Abstract
Background
Metabolic surgery is an effective treatment method for glycemic control and weight loss in obese patients with type 2 diabetes mellitus (T2DM). This study aimed to present the mid-term metabolic effects and weight loss results of the patients with T2DM who underwent transit bipartition with sleeve gastrectomy (TB-SG).
Methods
A total of 32 obese patients with T2DM who underwent TB-SG were included in the study. The T2DM remission status after surgery was evaluated. The postoperative glycemic variables, weight loss, lipid profile, and nutritional profile were also compared with the baseline values.
Results
At 36 months after surgery, T2DM remission occurred in 27 patients (84.3%) and the mean BMI decreased from 44.70 ± 9.34 to 29.75 ± 2.19 kg/m2. The percentage of total weight loss (TWL) and excess weight loss (EWL) was 33.84% and 77.19%, respectively. The mean LDL values significantly decreased compared to baseline; however, the mean HDL did not significantly differ. No significant difference was observed regarding the mean albumin, vitamin B12, and folic acid levels.
Conclusion
TB-SG procedure seems promising in terms of T2DM remission and weight loss with less malnutrition and vitamin deficiency in treating obese patients with T2DM.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease mostly characterized by peripheral insulin resistance and is closely related to obesity [1]. Besides, a decrease in insulin production and/or insulin activity in the target tissue can play role in the pathophysiology of the disease. Mortality and morbidity rates are seen to increase in T2DM patients when accompanied by obesity [2].
Adequate glycemic control cannot be achieved with pharmacotherapy in more than half of the patients with moderate to severe T2DM [3], and the majority of the patients in this group are obese T2DM patients [4]. Weight loss facilitates glucose metabolism regulation in diabetic patients and reduces the likelihood of developing the disease in patients with a predisposition to diabetes. Weight loss of 1 kg provides a 16% relative risk reduction of progression to T2DM in individuals with impaired glucose tolerance [5].
Metabolic surgery is an effective treatment method in terms of glycemic control and weight loss in obese T2DM patients [4, 6,7,8]. In the first Diabetes Surgery Summit (DSS-I) held in 2007, it was stated that metabolic surgery could be considered as a treatment method in class I obese T2DM patients [9]. The second meeting (DSS-II) was held in 2019, and at that time, metabolic surgery was emphasized as a potent treatment modality in the same patient group [10]. Several metabolic surgery techniques such as biliopancreatic diversion (BPD), Roux-en-Y gastric bypass (RYGB), ileal interposition, and sleeve gastrectomy (SG) have been performed and achieved remarkable T2DM remission and weight loss results for years [4, 11]. Despite the satisfactory results, it is a fact that there are still problems such as early complications and long-term malnutrition that need to be overcome.
Transit bipartition with sleeve gastrectomy (TB-SG) is a relatively novel metabolic procedure. It has similar metabolic effects as conventional procedures, promising less malnutrition and vitamin deficiency [4, 12,13,14]. This study aimed to present the mid-term metabolic effects and weight loss results of patients who underwent TB-SG.
Materials and Methods
Patients and Study Design
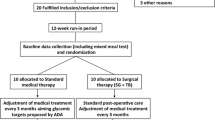
This present retrospective study was conducted with the 3-year follow-up data obtained from the medical charts of the patients who underwent TB-SG operation in our bariatric surgery department between May 2014 and January 2017. The study was designed in accordance with the Declaration of Helsinki, and approval for the study was granted by the Institutional Review Board (No: 2020/546).
Inclusion and Exclusion Criteria
The inclusion criteria for the surgical procedure were defined as T2DM for ≥ 2 and ≤ 10 years, glycated hemoglobin (HbA1c) level > 7% (53 mmol/mol), and body mass index (BMI) ≥ 35 kg/m2.
Patients with American Society of Anesthesiologists score of IV were excluded from the surgery. Other exclusion criteria were c-peptide level < 1.5 ng/mL, anti-glutamic acid decarboxylase antibody or islet cell antibody positivity, malignancy diagnosis, acute or chronic renal failure, alcohol or drug abuse, diagnosis of psychiatric eating disorder, or hiatal hernia.
Preoperative Evaluation and Postoperative Follow-up
All patients were informed about the surgical procedure, possible complications, postoperative course, and the follow-up period. Written informed consent forms were obtained before the surgery. A multidisciplinary team evaluated all patients, including a metabolic surgeon, endocrinologist, psychiatrist, cardiologist, and anesthesiologist in terms of suitability for metabolic surgery. Upper gastrointestinal endoscopy and hepatobiliary system ultrasound were performed before the surgery.
In the study, evaluations were made of BMI, HbA1c, fasting blood glucose (FBG), fasting insulin (FI), HOMA-IR [fasting glucose (mmol/L) × fasting insulin (mIU/mL)/22.5], LDL, HDL, albumin, vitamin B12, and folic acid values. The values were obtained and recorded preoperatively and at the postoperative 1st, 3rd, 6th, 12th, 24th, and 36th-month follow-up examination.
All patients were trained by an experienced nurse before discharge about recording FBG (at least once a day) and reporting high values of FBG over the phone. They were also informed on discharge to stop taking anti-hyperglycemic medication and have a liquid diet for the first week. Postoperatively, a multivitamin complex (Supradyn energy fast action sachet, Bayer, Germany) was given once a day for 6 months.
Definition of T2DM Remission and Assessment of Weight Loss
Remission definitions of T2DM were made according to the American Society for Metabolic and Bariatric Surgery outcome reporting standards [15]. Complete remission was defined as a level of FBG < 100 mg/dL (5.6 mmol/L) and HbA1c < 6% (42 mmol/mol) in the absence of antidiabetic medications. Partial remission was defined as a level of FBG 100–125 mg/dL (5.6–6.9 mmol/L) and HbA1c < 6.5 % (48 mmol/mol) in the absence of active pharmacological therapy. Improvement of glycemic control was defined as a statistically significant reduction in HbA1c and FBG (not meeting criteria for remission) or decrease in antidiabetic medications requirement (by discontinuing insulin or one oral agent, or ½ reduction in dose).
The weight loss assessment of the patients was made according to the percentage of total weight loss (%TWL) and percentage of excess weight loss (%EWL) [15].
Ideal body weight was defined as the weight corresponding to a BMI of 25 kg/m2.
Surgical Technique
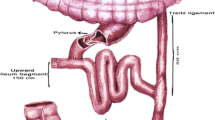
Antibiotic prophylaxis (cefazolin 2 g) and an intermittent pneumatic compression device were applied before the surgery. TB-SG was performed laparoscopically as previously described [4] (Fig. 1). After sleeve gastrectomy was performed using laparoscopic linear staplers, the ileum segment was marked at a distance of 80 cm proximal to the ileocecal valve with a single suture. Then, single-layer gastro-ileal anastomosis was performed between the residual antral site and the jejunum at a distance of 150 cm from the common channel (230 cm from the ileocecal valve) in an ante colic position. Finally, the small bowel cranial to the gastro-ileal anastomosis was transected and anastomosed to the ileum previously located (80 cm from the ileocecal valve) using the laparoscopic linear stapler. All potential internal hernia defects of the mesenteric fault were closed with barbed sutures.
Statistical Analysis
Data obtained in the study were analyzed statistically using Statistical Package for the Social Sciences vn. 15.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were stated as mean and standard deviation (SD) values. The data obtained in each follow-up period were compared with the preoperative and preceding follow-up values. The paired samples t-test and the Wilcoxon signed-rank test were used in the analyses. At the 12-month and 36-month follow-ups, patients were evaluated in 2 groups according to HbA1c values (complete remission and partial remission group). A value of p <0.05 was accepted as statistically significant.
Results
Patient Characteristics
Between the specified dates, 37 patients who met the inclusion criteria underwent TB-SG. Due to incomplete follow-up data, five patients were excluded, so the evaluation was performed on 32 patients (17 females and 15 males). The overall follow-up rate was calculated as 86.48%. The average follow-up time was 30.22 ± 6.74 months following the surgery. The baseline characteristics of the patients are shown in Table 1. The values of BMI, glycemic variables, lipid profile, and nutritional profile of the patients for preoperative and each follow-up period are shown in Table 2.
Glycemic Variables
Preoperatively, the mean duration of T2DM was 7.7 ± 2.3 years, and all patients were under insulin therapy. The mean HbA1c level of the patients was 9.25 ± 1.86% (77.6 ± 20.3 mmol/mol) preoperatively, and this started to decrease from the first postoperative month significantly fell below 6% (42 mmol/mol) by the 6th month. A significant decrease was also observed in the mean fasting insulin, fasting glucose, and HOMA-IR values in the first 6 months.
At the 12-month follow-up, the complete and partial remission rates were 75% (24 patients) and 12.5% (four patients), respectively. Although insulin therapy was still needed in the remaining four patients (12.5%), a significant decrease of insulin dose was observed as improved glycemic control in 2 of them. One of the 24 patients in complete remission in the postoperative 12th month regressed to partial remission in the 36th month. In the same follow-up period of the four patients in partial remission in the 12th month, one progressed to complete remission, and the other regressed out of remission. At the 36th month, the complete and partial remission rates were 75% (24 patients) and 9.3% (three patients), respectively. Although insulin therapy was still needed in the remaining five patients (15.6%), a significant decrease of insulin dose was observed in three of them as an improvement of glycemic control. If the remission status of T2DM was evaluated without classifying it as complete or partial, the remission rate was calculated as 87.5% in the 12th month and 84.3% in the 36th month.
Weight Loss
The mean preoperative BMI was 44.70 ± 9.34 kg/m2. Up to the 12th month, a significant decrease of BMI was observed at each follow-up evaluation. However, there was no significant change in subsequent follow-ups. TWL% and EWL% values were calculated at each follow-up and are presented in Fig. 2. The TWL% and EWL% in the 12th month were 33.07% and 75.43%, respectively. At the 36th month follow-up, TWL was calculated as 33.84%, and EWL as 77.19%.
Lipid and Nutritional Profile
There was a significant decrease in the LDL values in the first two postoperative follow-ups (1st and 3rd months). No significant change was observed in the subsequent controls. None of the seven patients using antihyperlipidemic drugs before the surgery needed medication after the 3rd month. The HDL values showed no significant change after the operation compared to the preoperative period.
There was no significant decrease in the mean albumin, folic acid, and vitamin B12 values in the postoperative period. However, vitamin B12 deficiency was detected in two (6.2%), and iron deficiency was detected in three (9.3%) patients during the follow-up. The mean iron level was significantly decreased at the 24th-month follow-up when compared with preoperative values. Similarly, this reduction was also observed in the 36th-month follow-up. The mean vitamin D values remained normal during all follow-up periods.
Complications
All operations were completed laparoscopically. No anastomotic leak, necrosis, internal herniation, or injury to other intestinal organs were observed in the perioperative period. In the early postoperative period, bleeding requiring blood transfusion was observed in 1 patient. The problem was resolved without any surgical intervention with drain follow-up. Transient nausea and/or vomiting were seen in 3 patients, and all were successfully treated with medical therapy. Four patients experienced gastroesophageal reflux (GER) disease during the follow-up and were treated with proper dietary advice and proton-pump inhibitors. One patient was diagnosed with cholelithiasis 6 months postoperatively, and laparoscopic cholecystectomy was performed.
Discussion
This study demonstrates that TB-SG can be seen as an effective metabolic surgery procedure that provides remarkable T2DM remission and weight loss with less malnutrition or vitamin deficiency. Furthermore, it improves the lipid profile of the patients.
Metabolic surgery has become more widely used in the treatment of obesity and T2DM in recent years. The number of metabolic surgeries performed worldwide in 2009 was 220,000 [16]. This number has increased over the years and was recorded as 833,000 per year in 2019 [17]. RYGB and BPD are the most commonly used metabolic surgeries with T2DM remission rates of 70–75% and 90–95%, respectively [18,19,20].
RYGB has been in use for about half a century as a metabolic procedure since it was first defined in 1969 [21]. Although it is still one of the most commonly performed metabolic procedures, difficulties in achieving and maintaining significant weight loss have been reported in patients with a BMI > 50 kg/m2 [22, 23]. BPD emerged in 1980 as a more effective surgical technique for treating super-obese patients [24]. It provides better weight loss and metabolic improvement results than the RYGB procedure [6, 25]; however, it does not have widespread popularity, presumably because of its complex surgical technique and long-term nutritional complications [7].
All metabolic procedures, to variable degrees, alter the anatomy and physiology of the gastrointestinal tract. As a result of these alterations, nutritional complications such as deficiencies of macro- and micro-nutrients can be observed. Vitamin B12 and folic acid deficiencies are potential complications that can contribute to anemia following BPD and RYGB. As a result of bypassing the proximal small intestine where folic acid is absorbed, folic acid deficiency occurs in a range of 9 to 39% [26, 27]. The prevalence of B12 deficiency, which results from inadequate secretion of intrinsic factors and limited gastric acidity, has been reported to be 19 to 35% [28, 29]. Malnutrition has been reported in 13% and 21% of patients who underwent RYGB and BPD, respectively [30].
The TB-SG procedure was developed by Santoro et al. in 2006, with the concept of not eliminating any small intestine passage to thereby decreasing malnutrition and vitamin deficiency complications of metabolic surgeries [31]. In this procedure, ingested nutrients enter the intestinal system with both the “short circuit” created by the gastro-ileal anastomosis and pylorus. As there is no unused intestinal area, malabsorption is a less expected condition [32]. However, there are insufficient data in the literature about the mid-long-term nutritional results.
The potential underlying mechanism of glycemic control following metabolic surgeries has been partly elucidated. There are two hypotheses (foregut and hindgut hypothesis) that have been proposed to explain the antidiabetic effects of metabolic surgeries [33]. It has been suggested with the foregut hypothesis that the exclusion of the proximal intestine leads to an increase in incretin levels and a reduction of the anti-incretin factors [34]. On the other hand, according to the hindgut hypothesis, the expedited arrival of nutrients to the distal bowel enhances the incretin secretion that improves glucose metabolism [35]. Besides, the restrictive parts of the surgery, such as SG, decrease food/calorie intake and contribute to weight loss, leading to improved insulin sensitivity [33]. It is most likely that both mechanisms explained by the foregut and hindgut hypothesis potentially improve glycemic control. Glucagon-like peptide-1 (GLP-1) has been considered the main incretin secreted from the distal part of the small intestine and responsible for increasing insulin secretion and sensitivity [36, 37]. Glucose-dependent insulinotropic polypeptide (GIP) is an incretin secreted from the proximal intestine with the transit of nutrients and considered to have a combined agonistic effect with GLP-1 on glucose metabolism regulation [38]. With the surgeries such as BPD and RYGB, bypassing the proximal small intestine deprives the patient both of nutrients absorbed from this area and GIP secretion. In the TB-SG procedure, in addition to less malabsorption, stimulation of GIP secretion continues as well as GLP-1.
T2DM remission rates of TB-SG have been reported as 86% and 86.5% in previous studies. [13, 39]. In both of those studies, T2DM remission was defined as a level of HbA1c <6.5% without the use of any antidiabetic medication. With the same cutoff value for HbA1c to define T2DM remission, the current study's remission rate was 87.5% in the 12th month and 84.3% in the 36th month, similar to the previous studies. In the present study, T2DM remission was categorized in 2 groups as partial and complete to evaluate the degree of glycemic control within the patients in remission and transitions between groups (switching to the other group or coming out of remission) during the follow-up period. The remission status change rate was found to be 4.2% (1 of 24 patients) in the complete remission group and 50% (2 of 4 patients) in the partial remission group between the last 2 follow-ups. These rates showed that the remission status of the partial remission group was more unstable. Therefore, it is important to keep follow-up intervals closer and make necessary diet and lifestyle adjustments for patients in partial remission to ensure that their current remission is maintained for a longer time.
One reason for the popularity of metabolic surgery is its positive effects on cardiovascular risk factors such as being overweight and the lipid profile [40, 41]. In the present study, a significant improvement was observed in LDL levels, especially in the first 3 months postoperatively. Similar findings on lipid profile have been reported in other metabolic surgical methods [18, 42].
Pancreatic burnout can be defined as endocrine insufficiency of the pancreas (HbA1c > 6.5%, 2 h plasma glucose > 200 mg/dL in oral glucose tolerance test) together with the absence of pain [43]. It might be a possible contributing factor to the deficiency of remission of T2DM after metabolic surgery. The serum c-peptide concentration is frequently employed to determine the sufficiency of pancreatic insulin secretion. The fasting c-peptide level’s normal range is 0.78 ± 1.89 ng/mL in healthy persons [44]. A criterion was defined as c-peptide level < 1.5 ng/mL to exclude the patients with low pancreatic endocrine reserve in the present study.
GER symptoms have been described following the laparoscopic sleeve gastrectomy (LSG) procedure in many studies. It is one of the most common indications for converting LSG to gastric bypass [45,46,47]. However, in high-volume trials, where strict criteria for patient selection for LSG were applied, it was stated that reflux symptoms could be overcome with medical treatment, and the need for conversion operation due to GER disease was reduced [48]. In the present study, with the preoperative gastroscopy, patients with endoscopic reflux signs and hiatal hernia were excluded. In the postoperative period, patients with GER disease were treated with proper dietary advice and acid suppression therapy.
The dumping syndromes (early and late) are well-known phenomena after gastric resections and bypass procedures due to altered gastrointestinal anatomy. They are closely related to the rapid delivery of the osmotically high nutrients to the small intestines [49, 50]. The two syndromes have distinct symptomatology and pathophysiology [51]. In the present study, late dumping syndrome that is mainly characterized by postprandial hypoglycemia was not observed. However, nausea and/or vomiting accompanied by vasomotor symptoms seen in three patients could be considered early dumping syndrome. These symptoms were treated with strict dietary recommendations and symptomatic medications.
Rapid weight loss in obese patients increases the risk of cholelithiasis [52]. After TB-SG, reported rates of cholecystectomy differ in a wide range from 2.8 to 21.9% [13, 14, 39]. This difference might be caused by different follow-up periods of the studies. In the current study, it was found to be 3.12%.
“Sleeve plus procedure” is a term that can be used to define the metabolic procedures in which SG is added to the surgery alongside anatomic route modifications [53, 54]. Some of the sleeve plus procedures are transit bipartition, biliopancreatic diversion, duodenojejunal bypass, mini-gastric bypass, and ileal interposition [6, 55,56,57]. In these procedures, except the surgical rerouting of nutrients to the distal part of the small intestine and hormonal changes, reducing food/calorie intake and ghrelin secretion that is provided with SG improves glycemic control by reducing insulin resistance [56, 58, 59]. However, in these procedures other than transit bipartition, the duodenum is anatomically excluded, and the endoscopic approach to the biliary tract is not possible with endoscopic retrograde cholangiopancreatography. Depending on the type of surgery performed, some complications are observed more frequently in some sleeve plus procedures. Bile reflux to the remnant gastric tube is a common concern after mini-gastric bypass procedure due to the single anastomosis to the antral gastric region [60]. Ischemia, necrosis, and internal herniation are more common in ileal interposition due to its relatively complex anatomy in which ileal segments are relocalized [4, 61]. In the present study, no bile reflux or internal herniation was observed.
The limitations of this study were its nonrandomized, retrospective design, and the lack of a control population. Despite the limitations, the present study contributes to the literature by presenting mid-term results of the TB-SG procedure, for which there is not much follow-up data.
In conclusion, the present study supports that the TB-SG procedure can be considered a safe and effective method in the treatment of obesity and T2DM. It seems that TB-SG may be an alternative to other metabolic surgeries with the advantages of the prevention of malnutrition and vitamin deficiency.
Limitations
The study was a retrospective analysis.
References
Rahier J, Guiot Y, Goebbels R, et al. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10:32–42.
Hossain P. Obesity and diabetes in the developing world-a growing challenge (vol 356, pg 213, 2007). N Engl J Med. 2007;356(9):973.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Yormaz S, Yılmaz H, Ece I, et al. Laparoscopic ileal interposition with diverted sleeve gastrectomy versus laparoscopic transit bipartition with sleeve gastrectomy for better glycemic outcomes in T2DM patients. Obes Surg. 2018;28(1):77–86.
Brown E, Wilding JP, Barber TM, et al. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev. 2019;20(6):816–28.
Kapeluto JE, Tchernof A, Masckauchan D, et al. Ten-year remission rates in insulin-treated type 2 diabetes after biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2020;16:1701–12.
Bianchi A, Pagan-Pomar A, Jimenez-Segovia M, et al. Biliopancreatic diversion in the surgical treatment of morbid obesity: long-term results and ,etabolic consequences. Obes Surg. 2020:1–9.
Turcotte A-F, Grenier-Larouche T, Lacombe J, et al. Association between changes in bioactive osteocalcin and glucose homeostasis after biliopancreatic diversion. Endocrine. 2020:1–10.
Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251(3):399–405.
Cohen RV, Shikora S, Petry T, et al. The diabetes surgery summit II guidelines: a disease-based clinical recommendation. Obes Surg. 2016;26(8):1989–91.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis. 2016;12(6):1144–62.
Ghio B, Jiménez A, Corcelles R, et al. Midterm effects of bariatric surgery in patients with insulin-treated type 2 diabetes. Surg Obes Relat Dis. 2017;13(12):2004–9.
Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256(1):104–10.
Karaca FC. Effects of sleeve gastrectomy with transit bipartition on glycemic variables, lipid profile, liver enzymes, and nutritional status in type 2 diabetes mellitus patients. Obes Surg. 2020:1–9.
Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. e5.
Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg. 2019;29(3):782–95.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85.
Misra S, Nandhini BD, Christinajoice S, et al. Is laparoscopic Roux-en-Y gastric bypass still the gold standard procedure for Indians? Mid- to long-term outcomes from a tertiary care center. Obes Surg. 2020;30(11):4482–93.
Lebel S, Dion G, Marceau S, et al. Clinical outcomes of duodenal switch with a 200-cm common channel: a matched, controlled trial. Surg Obes Relat Dis. 2016;12(5):1014–20.
Mason EE, Ito C. Gastric bypass. Ann Surg. 1969;170(3):329–39.
Topart P, Becouarn G, Ritz P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg Obes Relat Dis. 2013;9(4):526–30.
Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22(1):70–89.
Scopinaro N, Gianetta E, Civalleri D, et al. The bilio-pancreatic bypass for functional surgical treatment of obesity. Minerva Med. 1979;70(52):3537–47.
Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147(9):847–54.
von Drygalski A, Andris DA, Nuttleman PR, et al. Anemia after bariatric surgery cannot be explained by iron deficiency alone: results of a large cohort study. Surg Obes Relat Dis. 2011;7(2):151–6.
Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031–7.
Blume CA, Boni CC, Casagrande DS, et al. Nutritional profile of patients before and after Roux-en-Y gastric bypass: 3-year follow-up. Obes Surg. 2012;22(11):1676–85.
Sala P, Belarmino G, Torrinhas RS, et al. Gastrointestinal transcriptomic response of metabolic vitamin B12 pathways in Roux-en-Y gastric bypass. Clin Transl Gastroenterol. 2017;8(1):e212.
Lupoli R, Lembo E, Saldalamacchia G, et al. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–74.
Santoro S, Malzoni CE, Velhote MC, et al. Digestive adaptation with intestinal reserve: a neuroendocrine-based operation for morbid obesity. Obes Surg. 2006;16(10):1371–9.
Santoro S. From bariatric to pure metabolic surgery: new concepts on the rise. Ann Surg. 2015;262(2):e79–80.
Reis CE, Alvarez-Leite JI, Bressan J, et al. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index< 35 kg/m2: a literature review. Diabetes Technol Ther. 2012;14(4):365–72.
Liu S, Zhang G, Wang L, et al. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256(6):1049–58.
Vella A. Enteroendocrine secretion after roux-en-Y gastric bypass: is it important? Neurogastroenterol Motil. 2013;25(1):1–3.
Mingrone G, Nolfe G, Gissey GC, et al. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52(5):873–81.
Azevedo FR, Santoro S, Correa-Giannella ML, et al. A prospective randomized controlled trial of the metabolic effects of sleeve gastrectomy with transit bipartition. Obes Surg. 2018;28(10):3012–9.
Asmar M, Holst JJ. Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: new advances. Curr Opin Endocrinol Diabetes Obes. 2010;17(1):57–62.
Bilecik T. Metabolic effects of sleeve gastrectomy with transit bipartition in obese females with type 2 diabetes mellitus: results after 1-year follow-up. Obes Surg. 2019;29(3):805–10.
Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Vest AR, Heneghan HM, Agarwal S, et al. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24):1763–77.
Leonetti F, Capoccia D, Coccia F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. 2012;147(8):694–700.
Hirth M, Weiss C, Hardt P, et al. Analysis of the course of chronic pancreatitis: pancreatic burnout rates are only increased in a subgroup of patients with alcoholic chronic pancreatitis. Pancreas. 2019;48(5):726–33.
Iwase H, Kobayashi M, Nakajima M, et al. The ratio of insulin to C-peptide can be used to make a forensic diagnosis of exogenous insulin overdosage. Forensic Sci Int. 2001;115(1-2):123–7.
Abdemur A, Han S-M, Menzo EL, et al. Reasons and outcomes of conversion of laparoscopic sleeve gastrectomy to Roux-en-Y gastric bypass for nonresponders. Surg Obes Relat Dis. 2016;12(1):113–8.
Iannelli A, Debs T, Martini F, et al. Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass: indications and preliminary results. Surg Obes Relat Dis. 2016;12(8):1533–8.
Boru CE, Greco F, Giustacchini P, et al. Short-term outcomes of sleeve gastrectomy conversion to RY gastric bypass: multi-center retrospective study. Langenbeck's Arch Surg. 2018;403(4):473–9.
Braghetto I, Korn O, Burgos A, et al. When should be converted laparoscopic sleeve gastrectomy to laparoscopic Roux-en-Y Gastric bypass due to gastroesophageal reflux? ABCD Arquivos Brasileiros de Cirurgia Digestiva (São Paulo). 2020;33(4)
Ukleja A. Dumping syndrome: pathophysiology and treatment. Nutr Clin Pract. 2005;20(5):517–25.
Hedberg J, Hedenström H, Karlsson FA, et al. Gastric emptying and postprandial PYY response after biliopancreatic diversion with duodenal switch. Obes Surg. 2011;21(5):609–15.
Kim TY, Kim S, Schafer AL. Medical Management of the Postoperative Bariatric Surgery Patient. [Updated 2020 Aug 24]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK481901/
Coupaye M, Calabrese D, Sami O, et al. Effectiveness of ursodeoxycholic acid in the prevention of cholelithiasis after sleeve gastrectomy. Obes Surg. 2019;29(8):2464–9.
Huang C-K, Liu C-C, Hsin M-C, et al. Sleeve and sleeve plus. Ann Laparosc Endosc Surg. 2017;2(1):24.
Huang C-K, Katakwar A. Sleeve plus procedures: need of time. Surg Today. 2019:1–4.
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11(3):276–80.
Huang C-K, Goel R, Tai C-M, et al. Novel metabolic surgery for type II diabetes mellitus: loop duodenojejunal bypass with sleeve gastrectomy. Surg Laparosc Endosc PercutanTechn. 2013;23(6):481–5.
Kumar KH, Ugale S, Gupta N, et al. Ileal interposition with sleeve gastrectomy for control of type 2 diabetes. Diabetes Technol Ther. 2009;11(12):785–9.
Langer F, Hoda MR, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15(7):1024–9.
Vidal J, Ibarzabal A, Nicolau J, et al. Short-term effects of sleeve gastrectomy on type 2 diabetes mellitus in severely obese subjects. Obes Surg. 2007;17(8):1069–74.
Saarinen T, Räsänen J, Salo J, et al. Bile reflux scintigraphy after mini-gastric bypass. Obes Surg. 2017;27(8):2083–9.
Gagner M. Laparoscopic sleeve gastrectomy with ileal interposition (SGIT): a modified duodenal switch for resolution of type 2 diabetes mellitus in lesser obese patients (BMI< 35). World J Surg. 2011;35(1):109–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The need for patient informed consent for this retrospective study was waived by the institutional review board.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• TB-SG leads significant weight loss and diabetes remission in obese T2DM patients
• TB-SG can be considered a safe and effective metabolic surgical method
• TB-SG may be a remarkable alternative to other metabolic surgeries
Rights and permissions
About this article
Cite this article
Calisir, A., Ece, I., Yilmaz, H. et al. The Mid-Term Effects of Transit Bipartition with Sleeve Gastrectomy on Glycemic Control, Weight Loss, and Nutritional Status in Patients with Type 2 Diabetes Mellitus: a Retrospective Analysis of a 3-Year Follow-up. OBES SURG 31, 4724–4733 (2021). https://doi.org/10.1007/s11695-021-05536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05536-1