Abstract
Purpose
Calcaneal fracture treatment is challenging. Implant failure is one problem encountered with plate osteosynthesis. Therefore a new “bionic” plate was developed, which imitates the trabecular orientation of the human calcaneus. The aim of this study was to biomechanically test this new plate in comparison to a “standard” calcaneal locking plate and present the first clinical results.
Methods
Six “bionic” and six “standard” calcaneal plates were biomechanically tested for stability and fatigue using synthetic calcanei. Between 4/2012 and 04/2013 the first ten consecutive patients meeting the inclusion criteria were treated with the novel implant and followed-up clinically and radiologically. The 12-month follow-up results are reported.
Results
The “bionic” plate design showed significantly higher fatigue life (68 %), load to failure (100 %) and reduced displacement under load (90 %) if compared to a “standard” locking plate. No major complications were seen; most notably there was no implant failure and no loss of reduction. Mean AOFAS/hindfoot score was 79 (69–86).
Conclusions
The novel plate architecture offers higher stability and load tolerance while being more resistant to fatigue. The preliminary clinical results are promising. These findings will have to be proved by larger clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcaneal fracture treatment is challenging [1–4]. Different techniques for reduction and fixation of calcaneal fractures are discussed today but open reduction and internal fixation (ORIF) with calcaneal plates may still be regarded the gold standard for most calcaneal fractures [1, 2, 5–8]. Several different plate designs have been advocated; however, mechanical or clinical data are not available for most of the implants used.
Following the successful use of contoured plates and locking screws for other complex fractures [9–11] these designs have recently been introduced for calcaneal fractures. Experimental studies showed that plates with locking screws provide higher stability during cyclic loading compared to plates without locking screws [12, 13]. Clinical results, however, await critical analysis and the specific indications for their use still have to be defined, as not all results were satisfactory. The higher rigidity of locking constructs may be a problem and an increasing incidence in plate fracture was reported [14].
Recently, Richter et al. reported superior results for polyaxially locked screws [13, 15]. This was attributed to the fact, that more locking screws could be placed into the sustentacular fragment. However, the plate used in this study still resembled the traditional design of the “AO plate”.
Based on these findings, a novel plate was designed: The Medartis Aptus® Foot Calcaneus 3.5 System. The plate has a robust construction due to the frame design; the locking holes are arranged in a way that the screws can easily be placed in areas with best bone quality. The alignment of the plate lugs is based on the direction of force, following the anatomic trajectories of the calcaneus to provide a high degree of strength despite the low profile. This is referred to as “bionic” plate design. In addition the TriLock® multidirectional (±15°) and angular stable locking technology was implemented (Fig. 1).
Objectives
The objectives of this study were to compare the basic mechanical properties: load, deformation, and mode of failure of the Medartis Aptus® calcaneal plate with “bionic” design to a “standard” locking calcaneal plate (Synthes® Calcaneal Locking Plate) in a fatigue test.
Moreover, we aimed to clinically evaluate the first ten consecutive patients treated with the Medartis Aptus® calcaneal plate.
The hypotheses were that “bionic plate design” results in a higher load resistance compared to the “standard design” of a calcaneal locking plate, and that a safe use of this new plate is possible.
Materials and methods
Biomechanical analysis
For mechanical testing an experimental setup previously described by Richter et al. [13, 15] for the testing of calcaneal plates was used with slight modifications.
The mechanical testing of the plates was performed on calcaneal models made of glass-reinforced polyamide (1zu1 Prototypen GmbH & Co KG) using rapid prototyping. The shape was modified using CAD software, and a Sanders type 2b fracture consisting of six fragments [16] was incorporated analogous to Richter et al. [13].
Calcaneal plate designs
Two different types of plates comparable in thickness and hole diameter but substantially different in terms of geometry and design were compared:
-
Bionic plate design: Medartis Aputs® Calcaneal Plate
The plate features 13 holes for 3.5 multidirectional locking or cortical screws having a diameter of 3.5 mm. Two holes are located in bendable tabs that may be adapted to fit the subtalamic region of the calcaneus and the anterior process. The plate also includes K-wire holes (ø 2 mm) for temporary fixation. The plate has a thickness of 1.4–2 mm and is made of pure grade 4 titanium.
-
Standard plate design: Synthes® Calcaneal Locking Plate
This plate features 15 holes for 3.5-mm unidirectional locking or cortical screws (ø 2.7 mm or 3.5 mm). It includes bendable tabs that may be adapted to fit the calcaneal anatomy. In addition, the plate features two bendable tabs that may be used to stabilize anterior process fragments and plantar fragments. The plate has a thickness of 1.3–2 mm and is made of pure titanium.
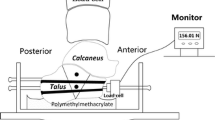
Both plates were used and mounted according to the manufacturer’s instructions. The osteosynthesis was performed according to the model described by Zwipp et al. [7, 17]. All plates were positioned with a 1-mm gap between the plate and the bone model to simulate worst-case conditions for the calcanal locking plates. All tests were performed using a Zwick/Roell dynamic testing machine (model LTM-1000) and testXpert II software. This setup allowed the application of defined forces on the specimens under quasi-physiological conditions. A movable yoke with two ball bearings transferred the load axially via the subtalar joint. Forces could exit through another bearing ball at the tuber of the calcaneus. The calcaneocuboid articulation was represented by a hinge joint allowing movement in the sagital plane. Ball bearings were used in order to minimize shear forces (Fig. 2).
After a previous power calculation six constructs were tested in each group. A modified Locati load profile was used (Table 1), starting at 100 N for 50,000 load cycles. Subsequently the force was increased by 50 N stepwise every 10,000 load cycles. Load cycled at 5 Hz in a sinusoidal fashion with a force ratio Fmin /Fmax = 0.1.
Forces and displacement at the point of load transfer were recorded. Additional video analysis allowed a precise determination of cause of failure. Failure was defined as:
-
Maximum axial displacement of more than 10 mm
-
Plate fracture
-
Screw failure (fracture)
Clinical evaluation
After IRB approval ten consecutive and consenting patients with calcaneal fractures were included into this preliminary study between 04/2012 and 04/2013 and treated operatively with the Medartis Aputs® Calcaneal Plate by a single surgeon (B.K.) following a standard protocol described previously in detail [3]. Briefly, fractures were diagnosed from plain X-rays and CT-scans with mulitplanar image reformation and classified according to Sanders [2]. Surgery was performed after soft tissue recovery (seven days [range four to 28]). A single extended lateral approach was used. Partial weight bearing (15 kp) was recommend for at least six weeks. No immobilization (cast or walker, etc.) was used. A cushioning insole was prescribed after six to 12 weeks for all patients.
Follow-up visits were scheduled after six, 12, 24 and 48 weeks. Inclusion criteria were: calcaneal fracture, Sanders type 2 and 3, informed consent. Exclusion criteria were: open fracture, multiple injuries, Calcaneal fractures Sander’s type 1 and 4, and age younger than 18 years. The evaluation included a structured questionnaire (assessing pain level, activity level, work ability, etc.), a clinical and a radiographic examination. The AOFAS hind-foot score was calculated to evaluate the clinical outcome.
The radiologic follow-up included lateral and dorso-plantar weight bearing and oblique radiographs of the foot as well as Saltzman and Broden’s views. Images were analysed for: quality of reconstruction and posttraumatic degenerative changes. Only the 12-month results are reported. Primary endpoints were complications during the observation period and any kind of implant failure. Secondary endpoints were function at 12 months measured with the AOFAS ankle/hind foot score and radiographic evidence of osteoarthritis.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science (SPSS) version 18.0 software. Student’s t-test was used to detect significant differences between the measured parameters. Statistical significance was assumed with p < 0.05. Power analysis for the biomechanical tests was performed with β = 0.20 in order to choose the sample size of six samples in each group. Post hoc analysis revealed a power of 95 %.
Results
Biomechanical results
Cycles to failure
The number of cycles completed is defined as the number of axial load cycles the construct was subjected to before it failed as defined by the criteria listed above. This parameter has proven to be a suitable indicator for fatigue susceptibility and can be used to approximately estimate the durability and load tolerance of an implant.
The “bionic” plate (Medartis Aptus® Calcaneal Plate) achieved an average of 89,250 cycles before failure, while the “standard” plate (Synthes® Calcaneal Locking Plate) completed an average of 53,100 cycles (t-test; p < 0.001).
Mode of failure
All samples were evaluated visually, and the mode of failure was analysed. The following modes of failure were observed: fractures of the plate and/or screws, material deformation or failure of the plate/screw unit. The videos were analysed to distinguish the primary cause of failure from secondary fractures of plates and/or screws. The “bionic” plate (Medartis Aptus® Calcaneal Plate) always failed by plate fracture at the upper tuber part of the frame (Fig. 3a). The “standard” plate (Synthes® Calcaneal Locking Plate) failed due to massive deformation of the plate: the osteosynthesis was destabilized and the tuber fragment was free to rotate relative to the other fragments until axial displacement exceeded 10 mm and thus met one of the defined criteria of failure (Fig. 3b).
Force and load levels
Axial forces at time of failure were measured. The “bionic” plate (Medartis Aptus Calcaneal Plate®) tolerated substantially higher forces compared to the “standard” plate (Synthes® Calcaneal Locking Plate). Fatigue life was defined as the last load level successfully completed without hardware failure. The “bionic” plate accomplished a significantly higher load level in comparison to the “standard” plate: 100 N vs. 250 N (250–350 N, median, range); Student t-test, p < 0.001.
Displacement
The “standard” plate showed significantly more axial displacement compared to the “bionic” plate (Fig. 4). Increase of displacement under cyclic loading (e.g. between 1,000 and 50,000 cycles) is a good indicator for plastic deformation. Axial displacement increased by 0.305 mm for the “standard” plate and by 0.035 mm for the “bionic” plate (t-test; p < 0.001). Therefore, the “bionic” plate has to be regarded more rigid compared to the “standard” plate.
Clinical results
The data of all ten patients could be analysed. The mean age of the patients was 52 years (25–70), consisting of eight males and two females. Trauma mechanisms were fall from height (n = 3), fall (n = 4), and traffic accidents (n = 3).
Fractures were classified as follows: Sanders type 2A (n =6) and type 3 AC (n = 4) (Fig. 5).
Surgery was performed an average (median) seven days (range four to 28) after trauma. All patients were treated with the “bionic” plate (Medartis Aptus® Calcaneal Plate). In one patient an intraarticular osteotomy in the primary fracture line was necessary due to the age of the fracture (the patient presented with a fracture four weeks old).
No major complications were seen during follow-up. Delayed wound healing was recognized in one patient, and none of the patients showed signs of superficial or deep wound infection. No patient had to be revised for any reason during the 12-month period. At the time of the last follow-up all implants were still in situ. No implant failure had occurred.
Bohler’s angel ranged from −20° to 25° pre-operatively with an average of 7° and 28° postoperatively (range 20–32°). The intra- or postoperative CT scans showed a step-off or gap of 1 mm in three patients, which was acceptable. No step-off or gap of more than 1 mm was seen.
During the last follow-up (12 months) all fractures had healed. No patient showed loss of reduction comparing Bohler’s angle (29° [range 20–34°]; p = 0.65), hint-foot alignment, height or width of the calcaneus. Six patients showed signs of initial osteoarthritis after one year (Grades 1 and 2 according to Paley [18]). The average AOFAS score after one year was 79 (range 69–86).
Discussion
This study was aimed at biomechanically testing two different plate designs for the treatment of calcaneal fractures. A second objective was to present the early clinical result of the first ten consecutive patients treated in a single centre to assess clinical safety.
The designs of both plates tested mechanically featured angular stability, however with different locking mechanisms and multidirectional angular stability for the “bionic” plate (Medartis Aptus® Calcaneal Plate). Yet the main difference of the plates was the “architectural” configuration. Whereas the “standard” plate (Synthes® Calcaneal Locking Plate) merely copies the design of older plates without locking, the Medartis Aptus® Calcaneal Plate features an anatomic design, where the struts of the plate mimic the trajectory architecture of the heel.
We modified the experimental setup previously described by Richter et al. [13, 15] to provide nearly physiological conditions and, at the same time, ensure reproducibility and comparability. Artificial calcanei were used for this purpose. Although the shape of the models imitated human anatomy, the properties of the plastic material differ from those of human bone [13, 19]. In addition, the test setup does not allow any conclusions on the influence of surrounding structures such as tendons, ligaments or muscles. Previous studies have used cadaver bones [20]; however, in spite of their physiological properties, cadaver bones are disadvantageous in that the results obtained are difficult to compare due to heterogeneity concerning anatomy, age and bone quality [12, 20, 21]. Therefore the value of tests using cadaver bones is limited. Since our test was developed to biomechanically compare two different plate designs and was not intended to evaluate screw/bone stability, artificial models were considered superior to cadaver bones. This corresponds to the findings of Richter et al. [13, 15].
One shortcoming of the study concerns the locking mechanisms of the two plates. Although both mechanisms proved to be effective, they differed substantially. While the Medartis Aptus® Calcaneal Plate uses polyaxial locking screws (90° ± 15°), which allow for insertion of two subthalamic locking screws, the uniaxial locking screws of the Synthes® Calcaneal Locking Plate do not support this additional central stability. It will be interesting to see how the Medartis Aptus® Calcaneal Plate design compares to plates with polyaxial locking plates with a design similar to the Synthes® Calcaneal Locking Plate in future studies.
The analysis revealed that the “bionic” implant tolerated a significantly larger number of load cycles than the “standard” calcaneal plate. Moreover, it withstood considerably larger forces before the construct failed. The “bionic” plate also underwent significantly less displacement and plastic deformation under cyclic loading. Failure of the “standard” implant occurred always due to a rotational movement in the central frame portion, where the tuber fragment rotated into varus. The stable design of the “bionic plate”, which imitates the trabecular orientation of the human calcaneus, prevented this rotation.
A standard protocol was used to treat and evaluate the patients [3]. Bilateral, open or severely displaced fractures were excluded, as well as multiple injured patients to get a more homogenous picture in a short time frame. This, however, is one of the shortcomings of this study and will limit the conclusions the can be drawn from this trial.
No major complications were recorded. No revision was necessary, and no implant failure occurred. All fractures had healed at the time of the last follow-up. This was an important finding, since one disadvantage of the plate could have been a possible delay in fracture healing due to the higher rigidity of the plate. No secondary loss of correction was noted. It will be interesting to see how this novel implant performs in fractures with greater defect zones and poor bone quality such as osteoporosis. This will be an important question for the future, as the mean age of patients affected increases [22].
The clinical outcome was comparable to that of other studies [4, 23, 24]. The current patient population, however, is too small to conduct a matched pair analysis with the results of prior studies [25].
One-year results are never in the range of healthy individuals, and the fate of a calcaneal fracture does not only depend on the implant used. However, the implant is an important factor and the additional risk due to an inappropriate implant should be avoided. Our experience in the past showed that implant failure (e.g. breakage or bending of the plate) contributes to an inferior outcome [14]. Therefore a more robust plate, without jeopardizing the advantages of a “low-profile” implant, was one of the main reasons developing this new implant. Not only for approval reasons mechanical analysis and clinical observation is indispensable.
Handling of the implant was easy, in terms of contouring the plate to patients’ anatomy. The fit of the six available sizes posed no problem to the treating surgeon. No changes in the operative technique, which still follows the recommendations made by Zwipp [17, 24], were necessary. The high rate of wound infections with open reduction and internal fixation using an extended lateral approach as reported recently by Backes et al. [25] was not seen in this small trial under the present protocol.
Future studies must show if the plate can indeed contribute to fewer complications due to implant failure. Therefore, it will be necessary to include more Sanders’ type 3 and 4 fractures and patients with more critical soft tissue conditions.
Conclusion
Our results showed that a calcaneal locking plate with “bionic design” was mechanically more resistant to axial loads compared to “standard” plate architecture. The clinical evaluation showed no problems with handling of the “bionic” plate, no specific complications and at least equal clinical results compared to results from literature. The future clinical use and a thorough clinical study with more patients have to prove the theoretical advantages due to the biomechanical superiority of the “bionic” plate design.
References
Rammelt S, Zwipp H (2004) Calcaneus fractures: facts, controversies and recent developments. Injury 35(5):443–461. doi:10.1016/j.injury.2003.10.006
Sanders R (2000) Displaced intra-articular fractures of the calcaneus. J Bone Joint Surg Am 82(2):225–250
Kinner B, Schieder S, Muller F, Pannek A, Roll C (2010) Calcaneocuboid joint involvement in calcaneal fractures. J Trauma 68(5):1192–1199. doi:10.1097/TA.0b013e3181b28b8c
Schepers T, Backes M, Schep NW, Carel Goslings J, Luitse JS (2013) Functional outcome following a locked fracture-dislocation of the calcaneus. Int Orthop 37(9):1833–1838. doi:10.1007/s00264-013-2065-4
Benirschke SK, Sangeorzan BJ (1993) Extensive intraarticular fractures of the foot. Surgical management of calcaneal fractures. Clin Orthop Relat Res 292:128–134
Boack DH, Wichelhaus A, Mittlmeier T, Hoffmann R, Haas NP (1998) Therapy of dislocated calcaneus joint fracture with the AO calcaneus plate. Chirurg 69(11):1214–1223
Zwipp H, Rammelt S, Barthel S (2004) Calcaneal fractures–open reduction and internal fixation (ORIF). Injury 35(Suppl 2):SB46–SB54. doi:10.1016/j.injury.2004.07.011
Jiang N, Lin QR, Diao XC, Wu L, Yu B (2012) Surgical versus nonsurgical treatment of displaced intra-articular calcaneal fracture: a meta-analysis of current evidence base. Int Orthop 36(8):1615–1622. doi:10.1007/s00264-012-1563-0
Arora R, Lutz M, Fritz D, Zimmermann R, Oberladstatter J, Gabl M (2005) Palmar locking plate for treatment of unstable dorsal dislocated distal radius fractures. Arch Orthop Trauma Surg 125(6):399–404. doi:10.1007/s00402-005-0820-8
Ehlinger M, Adam P, Bonnomet F (2010) Minimally invasive locking screw plate fixation of non-articular proximal and distal tibia fractures. Orthop Traumatol Surg Res 96 (7):800–809. doi:10.1016/j.otsr.2010.03.025
Lee T, Blitz NM, Rush SM (2008) Percutaneous contoured locking plate fixation of the pilon fracture: surgical technique. J Foot Ankle Surg 47(6):598–602. doi:10.1053/j.jfas.2008.06.009
Illert T, Rammelt S, Drewes T, Grass R, Zwipp H (2011) Stability of locking and non-locking plates in an osteoporotic calcaneal fracture model. Foot Ankle Int 32 (3):307–313. doi:10.3113/FAI.2011.0307
Richter M, Gosling T, Zech S, Allami M, Geerling J, Droste P, Krettek C (2005) A comparison of plates with and without locking screws in a calcaneal fracture model. Foot Ankle Int 26(4):309–319
Kinner B, Roll C, Dienstknecht TM (2006) N Plattenbrüche nach winkelstabiler Calcaneusosteosynthese. In: Deutscher Kongress für Orthopädie und Unfallchirurgie. 70. Jahrestagung der Deutschen Gesellschaft für Unfallchirurgie, 92. Tagung der Deutschen Gesellschaft für Orthopädie und Orthopädische Chirurgie und 47. Tagung des Berufsverbandes der Fachärzte für Orthopädie, Berlin. German Medical Science
Richter M, Droste P, Goesling T, Zech S, Krettek C (2006) Polyaxially-locked plate screws increase stability of fracture fixation in an experimental model of calcaneal fracture. J Bone Joint Surg (Br) 88(9):1257–1263. doi:10.1302/0301-620X.88B9.17822
Sanders R, Fortin P, DiPasquale T, Walling A (1993) Operative treatment in 120 displaced intraarticular calcaneal fractures. Results using a prognostic computed tomography scan classification. Clin Orthop Relat Res 290:87–95
Zwipp H, Rammelt S, Barthel S (2005) Fracture of the calcaneus. Surgical technique. Unfallchirurg 108(9):749–760. doi:10.1007/s00113-005-1001-5
Paley D, Hall H (1993) Intra-articular fractures of the calcaneus. A critical analysis of results and prognostic factors. J Bone Joint Surg Am 75(3):342–354
Gefen A, Seliktar R (2004) Comparison of the trabecular architecture and the isostatic stress flow in the human calcaneus. Med Eng Phys 26(2):119–129. doi:10.1016/j.medengphy.2003.10.003
Lin PP, Roe S, Kay M, Abrams CF, Jones A (1998) Placement of screws in the sustentaculum tali. A calcaneal fracture model. Clin Orthop Relat Res 352:194–201
Blake MH, Owen JR, Sanford TS, Wayne JS, Adelaar RS (2011) Biomechanical evaluation of a locking and nonlocking reconstruction plate in an osteoporotic calcaneal fracture model. Foot Ankle Int 32 (4):432–436. doi:10.3113/FAI.2011.0432
Gaskill T, Schweitzer K, Nunley J (2010) Comparison of surgical outcomes of intra-articular calcaneal fractures by age. J Bone Joint Surg Am 92(18):2884–2889. doi:10.2106/JBJS.J.00089
Kinner B, Tietz S, Muller F, Prantl L, Nerlich M, Roll C (2011) Outcome after complex trauma of the foot. J Trauma 70(1):159–168. doi:10.1097/TA.0b013e3181fef5eb, discussion 168
Zwipp H, Rammelt S, Amlang M, Pompach M, Durr C (2013) Operative treatment of displaced intra-articular calcaneal fractures. Oper Orthop Traumatol 25(6):554–568. doi:10.1007/s00064-013-0246-3
Backes M, Schepers T, Beerekamp MS, Luitse JS, Goslings JC, Schep NW (2014) Wound infections following open reduction and internal fixation of calcaneal fractures with an extended lateral approach. Int Orthop 38(4):767–773. doi:10.1007/s00264-013-2181-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinner, B., Kerschbaum, M., Bley, C. et al. Bionic plate design for calcaneal fracture treatment. A biomechanical analysis and first clinical results. International Orthopaedics (SICOT) 39, 111–117 (2015). https://doi.org/10.1007/s00264-014-2561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2561-1