Abstract
Background
The surgical treatment for obesity promotes massive weight loss and early improvement in co-morbid conditions such as type-2 diabetes. Because surgically mediated glycemic improvements are immediate, the mechanisms involved appear to be weight loss independent. Ileal interposition has been used to gain understanding of the relative role that the lower intestine plays in mediating metabolic improvement. Here, we report that ileal interposition is sufficient for improving glucose tolerance in a low-dose streptozotocin-treated diabetic rat model as well as in normal rats with no effect on body weight.
Methods
Male Long–Evans rats were treated with streptozotocin (35 mg/kg) or left untreated and then received sham or ileal interposition. Body weight was measured as well as glucose and insulin tolerance. Plasma insulin and gut hormones were measured during the glucose tolerance test.
Results
Streptozotocin treatment resulted in hyperglycemia within 48 h after treatment. Diabetic rats with ileal interposition showed improvement in glucose tolerance as early as 4 weeks after surgery compared to sham (p < 0.05). By 11 weeks after surgery glucose and insulin tolerance was markedly improved in interposed-diabetic compared to sham-diabetic rats (p < 0.05). Normal non-diabetic rats showed improved glucose tolerance after ileal interposition compared to sham (p < 0.05). Insulin secretion was increased in interposed rats following glucose administration (p < 0.05). The ileal-derived hormones glucagon like peptide-1 (GLP-1), peptide YY (PYY), and glucagon were all significantly elevated in the ileal interposed rats (p < 0.01). Gastric inhibitory polypeptide (GIP) was unchanged. In neither study did body weight between the surgical groups differ at any time point.
Conclusions
Ileal interposition effectively improves glucose tolerance in streptozotocin-diabetic and euglycemic rats. Enhanced insulin secretion can explain the lowered glucose concentrations in euglycemic rats following ileal interposition. Ileal interposition is associated with dramatically elevated ileal hormones, GLP-1, PYY, and glucagon (p < 0.01) with no change in the duodenal hormone GIP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity surgery is currently the most effective treatment for morbid obesity. Weight loss is typically massive and co-morbid conditions show dramatic amelioration following surgery. Specifically, patients demonstrate immediate and long-standing improvement or resolution of type 2 diabetes following specific surgical procedures that appears to be independent of weight loss. Gastric bypass and biliopancreatic diversion result in 84% and 99% resolution of type 2 diabetes respectively [1]. Restrictive procedures such as gastric banding also result in improvement in type 2 diabetes; however, with a later appearance, that is likely a consequence of weight loss.
Anatomically, the surgical procedures that are most effective in resolving type 2 diabetes include nutrient diversion to the lower small intestine. This interesting commonality has led to the “hind-gut” hypothesis that suggests that increased nutrient delivery to the lower small intestine leads to the secretion of hormones or factors that act as mediators in the diabetic improvement. To isolate the effects of lower intestinal stimulation in the absence of restriction or a bypassed intestinal tract, we utilized a novel surgical model called ileal interposition. Ileal interposition (formerly transposition) involves the relocation of a short portion of lower intestine, primarily ileum, to a more proximal region within the jejunum [2]. The hypothesis behind the model, first proposed by Mason, is that lower intestinal stimulation may be an important component in mediating the post-surgical improvement in type 2 diabetes [3].
Previous studies have demonstrated improved glucose homeostasis in diabetic humans and diabetic rodent models following ileal interposition surgery [4, 5]. Possible factors involved in the diabetic improvement include increased ileal-produced hormones such as glucagon like peptide-1 (GLP-1). GLP-1 is markedly increased following ileal interposition [6]. Because GLP-1 and GLP-1 receptor agonists promote beta cell proliferation, neogenesis, and reduced apoptosis, it is possible that increased beta cell mass or ‘health’ results from ileal interposition surgery [7–9]. Streptozotocin treatment reliably results in beta cell destruction and consequently causes severe hyperglycemia in rats [10]. Because ileal interposition results in increased GLP-1 secretion, we predicted hyperglycemia would be improved in streptozotocin-treated diabetic rats with ileal interposition compared to sham-operated rats. Further, we examined whether ileal interposition would improve glucose tolerance in normal euglycemic rats.
Methods
Animals
Age-matched male Long–Evans rats (Harlan, Indianapolis, IN, USA) were used for all experiments. Rats were individually housed in the University Vivarium under a light:dark cycle of 12:12. Rats were allowed free access to Purina rodent chow (Harlan Teklad) and water unless specified. All experiments were approved by the University IACUC committee.
Ileal Interposition Surgery
Rats received either sham or ileal interposition as described by Strader et al. [6]. Briefly, a midline abdominal incision was made and the cecum was externalized. Intestinal transections were made at 5 and 15 cm proximal to the ileocecal valve to isolate a 10-cm segment of ileum. A single anastomosis was made using 7-0 silk (Ethicon) at the site of the segment removal. The segment was laid aside and kept moist with warmed 0.9% saline while the remaining intestines were externalized to locate the Ligament of Treitz. The jejunum was transected 5 cm distal to the ligament of Treitz and the segment was inserted using anastomosis in an iso-peristaltic direction. The intestines were bathed in 0.9% saline and re-inserted into the abdominal cavity. Sham-operated rats received three transections in the same locations as the ileal interposition group, which were immediately re-joined by anastomosis. The duration of both surgical procedures was approximately 45 min.
Experiment 1: Effects of Ileal Interposition or Sham Surgery on Glucose Homeostasis in STZ-treated Diabetic Rats
Rats were given a single intraperitoneal injection of streptozotocin (STZ) (Sigma-Aldrich) (35 mg/kg dissolved in ice cold citrate buffer pH 4.5 to induce hyperglycemia). It has been shown that there is a dose–response effect following STZ and that a 35 mg/kg dose results in an 80% reduction in plasma insulin immunoreactivity [10]. It was important that some beta cell mass be preserved as to not create an insulin-deficient diabetic model.
Forty-eight hours following the STZ treatment, non-fasting glucose was measured in duplicate from tail vein blood using a handheld (Freestyle, Alameda, CA, USA) glucometer to assess hyperglycemia. One week after STZ treatment, rats were given an oral glucose tolerance test (OGTT; 1 g/kg, 20% dextrose). Rats were fasted overnight (16 h) followed by an oral gavage of 20% dextrose in the concentration of 1 g/kg. Blood samples were taken from the tail vein for the measurement of blood glucose in duplicate at times 0, 15, 30, 45, 60, and 120 min. Two weeks after STZ treatment, all rats received either sham (n = 6) or ileal interposition (n = 5) surgery. Body weights were measured at various time points following surgery. Two additional OGTTs were performed at 4 and 11 weeks following surgery. For the OGTT at 11 weeks, plasma insulin was measured in duplicate in each of the samples using the commercially available rat insulin ELISA kit from Crystal Chem Inc. (Downer’s Grove, IL, USA). An insulin tolerance test was performed at 13 weeks. For the insulin tolerance test, rats were fasted overnight (16 h) followed by an intraperitoneal injection of insulin (0.75 U/kg). Tail vein blood samples were taken at 0, 15, 30, and 60 min for glucose measurement.
Experiment 2: Effects of Ileal Interposition or Sham Surgery on Glucose Homeostasis in Non-diabetic Rats
Seventeen mature male Long–Evans rats received either sham (n = 9) or ileal interposition surgery (n = 8). During the recovery period all rats were provided with ad libitum access to Purina rodent chow and water. At 12 weeks following surgery, an oral glucose tolerance test was performed to assess glucose tolerance. Following an overnight fast (16 h), rats received an oral gavage of 20% dextrose (2 g/kg) and blood samples were collected for measurement of blood glucose (time 0, 15, 30, 45, 60, and 120 min) plasma insulin and the gut hormones GLP-1, peptide YY (PYY), gastric inhibitory polypeptide (GIP), and glucagon (time 0, 15 and 30 min). Glucose was measured in duplicate using a handheld glucometer (Freestyle) and plasma insulin was measured in duplicate using a rat insulin ELISA (Crystal Chem). Total bile acids were measured from the time 0 sample from the OGTT.
Assays
Total Bile Acids Assay
Enzyme Cycling Method
Total bile acids levels were measured in plasma containing a DPPIV (Linco Research Inc.) inhibitor, using an enzyme cycling-based Total Bile Acids Assay Kit from Diazyme (catalog number DZ042A, San Diego, CA, USA). The assay was performed according to the manufacturer’s protocol, with slight modifications. Briefly, the assay was run in 96-well microtiter plates. The enzyme cycling assay is a method that allows for signal amplification through cycled regeneration reactions. Serum bile acid molecules are repeatedly oxidized and reduced by the enzyme 3-α-hydroxysteroid dehydrogenase (3-α-HSD) with a concomitant accumulation of reduced co-enzyme thio-NADH. The rate of thio-NADH formation is proportional to the amount of total bile acids in the sample. Measurement of thio-NADH was performed at 405 nm using a SpectraMax plate reader.
Multiplex Assay
Plasma levels of insulin, PYY, and GIP were measured in plasma containing a DPPIV inhibitor (Linco Research) using the rat gut hormone LINCOplex assay (catalog number RGT-88K, Linco Research). The assay was performed according to the manufacturer’s protocol. The LincoPlex assay is based on conventional sandwich assay technology. The antibody specific to each hormone is covalently coupled to Luminex microspheres with each antibody coupled to a different microsphere uniquely labeled with a fluorescent dye. The microspheres can be discriminated by using the dual-laser based Luminex xMAP technology. This allows for the measurement of multiple hormones in a single assay using limited amount of material. Briefly, microspheres were incubated with standards, controls, and samples (25 μl) in a 96-well microtiter filter plate overnight (16–18 h) at 2–8°C while shaking. After incubation, the plate was washed and the detection antibody was added. After 30-min incubation at room temperature, streptavidin–phycoerythrin was added for an additional 30 min. After washing, the beads were resuspended and read on the Lumine×100 instrument to determine the concentration of the hormones of interest. All samples were tested in duplicate wells and results reported as the mean of the duplicates.
Total GLP-1 and Glucagon, LOCI Assays
Plasma levels of total GLP-1 and glucagon were measured in plasma using a Luminescence Oxygen Channeling Immuno assay (LOCI). LOCIis a homogenous bead based sandwich assay which include donor and acceptor beads and a biotinylated antibody (which is one of the antibodies in the sandwich). The donor beads are coated with streptavidin and contain a photosensitive dye. The acceptor beads are coated with the other antibody making up the sandwich. During the assay the three reactants combine with analyte to form a bead-aggregate-immune complex. Illumination of the complex releases singlet oxygen from the donor beads which channels into the acceptor beads and triggers chemiluminescence which is measured in an EnVision plate reader. The amount of light generated is proportional to the concentration of hormone in the sample.
Two antibodies recognizing different sites of the hormone of interest (total GLP-1 or glucagon) were used. One was biotinylated and the other was used to coat the acceptor beads. Briefly, biotinylated antibody and antibody coated acceptor beads (15 μl) were incubated with calibrator, control, or analyte (2 μl) in a 384-well microtiter plate covered with a black lid for 1 hour at room temperature. After incubation, 30 μl of streptavidin coated donor beads was added for an additional 30 min. The plate was read in an EnVision Turbo Alpha plate reader from Perkin Elmer to determine the concentration of total GLP-1 or glucagon in the sample. All samples were tested in replicate wells and results reported as the mean of the replicates.
Statistics
Body weight changes over time were analyzed using a two-way ANOVA with repeated measures. Fasting glucose concentrations between ileal interposed and sham-operated rats were compared using a t test. All glucose tolerance, insulin, and gut hormone data were analyzed using a two-way ANOVA with repeated measures. A Bonferroni test was used for post hoc analysis. Area under the curve was analyzed using a t test. All statistics were performed using Prism Statistical Software and significance was set at p < 0.05. All data are expressed as mean ± SEM.
Results
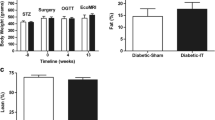
Low-dose streptozotocin-treated Long–Evans rats were utilized for the present study to determine whether ileal interposition would improve hyperglycemia. In experiment 1, the rats were given a 35 mg/kg intraperitoneal injection of streptozotocin. As shown previously, the present dose of STZ resulted in significant hyperglycemia in all rats (Fig. 1a,b). The average non-fasting blood glucose concentration before surgery for each group was 345.3 ± 16 (sham) and 337.5 ± 19.8 (interposition) (Fig. 1a). A pre-surgical fasting oral glucose tolerance test was performed to show that all rats were sufficiently hyperglycemic (Fig. 1b). At the onset of the experiment, rats did not differ in body weight and continued to show similar body weights following STZ treatment (Fig. 2). Interestingly, following surgery, sham and ileal interposition rats lost and re-gained the same amount of body weight (Fig. 2). At no point during the 13-week experiment was there a difference in body weight between the groups.
To determine whether improved glycemia following obesity surgery was a consequence of body weight loss, a glucose tolerance test was performed. Because all rats in our present study remained similar in body weight despite their surgical treatment, this question could be answered. At 4 weeks following surgery, rats that received ileal interposition showed improved glucose tolerance (AUC) compared to sham-operated rats (Fig. 3). Although a two-way ANOVA failed to identify individual time points that were significantly different during the OGTT, a t test determined that the area under the curve was significantly lower for the interposed rats compared to sham (8,772.8 ± 1027.7 vs. 10,296.3 ± 1,595.9; p < 0.05, Fig. 3b). A second OGTT performed at 11 weeks following surgery revealed significantly improved glucose tolerance in the interposed surgical group compared to sham. Fasting as well as post-glucose gavage time points (30, 45, 60, and 120 min) during the OGTT were significantly lower in the interposed group (two-way ANOVA repeated measures, p < 0.05, Fig. 4a). Total glucose excursions expressed as area under the curve were lower for the interposed group (t test, p < 0.05, Fig. 4c). Insulin concentrations were measured and were similar at all time points during the OGTT (Fig. 4b). An insulin tolerance test at 13 weeks following surgery showed that rats with ileal interposition had lower fasting blood glucose (p < 0.01) prior to the test as well as lower glucose 15 min following insulin administration (two-way ANOVA repeated measures, p < 0.05, Fig. 5). The insulin dose was similar in both groups as body weights were very similar (0.75 U/kg).
Glucose tolerance test 4 weeks after ileal interposition or sham surgery. STZ-treated fasted rats were given a 1 g/kg d-glucose gavage and blood glucose was measured in duplicate at times 0, 15, 30, 45, 60, and 120 min (a). Area under the curve was determined using trapezoidal analysis and showed a significant improvement in rats with ileal interposition surgery (b)
Glucose tolerance test 11 weeks after ileal interposition or sham surgery. STZ-treated fasted rats were given a 1 g/kg d-glucose gavage and blood glucose was measured in duplicate at times 0, 15, 30, 45, 60, and 120 min (a). Insulin was measured at each time point during the test (b). Area under the curve was determined using trapezoidal analysis and showed a great improvement in rats with ileal interposition (c)
Experiment 2 tested whether normal chow-fed rats would show improvements in glucose tolerance following ileal interposition. Chow-fed rats showed a significant improvement in glucose tolerance 12 weeks after interposition surgery compared to sham. Specifically, significance was determined at 45 and 60 min after the glucose administration (two-way ANOVA repeated measures, p < 0.001, Fig. 6b). Area under the curve was approximately 25% lower for interposed rats compared to sham (t test, p < 0.05, Fig. 6d). The lowered glucose excursions may be partially due to increased insulin secretion during the test as plasma insulin was significantly elevated in the interposed surgical group (two-way ANOVA repeated measures, p < 0.001, Fig. 6c). Lastly, there were no differences in body weight between the surgical groups (Fig. 6a).
Body weight and glucose tolerance data in euglycemic rats 12 weeks after ileal interposition or sham surgery. No difference was noted in body weight between the surgical groups (a). A glucose tolerance test was administered to fasted rats and blood glucose was measured in duplicate at times 0, 15, 30, 45, 60, and 120 min (b). Insulin was measured at times 0, 15, and 30 min (c). Area under the curve was determined using trapezoidal analysis and was improved in rats with ileal interposition (d)
During the glucose tolerance test additional blood samples were taken at 0, 15, and 30 min for measurement of plasma hormones. All of the ileal-derived hormones (glucagon like peptide-1, peptide YY, and enteric glucagon) were significantly elevated following glucose administration at 15 and 30 min (Fig. 7a–c, two-way ANOVA repeated measures, p < 0.01). No differences were seen between surgical groups at the baseline (time 0) time point. In addition, no differences were noted at any time point for the duodenal hormone gastric inhibitory polypeptide (Fig. 7d). Total bile acids in plasma were measured in fasted rats and a 3-fold elevation was noted in the rats with ileal interposition (t test, p < 0.05, Fig. 8) compared to sham.
Discussion
Gastric bypass surgery involves a large degree of gastrointestinal restructuring including exclusion of the proximal small intestine and increased nutrient delivery to the lower small intestine. To isolate the effects of lower intestinal stimulation on weight loss and metabolism, we chose the surgical model ileal interposition. Ileal interposition has been previously studied primarily in rodents but more recently in humans for its usefulness as a potential ‘anti-diabetic’ surgery [2, 4–6, 11]. The present findings contribute to the existing literature in support of ileal interposition or ‘hind-gut’ stimulation as a possible future intervention for type 2 diabetes.
Streptozotocin treatment renders rodents hyperglycemic through the selective destruction of beta cell mass [10]. A dose–response effect exists for streptozotocin with low doses sparing a small number of functional beta cells capable of secreting insulin. A dose of 35 mg/kg has been estimated to destroy approximately 4/5 of insulin immunoreactivity in the rat [10]. In the present study, rats treated with this dose of streptozotocin achieved non-fasting glucose concentrations above 300 mg/dl within 48 h (Fig. 1a). In addition, prior to ileal interposition or sham surgery, both groups showed similar peak glucose concentrations during a glucose tolerance test (Fig. 1b). Following surgery, glucose tolerance was evaluated and the area under the curve showed a small but significant improvement at 4 weeks (Fig. 3b), but a more dramatic improvement at 11 weeks (Fig. 4c) in the ileal interposition group. Diabetic rats with ileal interposition had an area under the curve that was 25% lower than diabetic rats with sham surgery. Similarly, by 13 weeks fasting glucose was lower in rats with ileal interposition compared with sham rats (Fig. 5). However, both surgical groups reached similar glucose levels during an insulin tolerance test. These findings lend further support that lower intestinal stimulation alone may provide relief for type 2 diabetes.
Ileal interposition has previously been shown to improve hyperglycemia in the type 2 diabetic Goto–Kakizaki (GK) rat [5, 12]. The GK rat is a non-obese model of type 2 diabetes [13], and while studies show that glucose tolerance is improved following ileal interposition in this model, no differences were noted in post-surgical body weight gain between sham-operated rats. Remarkably, the effects we describe in both the STZ-diabetic and euglycemic rats were also achieved in the absence of any difference in body weight compared to sham-operated rats during the entire study period (Fig. 2 and Fig. 6a). The fact that glucose tolerance was improved independent of body weight is important as this is similar to the early improvement seen in humans following gastric bypass or biliopancreatic diversion [14]. Patients often demonstrate improvement within days after the above procedures, well before any measurable weight loss has occurred [15]. In the present study, we report that glucose tolerance is improved in the STZ-diabetic rat as early as 4 weeks. Previous studies in the GK rat show a similar time course for improvement with reductions in glucose (AUC) noted at 30 days [5]. However, fasting glucose shows a more rapid improvement at 1 week following surgery [12]. Finally, the idea that diabetes can be ameliorated in a diabetic rodent model using bariatric surgery independent of body weight changes was noted many years prior in a study showing that a partial (50%) jejuno-ileal bypass can immediately (1 week) resolve diabetes in the STZ-diabetic rat [16]. In contrast to the present study, jejuno-ileal bypass is also associated with significant malabsorption. Notably, jejuno-ileal bypass, like ileal interposition, involves lower intestinal stimulation without the exclusion of any proximal intestine.
One interesting difference between the previous rodent studies examining glucose tolerance improvement following ileal interposition and the present findings are in regards to the euglycemic rat. If nutrient delivery to the lower intestine reliably results in the release of beneficial factors or hormones, we hypothesized that glucose tolerance should be better in the interposed rats, despite the presence of a metabolic defect before surgery. Here we showed that chow-fed euglycemic Long–Evans rats with interposition secrete more insulin and therefore exhibited lower glucose excursions during a GTT compared to sham-operated rats. This is in contrast to that of Patriti et al. who failed to detect differences in glucose tolerance in euglycemic Sprague Dawley controls. These differences suggest the possibility of underlying species variability. Alternatively, the amount of glucose delivered during the glucose tolerance test may not have been sufficient to identify an effect of the surgery. In the present study, we chose 2 g/kg for the OGTT in euglycemic rats while Patriti et al. selected 1 g/kg. We too have previously failed to identify an improvement in glucose tolerance using a lower dose of 0.75 g/kg in the diet-induced obese rat model [6].
Regardless of any potential differences in species or metabolic model, glucagon like peptide-1 produced from ileal “L” cells is a likely candidate as a mechanism underlying early and prolonged improvements in post-surgical glucose control. We have previously demonstrated that fasting levels of proglucagon mRNA and glucose-stimulated GLP-1 are greatly elevated following ileal interposition compared to sham surgery in the diet-induced obese rat model [6]. Here again, we demonstrate in a euglycemic chow-fed rat that the ileal-produced hormones GLP-1 and PYY (Fig. 7) are greatly increased following ileal interposition. It is not surprising that secretion of GIP was unchanged since ileal interposition does not involve disruption or exclusion of the duodenum. In addition, glucagon was elevated and likely represents enteric glucagon or oxyntomodulin secretion. There is 80% and 20% cross reactivity for oxyntomodulin and glicentin, respectively for the glucagon antibody in the assay used in the present study. The contribution of enteroglucagon-derived hormones in post-surgical intestinal changes has been documented over 30 years ago in which patients with jejuno-ileal bypass surgery showed an increase in enteroglucagon within 3 weeks after surgery [17]. In humans, glucose-stimulated GLP-1 is elevated after both gastric bypass and biliopancreatic diversion [18, 19].
GLP-1’s roles as an incretin and a trophic factor on the beta cell are both consistent with the physiological changes seen after gastric bypass and possibly ileal interposition. Although GLP-1 was not measured in the STZ-diabetic rats, it is tempting to speculate that prolonged endogenous elevations in GLP-1 following ileal interposition may have resulted in a proliferative effect on the beta cell mass in the STZ-diabetic rats and perhaps the euglycemic rats as well. It has been shown that GLP-1 and the GLP-1 receptor agonist exendin-4 will ameliorate STZ-induced diabetes in rodents [20, 21].
The ileum is not only an important site of hormone synthesis and secretion, but it is also the key site for bile acid re-uptake. We hypothesized that with ileal interposition, the ileum is in a more proximal location within the small intestine and the ileal mucosa will proliferate upon nutrient stimulation which then presumably results in a higher absorptive capacity for bile acids. Here we show that euglycemic rats demonstrate elevated total bile acids in the plasma following ileal interposition. It has been previously shown that rats with ileal interposition have premature bile salt uptake at the site of the interposed segment, resulting in attenuated cholesterol absorption and transport [22]. A role for altered bile acid circulation in the resultant improvement in glucose homeostasis is highly likely as it has been shown that surgical diversion of only the common bile duct to the distal small intestine resolves STZ-induced diabetes in rodents and improves glucose tolerance in normal rats [23, 24]. Recent studies have identified elevated bile acid concentrations in the plasma of humans after gastric bypass surgery [25].
Because ileal interposition does not promote massive weight loss, as it is neither restrictive nor causes malabsorption, it is currently being performed in humans as a metabolic surgery due to its GLP-1-producing capabilities. Diabetic patients with BMIs below 35 kg/m2 showed great glycemic control (87% of patients) following ileal interposition with the remaining 13% of patients showing improvement [26]. However, the long-term effects of augmented GLP-1 secretion are unknown in both humans and rodents. Widespread use of ileal interposition in humans should be approached with caution since the elevations in GLP-1 following gastric bypass surgery has been implicated in post-surgical reports of hyperinsulinemic hypoglycemia with nesidioblastosis [27]. In addition, thorough patient screening to insure a diagnosis of type 2 diabetes opposed to slow onset type 1 diabetes appears to be critical for long-term control [28]. While improvement of type 2 diabetes is obviously an exciting consequence of ileal interposition surgery in humans, the long-term effects are unknown. Since ileal interposition is not a substitute for gastric bypass surgery, which also involves gastric restriction and duodenal exclusion and may lend additional important metabolic changes after surgery, it should still be considered a novel but promising potential treatment for type 2 diabetes in humans.
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama 2004;292:1724–37.
Atkinson RL, Whipple JH, Atkinson SH, et al. Role of the small bowel in regulating food intake in rats. Am J Physiol 1982;242:429–33.
Mason EE. Ileal [correction of ilial] transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg 1999;9:223–8.
Depaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc 2008;22:706–16.
Patriti A, Facchiano E, Annetti C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 2005;15:1258–64.
Strader AD, Vahl TP, Jandacek RJ, et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005;288:E447–53.
Perfetti R, Zhou J, Doyle ME, et al. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000;141:4600–5.
Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–6.
Li Y, Hansotia T, Yusta B, et al. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003;278:471–8.
Junod A, Lambert AE, Stauffacher W, et al. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest 1969;48:2129–39.
Koopmans HS, Ferri GL, Sarson DL, et al. The effects of ileal transposition and jejunoileal bypass on food intake and GI hormone levels in rats. Physiol Behav 1984;33:601–9.
Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg 2008;247:968–75.
Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 1976;119:85–90.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–81.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–50. discussion 350-2.
Morsiani E, Carpanelli MC. Observations on the metabolic effects of partial jejunoileal bypass in streptozotocin-treated rats. Eur Surg Res 1985;17:25–32.
Barry RE, Barisch J, Bray GA, et al. Intestinal adaptation after jejunoileal bypass in man. Am J Clin Nutr 1977;30:32–42.
Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91:1735–40.
Valverde I, Puente J, Martin-Duce A, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg 2005;15:387–97.
Tourrel C, Bailbe D, Meile MJ, et al. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 2001;50:1562–70.
Malendowicz LK, Macchi C, Nussdorfer GG, et al. Effects of prolonged exendin-4 administration on entero-insular axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med 2003;11:763–6.
Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol 1996;271:G681–91.
Ermini M, Iaconis E, Mori A. The effects of bilio-jejunal diversion on streptozotocin diabetes in the rat. Acta Diabetol Lat 1991;28:79–89.
Manfredini G, Ermini M, Scopsi L, et al. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol 1985;249:G519–27.
Patti ME, Bernier R, Bianco AC, et al. Gastric bypass surgery increases plasma bile acid levels: potential contribution to improved glucose tolerance. Chicago, Illinois, American Diabetes Association Annual Meeting; 2007.
DePaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc 2008;22:706–16.
Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005;353:249–54.
Deitel M. Surgery for diabetes at lower BMI: some caution. Obes Surg 2008;18:1211–4.
Acknowledgement
The present studies were funded in part through a research grant to A.D.S. from the American Society for Metabolic and Bariatric Surgery. We also thank Gitte Koelander Hansen and Vibeke Nielsen for technical assistance, Johannes Josef Fels and Hanne Skov Pedersen for carrying out glucagon analysis and Christian Rosenquist and Susanne Halkier for carrying out total GLP-1 analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strader, A.D., Clausen, T.R., Goodin, S.Z. et al. Ileal Interposition Improves Glucose Tolerance in Low Dose Streptozotocin-treated Diabetic and Euglycemic Rats. OBES SURG 19, 96–104 (2009). https://doi.org/10.1007/s11695-008-9754-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9754-x