852
Gault VA, Irwin N, Green BD et al (2005) Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 54:2436–2446
Ferrannini E, Cobelli C (1987) The kinetics of insulin in man. I. General aspects. Diabetes Metab Rev 3:335–363
Vilsboll T, Holst JJ (2004) Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia 47:357–366
Aasheim ET, Hofso D, Hjelmesaeth J et al (2008) Peripheral neuropathy and severe malnutrition following duodenal switch. Obes Surg 18:1640–1643
Varma S, Baz W, Badine E et al (2008) Need for parenteral iron therapy after bariatric surgery. Surg Obes Relat Dis 4:715–719
Shikora SA, Kim JJ, Tarnoff ME (2007) Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract 22:29–40
El-Kadre L, Rocha PR, de Almeida Tinoco AC et al (2004) Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg 14:1062–1066
Strader AD (2006) Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav 88:277–282
DePaula AL, Macedo AL, Schraibman V et al (2009) Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20–34. Surg Endosc 23:1724–1732
DePaula AL, Macedo AL, Rassi N et al (2008) Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc 22:2670–2678
Kojima M, Hosoda H, Date Y et al (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Cummings DE, Weigle DS, Frayo RS et al (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630
Williams D, Cummings D (2005) Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr 135:1320–1325
DePaula AL, Macedo AL, Rassi N et al (2008) Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc 22:706–716
DePaula AL, Macedo AL, Mota BR et al (2009) Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg Endosc 23:1313–1320
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33:62–69
American Diabetes Association (2010) Executive summary: standards of medical care in diabetes—2010. Diabetes Care 33:S4–S10
Turton MD, O’Shea D, Gunn I et al (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72
Verdich C, Flint A, Gutzwiller JP et al (2001) A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389
Kastin AJ, Akerstrom V, Pan W (2002) Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18:7–14
Nauck MA, Kleine N, Orskov C et al (1993) Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744
Drucker DJ (2003) Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 26:2929–2940
Buchwald H, Williams SE (2004) Bariatric surgery worldwide 2003. Obes Surg 14:1157–1164
Wittgrove AC, Clark GW (2000) Laparoscopic gastric bypass, Roux-en-Y—500 patients: technique and results, with 3–60 month follow-up. Obes Surg 10:233–239
Sugerman HJ, Wolfe LG, Sica DA et al (2003) Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237:751–756
Schauer PR, Burguera B, Ikramuddin S et al (2003) Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238:467–484
Kim S, Richards WO (2010) Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann Surg 251:1049–1055
Torquati A, Lutfi R, Abumrad N et al (2005) Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 9:1112–1116
DiGiorgi M, Rosen DJ, Choi JJ et al (2010) Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 6:249–253
Chikunguwo SM, Wolfe LG, Dodson P et al (2010) Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 6:254–259
Rubino F (2009) Access to bariatric surgery and patients with diabetes. JAMA 302:1055–1056
Buchwald H, Oien DM (2009) Metabolic/bariatric surgery worldwide 2008. Obes Surg 19:1605–1611
Buchwald H, Williams SE (2006) Bariatric surgery training in the United States. Surg Obes Relat Dis 2:52–55
Todkar JS, Shah SS, Shah PS et al (2010) Long-term effects of laparoscopic sleeve gastrectomy in morbidly obese subjects with type 2 diabetes mellitus. Surg Obes Relat Dis 6:142–145
Rosenthal R, Li X, Samuel S et al (2009) Effect of sleeve gastrectomy on patients with diabetes mellitus. Surg Obes Relat Dis 5:429–434
DePaula AL, Stival AR, DePaula CC et al (2010) Impact on dyslipidemia of the laparoscopic ileal interposition associated to sleeve gastrectomy in type 2 diabetic patients. J Gastrointest Surg 14:1319–1325
Kumar KV, Ugale S, Gupta N et al (2009) Ileal interposition with sleeve gastrectomy for control of type 2 diabetes. Diabetes Technol Ther 11:785–789
Pournaras DJ, Jafferbhoy S, Titcomb DR et al (2010) Three hundred laparoscopic Roux-en-Y gastric bypasses: managing the learning curve in higher risk patients. Obes Surg 20:290–294
Suter M, Giusti V, Heraief E et al (2003) Laparoscopic Roux-en-Y gastric bypass: initial 2-year experience. Surg Endosc 17:603–609
Strader AD, Vahl PV, Ronald J et al (2004) Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:447–453
Strader AD (2006) Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav 88:277–282
Strader AD, Clausen TR, Goodin SZ et al (2009) Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg 19:96–104
Strader AD, Vahl TP, Jandacek RJ et al (2005) Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:E447–E453
Patriti A, Annetti C, Sidoni A et al (2007) How the hindgut can cure type 2 diabetes: ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery 142:74–85
Patriti A, Facchiano E, Annetti C et al (2005) Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 15:1258–1264
Koopmans HS, Sclafani A, Fichtner C et al (1982) The effects of ileal transposition on food intake and body weight loss in VMH-obese rats. Am J Clin Nutr 35:284–293
Boza C, Gagner M, Devaud N et al (2008) Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): a new surgical procedure as effective as gastric bypass for weight control in a porcine model. Surg Endosc 22:1029–1034
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tinoco, A., El-Kadre, L., Aquiar, L. et al. Short-Term and Mid-Term Control of Type 2 Diabetes Mellitus by Laparoscopic Sleeve Gastrectomy with Ileal Interposition. World J Surg 35, 2238–2244 (2011). https://doi.org/10.1007/s00268-011-1188-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1188-2