Abstract
Contrast-enhanced ultrasound (CEUS) is an extension and an enhanced form of ultrasound that allows real-time evaluation of the various structures in different vascular phases. The last decade has witnessed a widespread expansion of CEUS applications beyond the liver. It has shown fair potential in kidneys and its diagnostic efficacy is comparable to CT and MRI. Ultrasound is the well-accepted screening modality for renal pathologies, however, it underperforms in the characterization of the renal masses. CEUS can be beneficial in such cases as it can help in the characterization of such incidental masses in the same sitting. It has an excellent safety profile with no risk of radiation or contract-related nephropathy. It can aid in the correct categorization of renal cysts into one of the Bosniak classes and has proven its worth especially in complex cysts or indeterminate renal masses (especially Bosniak Category IIF and III). Few studies also describe its potential role in solid masses and in differentiating benign from malignant masses. Other areas of interest include infections, infarctions, trauma, follow-up of local ablative procedures, and VUR. Through this review, the readers shall get an insight into the various applications of CEUS in kidneys, with imaging examples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contrast-enhanced ultrasound (CEUS) is an extension and an enhanced form of ultrasound that allows real-time evaluation of the various structures in different vascular phases. It has recently gained popularity and is widely practiced by radiologists worldwide [1]. It was initially designed to study the cardiovascular system. Later with the formulation of more stable second-generation ultrasound contrast agents (UCAs), its scope widened. It has proven potential in imaging evaluation of various abdominal viscera, more so for the liver. Other applications are seen in the spleen, kidneys, pancreas, abdominal aorta, lower genitourinary tract, bowel, etc. [2]. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) has laid various guidelines for its use in the liver and other abdominal viscera including kidneys in the year 2012 and updated in 2017 [3].
The pioneer of contrast in ultrasound was Joyner who observed increased enhancement of intracardiac echoes with saline injection during echocardiography in the year 1960. The first case report was published by Gramiak and Shah in 1968 who used an intracardiac injection of indocyanine green/saline in contrast echocardiography [4]. The compressed gas in the core of the microbubble caused the backscattering of ultrasound waves, resulting in enhancement. It allows real-time assessment of the macro and microvasculature along with the contrast enhancement pattern of the lesion in question. The diagnostic accuracy of the contrast-enhanced ultrasound in kidneys is comparable to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) with an excellent safety profile [5]. The rate of serious adverse reaction was reported to be around 0.0086% by a large retrospective study [6]. It is not metabolized by kidneys and hence can be used in patients with chronic kidney disease (CKD), unlike iodinated and gadolinium-based contrast agents which are to be used with caution in CKD indeed [7]. There is no risk of iodinated contrast-related nephrotoxicity, gadolinium-related nephrogenic systemic fibrosis, or radiation-related injury. It can be performed at the bedside in non-ambulatory patients [8]
It can be performed in kidneys for accurate classification of a cyst into Bosniak category, characterize solid and indeterminate renal masses especially can differentiate hypovascular renal tumors from cysts which are even undetermined at contrast-CT scanning), renal infarction, infections, renal transplant recipient, for follow-up after ablative treatment [3, 9] which shall be covered in this review.
CEUS principle
UCAs are composed of gas microbubbles that are surrounded by a shell of protein, lipid, or polymer [10]. The shell thickness is 10–200 nm and may be hard, as in denatured albumin, or soft and flexible as in lipids. The core gas may be air, perfluorocarbon, or sulfur hexafluoride. Over 30 different UCAs are available based on the differences in shell material and core gas [11]. Ideally, the core gas should be inert having low diffusibility and solubility with high vapor pressure to persist in microcirculation for a longer time. First-generation contrast agents have air microbubbles in the core. As the air gets easily dissolved in the blood, it is not being used these days. Second-generation contrast agents can have perfluorocarbon or sulfur hexafluoride which shows low solubility in blood and does not diffuse through the shell easily, so they are preferred. This particular composition allows these agents to last for a period of ~ 5–7 min inside the blood vessels. The microbubble size ranges from 3 to 5 μm, about the same size as the red blood cell, which allows them to be strictly pure blood pool agents [12].
Two basic principles govern the functioning of CEUS, one is an increase in backscatter, and the second is background signal suppression. When exposed to ultrasound waves, these bubbles undergo contraction and expansion causing a doubling of their size at a frequency close to diagnostic ultrasound frequency. At this frequency, they reflect the transducer with higher energy than the passive reflectors. They significantly enhance the backscatter owing to the major difference of acoustic impedance at the gas fluid/tissue surface interface. The size of microbubbles is in the range of 3–5 μm so they resonate maximally at the general frequency used in ultrasound. Background suppression is done with the technique of pulse inversion, in which two signals which are mirror images of each other but are of opposite phases are sent through the same scan line. The echoes, produced are then received by the transducer. Tissues with microbubbles behave as non-linear reflectors and produce a net signal, whereas the tissues with no contrast agent behave as linear reflectors with no net signal. When the ultrasound beam reaches the tissue with microbubbles, there is strong backscatter which results in a high signal against a dark background. These microbubbles undergo disruption after several minutes inside the blood vessel and the internal gas gets exhaled out by the lungs while the coating particles are metabolized in the liver [13]. Since they do not undergo renal metabolism, they can be used in patients with renal impairment as well. Contrast dynamics, flow rates, and perfusion can be evaluated with this technique [14].
These agents can detect microvasculature that is too small with blood flow very slow to be detected by color and power Doppler. The sensitivity of CEUS to show blood vessels is higher than the power and color doppler [15]. Furthermore, the temporal resolution is higher than the CT and MR as real-time evaluation is done. Another advantage is that with real-time CEUS, there is continuous scanning of the organ of interest during various contrast phases while lesions may be missed or be incorrectly characterized due to inappropriate timing of CT or MR acquisition phases [2].
Special ultrasound equipment is required for performing CEUS which uses the principle of phase inversion and with a low mechanical index (MI) which prevents disruption of these microbubbles [16].
EFSUMB recommendations for reducing bioeffects of CEUS are to use low MI, higher frequencies, reducing total acoustic exposure time, reducing contrast dose, and adjusting cardiac triggering to avoid end-systolic triggering to prevent ventricular arrhythmias [3].
Dose in kidney
Although not specified in the EFSUMB guidelines, the contrast dose to be used for the study of the renal parenchyma (Sulfur hexafluoride) generally ranges from 1.4 to 2.4 ml, depending upon the habitus of the patient, depth of the lesion, and the type of ultrasound scanner [17]. In our institute, for renal application, 1vial containing 25 mg of lyophilized powder of Sonovue was reconstituted with 6 ml of saline. 2 ml of this mixture is injected followed by a saline flush of 5 ml which gives fairly good results.
Contra-indications
Currently, the only contra-indication of the CEUS agent is hypersensitivity to the agent. In patients with severe pulmonary hypertension and unstable cardiopulmonary disease, these agents should be used with caution. Rarely, serious cardiopulmonary reactions may occur following the administration of CEUS agents [18]. Limited data are available on its use in pregnancy, breastfeeding, and in the pediatric population, however, no secondary effects have been described in these groups of patients. Trained personnel with resuscitation kits should be available in the US room to manage any such rare occurrence.
Few animal studies have been performed to evaluate the side effects specifically pertaining to CEUS in kidneys. These were performed on rats and pigs. Histological findings of glomerular capillary hemorrhage, surface bruising, and microhematuria were observed in the kidneys. However, these studies were performed using high MI, high dose of contrast agent, different frequency, and longer times of exposure [19].
Phases of enhancement in kidney
Immediately after the administration of CEUS, there is a rapid enhancement of the main renal artery followed by the renal branches. Thereafter there is the enhancement of the cortex of the kidney followed by medullary perfusion. The cortical phase begins 10–15 s after injection and lasts 20–40 s, whereas the medullary enhancement lasts for 45–120 s. First, the outer medulla enhances, and then the pyramids. Since the contrast is not excreted by the kidneys, no excretory phase is observed. As the concentration of contrast in the main circulation decreases, the enhancement in kidneys also diminishes [20, 21]. Both vascular architecture and dynamic visualization of any lesion in question can be assessed on different vascular phases.
Applications in kidneys
Cystic renal masses
Bosniak classification is the most widely accepted classification for cystic renal masses originally published in 1986 [22] and updated in 2019 [23]. Originally described on contrast-enhanced CT, the latest version proposed in 2019 has incorporated MRI features of renal lesions in classification [23]. It is to be noted that contrast-enhanced ultrasound (CEUS) has not been included in the Bosniak classification system. Recently CEUS adapted Bosniak cyst categorization has been proposed by Cantisani et al. [24]. Except for simple cysts with thin septa (< 3) and with calcifications, determination of the malignant potential of other cysts would require the administration of UCA. Based on wall thickness, number, enhancement of septa, and enhancing solid nodule, cystic renal masses are classified into various categories. A higher class corresponds to an increased likelihood of cystic renal mass being malignant. CEUS has a higher spatial and temporal resolution and has been reported in various studies to detect the presence or absence of contrast enhancement of the cyst wall, septa, and solid component which may up or downgrade the class (according to Bosniak classification) appropriately [25,26,27]. Thereby, owing to contrast enhancement, it is more reliable in evaluating complex renal lesions than conventional ultrasound [28].
According to this proposed Bosniak Classification based on the multiparameter US, five categories of cysts are defined with increased risk of malignancy. Category 1 cyst is a simple cyst with sharp margins, a thin wall (< 2 mm), anechoic contents, posterior acoustic enhancement, and the absence of calcification or wall irregularity (Fig. 1). The point to note is that these cysts are well characterized on B mode US and do not require CEUS. These are benign and require no further management.
Category II cysts are minimally complex cysts which are like simple cysts but with 1–3 thin septa (< 2 mm) with no irregularities along with calcification in septa or wall which does not hinder cyst evaluation. CEUS is not necessary for these cysts which do not demonstrate any enhancement or may show individual microbubbles through the septa. The presence of internal debris, echogenic contents, or mixed echotexture of the cyst will also fall in Category 2 cysts, however, they warrant CEUS which demonstrates no enhancement except for individual microbubbles through the septa. These lesions are benign and do not require further management.
Category II F lesions are presumably benign and include cysts with multiple septa, slightly thickened smooth septa or wall (2–3 mm), internal debris, echogenic content, mixed appearance, and calcification which slightly hampers the cyst evaluation. CEUS will also show minimally thickened septa or walls (Fig. 2). Completely intrarenal category II cysts will also fall in this category. Follow-up is necessary for such non-surgical lesions.
Category III cysts show smooth thickened walls or septa (> 4 mm) or irregular thickened (> 3 mm) septa or enhancing wall and absence of solid enhancing component on the contrast-enhanced US. Almost 50% of Bosniak III lesions are malignant and European urological guidelines recommend surgery or active surveillance for these lesions.
Category IV lesions are likely malignant. These have the characteristic of category III cysts along with soft tissue nodules which if obtuse-angled should measure more than 4 mm and if acute, could be of any size. CEUS will depict enhancement of the septa, wall, and solid nodules (Fig. 3).
Several studies have found CEUS more sensitive than CT in depicting the cystic wall and septa vascularity [25, 29, 30].In a study comparing CEUS and contrast-enhanced CT for classifying cystic renal masses, high interobserver agreement (k = 0.86, p < 0.001) was found with the US, and complete concordance between the two modalities was found in the differentiation of surgical and non-surgical candidates [31].
In a recent study, the diagnostic performance of CEUS for assessment of Bosniak III cystic masses was found to correlate well with histopathological diagnosis [32]
A study by Lan et al. showed the sensitivity of CEUS to be higher in comparison with CECT, but with slightly lower specificity of CEUS [33]. In a study done by Chen et al. to compare the diagnostic performance of CEUS with MRI for complex cystic renal masses, they found a higher diagnostic sensitivity and accuracy but lower specificity than MRI (sensitivity, specificity, and overall accuracy of CEUS in the assessment of masses were 97.2%, 71.4%, and 84.5% versus 80.6%, 77.1%, and 78.9% for MRI) [34].
In a recent study, the beneficial and promising role of CEUS for follow-up of Bosniak 2F lesions was demonstrated [35].
Though the staging of malignant cystic lesions is best done by CT, however, CEUS is advantageous for the follow-up of non-surgical complex cystic lesions [36]. This will avoid unnecessary radiation exposure to the patient.
So, CEUS has a potential role in the categorization of Bosniak IIF, III, IV cystic masses, and for follow-up of complex cystic masses (Fig. 4) which are managed with active surveillance.
Patients with chronic kidney disease, especially those on long-term dialysis, frequently have multiple and bilateral renal cysts [37]. These patients are also at an increased risk of developing renal malignancy [38,39,40]. As both CT and MRI contrast agents pose a risk to cause further renal impairment to these patients, CEUS (by virtue of non-renal excretion of US contrast agents) can be a potential alternative modality for the evaluation of renal cystic masses. Therefore, CEUS assumes a crucial role in the assessment of the renal cysts in these patients (Fig. 5).
A study conducted by Chang et al. showed a high sensitivity for diagnosing renal malignancy in patients with chronic kidney disease. CEUS sensitivity was comparable to the reported sensitivity of CT (83–100%) and MR (81–100%) among patients with and without CKD [41]. They found sensitivity to be high as 90% (95% CI 56%, 98%), and specificity was 55% in CKD patients.
In a retrospective study conducted by Yong et al. for evaluating the performance of contrast-enhanced ultrasound (CEUS) in the risk stratification of indeterminate renal lesions picked up incidentally, in CKD patients, they found a high sensitivity of 95.5% (95% CI 77.2–99.9%), specificity of 94.2% (95% CI 84.1–98.8%), positive predictive value (PPV) 87.5% (95% CI 67.6–97.3), and negative predictive value (NPV) 98.0% (95% CI 89.4–100%), concluding that CEUS has a high diagnostic performance in predicting benignity of renal lesions in CKD patients, with sensitivity and NPV approaching 100% [36].
However, it is not always easy to identify dubious lesions in kidneys with chronic renal failure. Indeed if we consider the dominant multicystic disease, due to the increase in size, does not allow us to identify the dubious lesion due to the poor panoramic view.
Solid renal masses
Over the last few years, the detection rate of incidental renal masses has improved with the increasing trend of routine ultrasounds being done. Solid renal tumors can be detected using the grayscale US; however, their characterization is difficult [42]. It is crucial to determine whether a mass is benign or malignant on detection of a solid renal mass in the US. Benign masses include angiomyolipoma, adenoma, and oncocytoma. Malignant renal masses could be renal cell carcinoma (RCC), metastasis, lymphoma, and urothelial tumors.
B mode the US, however, may at times fail to distinguish solid vs complex cystic lesions. Internal echoes with a cyst can be dense and do not always demonstrate layering on grayscale imaging and can therefore mimic a solid tumor on grayscale. CEUS may be of help in such scenarios as it can differentiate between the complicated cyst and solid tumor. There shall be no enhancement of the echoes within the cyst but any solid component shall show persistent enhancement [43] (Fig. 6).
Once the presence of solid renal mass is confirmed, the next step is to categorize it into a benign or malignant mass. This is essential as almost 30% of the incidental renal masses which undergo surgery, turn out to be benign lesions and 25% are low-grade malignancies [44]. Accurate preoperative diagnosis can avoid unnecessary surgeries especially in patients with multiple co-morbidities. Few studies have researched the application of CEUS in the characterization of solid masses and have obtained encouraging yet conflicting results. According to a recent meta-analysis done by Pan et al. pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio odd of CEUS in RCC were 0.97, 0.86, 6.8, and 0.04 [45]. A meta-analysis was done on CEUS to differentiate malignant vs. benign renal masses obtained a sensitivity of 88% and a specificity of 80% [46]. Another recent study by Geyer et al. [47], done in histopathologically proven 96 cases of RCC and 18 cases of benign renal mass, however, found no specific feature on CEUS to accurately distinguish solid renal masses.
RCC on B mode in the US usually depicts heterogeneous mass with internal areas of necrosis, calcification, and hemorrhage. Rarely they may be small and homogenous. The enhancement pattern of clear cell variety of RCC has not been uniform among various studies. The difference could be attributed to the different morphology of RCC, different contrast media used, or differences in the terminology of various phases. Most of the studies have described early hyperenhancement of clear cell RCC (ccRCC) with wash-out of the UCA in the delayed phase (Fig. 7). Peritumoral rim enhancement or formation of the pseudo capsule is fairly specific for RCC [48]. The papillary variant of RCC (pRCC) on the other hand is usually homogenous and hypoechoic on B mode ultrasound and remains hypovascular in all the phases of contrast enhancement on CEUS [48].
About 70% of renal carcinoma are Clear cell types that are easy to diagnose based on their hypervascularity and show intense contrast enhancement on contrast-enhanced CT [49]. However, hypovascular RCCs pose a real diagnostic challenge. These include papillary, cystic, and chromophobe renal cell carcinoma (chRCC), spindle cell carcinoma, clear cell sarcoma, and collecting duct carcinoma. In these patients with a hypovascular mass, the next step is usually to conduct an FNAC or biopsy which are invasive and carry a risk of tumor seeding or bleeding to reach a definitive diagnosis and carefully follow-up observation. A study by Tamai et al. showed no marked difference between contrast CT and CEUS in the ability to diagnose clear cell carcinoma; however, CEUS was found to supersede the diagnostic ability of CECT in the diagnosis of hypovascular renal tumors [50]. A prospective study conducted on 50 patients concluded that CEUS significantly improves diagnostic confidence to assess solid renal masses. Histopathology or MRI follow-up was used as a reference for definitive diagnosis.
Few recent studies have also evaluated the role of quantitative parameters for differentiation of various subtypes of RCC and a few benign tumors like Angiomyolipoma (AML). In one of the recent study by Hiu et al. various quantitative parameters like the peak intensity (PI), slope (SL), area under the curve (AUC), area under the wash-in curve (AWI), area under the wash-out curve (AWO), time to peak intensity (TTP), and the mean transit time (MTT) were studied of the maximum enhancing component of the renal tumor and the adjacent cortex were studied [51]. A combination of these parameters could differentiate RCC from AML with sensitivity and specificity of 100% and 81.2%, respectively. Likewise, sensitivity and specificity of 85.71% and 85.92% were obtained in differentiating ccRCC from pRCC and chRCC. In RCC, differentiation of bland vs. malignant thrombus can also be confidently done with CEUS. Malignant thrombus will show enhancement like the tumor, whereas bland thrombus will not have any enhancement (Fig. 8).
Early hyperenhancement is however also shown by benign tumors like AML and oncocytoma. AML is typically composed of fat, smooth muscle, and abnormal blood vessels. Depending on the concentration of fat, they can be classified as fat predominant AML and minimal fat AML. It is the minimal fat AMLS that pose a diagnostic dilemma as they closely mimic RCC on imaging. Most AMLs will be hyperechogenic on the b mode US and will show homogeneous and sustained tumor enhancement on CEUS [52]. According to a study by Lu et al. minimal fat AML showed centripetal enhancement in the cortical phase, had homogeneous peak enhancement, and in the parenchymal phase, they showed isoenhancement [53]. These features were in contrast to the enhancement pattern of clear cell RCC and helped in differentiating the two entities. Pseudocapsule formation was seen in 40% of the cases of an epithelioid variety of minimal fat RCC which is an aggressive mass requiring surgical management [48].
Oncocytoma is also a close mimicker of ccRCC. They are spherical tumors having a central scar on B mode US and may show central radiating vessels on Doppler interrogation [41]. Wu et al. and Mittal et al. found similar CEUS findings of oncocytomas which is a spoke wheel/centripetal pattern of hyperenhancement or a central scar with sustained enhancement in the delayed phase [50, 54].
Metastasis and lymphoma generally show multiple focal lesions in both the kidneys which are < 3 cm and lack a capsule, though they can present as a solitary mass of variable size they show hypoenhancement in all the phases on CEUS [41].
To summarize, washout is suggestive of malignancy, and isoenhancement in the venous phase is suggestive of benignancy. However, despite the revised literature using CEUS for the characterization of solid masses, EFSUMB guidelines do not recommend this characterization.
Indeterminate renal masses
Occasionally incidental renal masses may be observed while doing abdominal CT scans or MRI for other indications. In such cases, repeat CT exposure can be avoided, which will increase the radiation dose of the patient, by performing CEUS in such indeterminate masses.
Pseudotumors are the ones that simulate a tumor but are composed of non-neoplastic tissue. Renal pseudotumors may be developmental, infectious, granulomatous, and vascular in nature [55]. Examples include prominent columns (septa) of Bertin, persistent fetal lobulation, dromedary hump, abscess, or arteriovenous malformation. Development pseudotumors can be differentiated from true renal tumors by comparing the enhancement of the lesion to normal background renal parenchyma. If the suspected lesion shows a different pattern of enhancement in at least one of the vascular phases, it is likely a tumor, whereas a pseudolesion will enhance similar to background renal parenchyma in all the phases [14, 21, 56] (Fig. 9). However, in approximately 5% of the cases, one may encounter an iso-enhancing renal tumor which enhances similar to renal parenchyma in all the phases [49]. In such cases, the pattern of the vascular architecture in the early arterial phase helps to make the differentiation. Pseudotumors shall have a vascular pattern like normal renal parenchyma, whereas any enhancing tumor shall cause displacement or distortion of the renal vascular architecture [3]. Very rarely additional investigations like CT or MRI may be required to differentiate pseudolesion from a neoplasm.
Renal infarction
CEUS has the excellent capability, equivalent to CECT and better than Doppler US, to demarcate areas of diminished or absent perfusion in the kidney signifying ischemia and infarction, respectively. Doppler in such cases would show absent flow. New modalities of color Doppler can sometimes differentiate between ischemia and infarction as they can detect very low flow. CEUS can distinctly show absent vs reduced enhancement in infarcted vs. ischemic area, thereby increasing the diagnostic confidence. Infarcted areas, classically appear as wedge-shaped non-enhancing areas in the peripheral part of the kidney (Fig. 10) [57]. Cortical necrosis can also be differentiated from renal infarction by determining the hilar vascularity which remains preserved in cortical necrosis. Furthermore, the visualization of the interlobar arteries allows differentiating the two identities as these are visible in the cortical damage with saving of the renal medulla. Cortical perfusion will be absent in both [58]. In addition, cortical necrosis usually involves the entire kidney and is usually bilateral while renal ischemia can be most commonly segmentary or less commonly diffuse.
Infective and inflammatory renal lesions
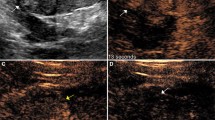
Renal infections are diagnosed primarily with help of clinical history and laboratory examinations, in these cases, imaging is done to identify the likely etiology and early detection of complications. Acute pyelonephritis may go undetected in the US. Focal pyelonephritis will appear as hypoechoic areas on B mode imaging and as hypoenhancing areas on CEUS (Fig. 11). These areas are most conspicuous in the parenchymal late phase. Focal pyelonephritis should be differentiated from abscess formation. Clinically, in cases of non-resolving infection even after treatment, areas of abscess formation should be evaluated which shall appear as a wedge or round-shaped, having no vascularity on CEUS with or without surrounding rim enhancement (Fig. 12). Follow-up of such cases can also be done by CEUS [59]. According to one of the recent studies by Jung et al. [60], the sensitivity and specificity of CEUS in detecting acute pyelonephritis is 87.5% and 80.0% as compared to CT with moderate Intra and inter-observer agreement.
In a case of UTI, CECT axial image a shows bulky hypoenhancing left kidney with an evolving abscess (arrow). Few enlarged retroperitoneal lymph nodes are seen. On B mode US image (b), the kidney appears hypoechoic with an anechoic area in the lower pole which on corresponding CEUS image c shows irregular peripheral enhancement, likely an evolving abscess in diffuse pyelonephritis
Renal transplant evaluation
CEUS has been used for the evaluation of graft kidneys in the early postoperative period for diagnosing parenchymal causes of graft dysfunction. In recent study patients undergoing renal transplantation were enrolled in a duration of 3 years excluding patients with urologic or vascular complications. All patients underwent CEUS (using sulfur hexafluoride) along with Doppler and Shear wave elastography and renal scintigraphic examination 3–10 days after transplantation. Quantitative CEUS parameters (generated from a time-intensity curve) were analyzed along with other US parameters to detect graft dysfunction and differentiate acute tubular necrosis (ATN) from acute rejection (AR). The study concluded that CEUS can be used to diagnose parenchymal causes of early graft dysfunction with reasonable diagnostic accuracy [61].
Conventionally, renal biopsy is performed for establishing the diagnosis of ATN in renal transplant patients. CEUS can assess real-time perfusion of the transplanted kidney and various perfusion parameters can be quantified. This can potentially reduce the need for biopsy which is an invasive procedure, however, more studies are required for its authentification.
Another important indication of CEUS in renal transplantation is to detect/confirm vascular complications such as a renal artery or renal vein thrombosis, cortical necrosis. It has the potential to detect subcapsular ischemia and infarction and complications like pseudoaneurysms, abscess, or hematoma after surgery [47, 62, 63].
Post ablative treatment assessment
Various ablation techniques like Radiofrequency ablation (RFA), microwave ablation, or cryoablation are being increasingly performed these days in the treatment of small renal tumors in patients who are not surgical candidates [64, 65]. The efficacy of the ablation is comparable to partial/complete nephrectomy. The procedure is traditionally performed under CT guidance [66]. As the procedure is not done in real-time with CT, multiple repeat scans are required for adequate placement of the probes, which results in unnecessary radiation exposure. Iodinated contrast needs to be administered for distinct recognition of the tumor margins and vascular relations, which is nephrotoxic. In a few places, it is also done under the US or combined US and CT guidance. Post ablative assessment cannot be done in the same sitting due to the presence of post-ablation inflammatory changes and also repeat contrast cannot be administered due to its known complications [67]. Many studies are coming up evaluating the role of CEUS guided RFA procedures for renal tumors. It allows precise localization of the target tissue. Also, it provides real-time guidance with no risk of radiation or side effects of iodinated contrast. Repeat injections of UCA can be administered to assess post ablative residual soft tissue immediately after the procedure after a gap of 5–10 min after the heat effect of the ablation sinks [68]. Any area of nodular or rim-like enhancement is considered as residual tissue [69, 70], whereas the presence of rim-like enhancement in the very early period can be secondary to inflammatory changes. The residual tissue can then be ablated at the same sitting. CEUS has equivalent efficacy to CT/MRI in determining any residual disease after the ablative procedure [70]. However, a recent study by Bertolotto et al. have demonstrated the persistence of enhancement even after 1 month of ablation on CEUS [71]. Another study by Bertolotto et al. on cryoablation of renal tumors shows that intratumoral enhancement on early CEUS examination may not signify residual viable tumor. Hence follow-up is advised in such cases. The main limitation of CEUS marks in the region which are not well assessed with ultrasound. The deep-seated areas like the upper pole of the left kidney may not be adequately seen or visualization is poor in very obese patients. In such scenarios, CEUS performs poorly.
Renal trauma
CEUS has a potential role in the assessment of solid visceral injury in the abdomen. B mode the US performs poorly in this area with a sensitivity of 45.7% [72], although it can detect free intraperitoneal fluid which is an indirect marker for solid organ injury with high sensitivity ranging from 63 to 99% [73]. However, in about 33% of cases, there can be solid visceral injury without evidence of hemoperitoneum [74], hence these injuries will be missed on the plain US. CECT with delayed imaging is the gold standard for initial evaluation of suspected renal injury in blunt abdominal trauma cases [75]. The usefulness of CEUS in initial imaging is limited due to its inherent properties and the need for a panoramic assessment of the abdominal organs. However, CEUS has a role in the follow-up of renal trauma, for monitoring high-grade injuries. It allows visual assessment of improvement of perfusion to injured areas, cortical volume loss estimation, and further follow-up for complications such as acute bleeding and characteristics such as parenchymal loss [76]. It is particularly useful in the follow-up of pediatric patients where concerns like nephrotoxicity, radiation exposure, and motion artifacts are associated with CECT. CEUS can assist in the evaluation of solid visceral injury in the same setting. During the post-contrast venous phase, any acute injury shall appear as a non-enhancing defect with a sharp distinction from the normal vascular tissue [77]. Any laceration will appear as a linear, branching defect that is perpendicular to the overlying capsule associated with the capsular breach [78]. Lenticular non-enhancing subcapsular hematoma can also be seen. Active extravasation of contrast can also well be appreciated on CEUS as microbubble extravasation into renal or perirenal space. Complete vascular injury due to pedicle avulsion is characterized by the complete absence of renal perfusion. According to a study by Regine et al. CEUS could correctly detect renal parenchymal injuries in all 28 patients [79]. As the UCAs are not excreted in the pelvicalyceal system (PCS), collecting duct injuries cannot be detected with CEUS and MDCT should be advised whenever there is clinical suspicion of PCS injury [67, 72].
Vesico ureteric reflux (VUR)
EFSUMB recommends endocavitatory use of CEUS in depicting VUR in boys and girls having high suspicion, for follow-up of VUR after conservative or surgical management, and in patients, high-risk patients like kidney transplant recipients with recurrent UTIs and siblings of patients with VUR. The contrast Sonovue is dilated with saline and injected into the bladder either through per urethral catheter or suprapubic puncture. Any reflux into the ureter or PCS can be picked up by repeated scanning of kidneys and ureter/bladder during straining and voiding (Fig. 13). CEUS is far superior to the conventional micturating cystourethrography done under fluoroscopy owing to its radiation-free nature (especially useful in the pediatric population) and higher sensitivity. Another advantage is evaluation of the entire urethra can also be performed in the same study [3, 80]. A study by Drudi et al. have shown the potential role of CEUS in depicting VUR in transplant kidneys as well and has suggested performing voiding cystourethrography only if the result is negative on Contrast-enhanced voiding ultrasonography [81].
Future applications
Few recent studies have been published in evaluating the role of CEUS in calyceal puncture during percutaneous nephrolithotomy (PCNL) and for guiding puncture for nephrostomies [82]. The results are encouraging. They have found more accuracy with fewer incidences of repeat punctures with CEUS. The time for puncture is also significantly reduced with lesser blood loss and lower complication rate [83, 84]. Studies are being conducted to evaluate perfusion parameters to assess renal function in children with ureteropelvic junction obstruction. Also complications like acute rejection in renal transplant recipients and also assessing their severity can be done with CEUS [85]. Few pilot studies have been done to predict renal outcomes in acute renal injury [86].
Conclusion
CEUS is an excellent modality to assess the perfusion of organs and lesions. There are very few adverse effects and can be safely used in renal dysfunction. The major role of CEUS in the kidney is to perform risk stratification in cystic Renal Masses and confidently differentiate cystic vs. Solid Renal Masses. More utilities of CEUS are becoming popular as the use of UCAs becomes more mainstream.
References
Dietrich CF, Averkiou M, Nielsen MB, et al (2018) How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open 4:E2–E15. https://doi.org/10.1055/s-0043-123931
Bertolotto M, Catalano O (2009) Contrast-enhanced Ultrasound: Past, Present, and Future. Ultrasound Clinics 4:339–367. https://doi.org/10.1016/j.cult.2009.10.011
Sidhu PS, Cantisani V, Dietrich CF, et al (2018) The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med 39:e2–e44. https://doi.org/10.1055/a-0586-1107
Gramiak R, Shah PM (1968) Echocardiography of the aortic root. Invest Radiol 3:356–366. https://doi.org/10.1097/00004424-196809000-00011
Zhang F, Li R, Li G, et al (2019) Value of Contrast-Enhanced Ultrasound in the Diagnosis of Renal Cancer and in Comparison With Contrast-Enhanced Computed Tomography: A Meta-analysis. J Ultrasound Med 38:903–914. https://doi.org/10.1002/jum.14769
Piscaglia F, Bolondi L, Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents (2006) The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 32:1369–1375. https://doi.org/10.1016/j.ultrasmedbio.2006.05.031
Girometti R, Stocca T, Serena E, et al (2017) Impact of contrast-enhanced ultrasound in patients with renal function impairment. World Journal of Radiology 9:10–16. https://doi.org/10.4329/wjr.v9.i1.10
Wilson SR, Greenbaum LD, Goldberg BB (2009) Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol 193:55–60. https://doi.org/10.2214/AJR.09.2553
Bertolotto M, Cicero C, Perrone R, et al (2015) Renal Masses With Equivocal Enhancement at CT: Characterization With Contrast-Enhanced Ultrasound. American Journal of Roentgenology 204:W557–W565. https://doi.org/10.2214/AJR.14.13375
Quaia E (2005) Classification and Safety of Microbubble-Based Contrast Agents. In: Quaia E (ed) Contrast Media in Ultrasonography: Basic Principles and Clinical Applications. Springer, Berlin, Heidelberg, pp 3–14
Dalla Palma L, Bertolotto M (1999) Introduction to ultrasound contrast agents: physics overview. Eur Radiol 9 Suppl 3:S338-342. https://doi.org/10.1007/pl00014069
Ignee A, Atkinson NSS, Schuessler G, Dietrich CF (2016) Ultrasound contrast agents. Endosc Ultrasound 5:355–362. https://doi.org/10.4103/2303-9027.193594
Wilson SR, Burns PN (2010) Microbubble-enhanced US in Body Imaging: What Role? Radiology 257:24–39. https://doi.org/10.1148/radiol.10091210
Quaia E (2011) Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 21:604–615. https://doi.org/10.1007/s00330-010-1965-6
Claudon M, Dietrich CF, Choi BI, et al (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 34:11–29. https://doi.org/10.1055/s-0032-1325499
Eckersley RJ, Chin CT, Burns PN (2005) Optimising phase and amplitude modulation schemes for imaging microbubble contrast agents at low acoustic power. Ultrasound Med Biol 31:213–219. https://doi.org/10.1016/j.ultrasmedbio.2004.10.004
Granata A, Campo I, Lentini P, et al (2021) Role of Contrast-Enhanced Ultrasound (CEUS) in Native Kidney Pathology: Limits and Fields of Action. Diagnostics (Basel) 11:1058. https://doi.org/10.3390/diagnostics11061058
Main ML, Goldman JH, Grayburn PA (2009) Ultrasound contrast agents: balancing safety versus efficacy. Expert Opin Drug Saf 8:49–56. https://doi.org/10.1517/14740330802658581
Miller DL, Dou C, Wiggins RC (2009) Glomerular Capillary Hemorrhage Induced in Rats by Diagnostic Ultrasound with Gas Body Contrast Agent Produces Intra-Tubular Obstruction. Ultrasound Med Biol 35:869–877. https://doi.org/10.1016/j.ultrasmedbio.2008.10.015
Tenant SC, Gutteridge CM (2016) The clinical use of contrast-enhanced ultrasound in the kidney. Ultrasound 24:94–103. https://doi.org/10.1177/1742271X15627185
Correas J-M, Claudon M, Tranquart F, Hélénon AO (2006) The kidney: imaging with microbubble contrast agents. Ultrasound Q 22:53–66
Bosniak MA (1986) The current radiological approach to renal cysts. Radiology 158:1–10. https://doi.org/10.1148/radiology.158.1.3510019
Silverman SG, Pedrosa I, Ellis JH, et al (2019) Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 292:475–488. https://doi.org/10.1148/radiol.2019182646
Cantisani V, Bertolotto M, Clevert D-A, et al (2021) EFSUMB 2020 Proposal for a Contrast-Enhanced Ultrasound-Adapted Bosniak Cyst Categorization - Position Statement. Ultraschall Med 42:154–166. https://doi.org/10.1055/a-1300-1727
Quaia E, Bertolotto M, Cioffi V, et al (2008) Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR Am J Roentgenol 191:1239–1249. https://doi.org/10.2214/AJR.07.3546
Clevert D-A, Minaifar N, Weckbach S, et al (2008) Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc 39:171–178
Graumann O, Osther SS, Karstoft J, et al (2016) Bosniak classification system: a prospective comparison of CT, contrast-enhanced US, and MR for categorizing complex renal cystic masses. Acta Radiol 57:1409–1417. https://doi.org/10.1177/0284185115588124
Qiu X, Zhao Q, Ye Z, et al (2020) How does contrast-enhanced ultrasonography influence Bosniak classification for complex cystic renal mass compared with conventional ultrasonography? Medicine (Baltimore) 99:e19190. https://doi.org/10.1097/MD.0000000000019190
Nicolau C, Bunesch L, Sebastia C (2011) Renal complex cysts in adults: contrast-enhanced ultrasound. Abdom Imaging 36:742–752. https://doi.org/10.1007/s00261-011-9727-8
Nicolau C, Buñesch L, Paño B, et al (2015) Prospective evaluation of CT indeterminate renal masses using US and contrast-enhanced ultrasound. Abdom Imaging 40:542–551. https://doi.org/10.1007/s00261-014-0237-3
Ascenti G, Mazziotti S, Zimbaro G, et al (2007) Complex cystic renal masses: characterization with contrast-enhanced US. Radiology 243:158–165. https://doi.org/10.1148/radiol.2431051924
Schwarze V, Rübenthaler J, Čečatka S, et al (2020) Contrast-Enhanced Ultrasound (CEUS) for the Evaluation of Bosniak III Complex Renal Cystic Lesions-A 10-Year Specialized European Single-Center Experience with Histopathological Validation. Medicina (Kaunas) 56:E692. https://doi.org/10.3390/medicina56120692
Lan D, Qu H-C, Li N, et al (2016) The Value of Contrast-Enhanced Ultrasonography and Contrast-Enhanced CT in the Diagnosis of Malignant Renal Cystic Lesions: A Meta-Analysis. PLoS One 11:e0155857. https://doi.org/10.1371/journal.pone.0155857
Chen Y, Wu N, Xue T, et al (2015) Comparison of contrast-enhanced sonography with MRI in the diagnosis of complex cystic renal masses. J Clin Ultrasound 43:203–209. https://doi.org/10.1002/jcu.22232
Rubenthaler J, Cecatka S, Froelich MF, et al (2020) Contrast-Enhanced Ultrasound (CEUS) for Follow-Up of Bosniak 2F Complex Renal Cystic Lesions--A 12-Year Retrospective Study in a Specialized European Center. Cancers 12:1fo–1fo
Yong C, Teo YM, Jeevesh K (2016) Diagnostic performance of contrast-enhanced ultrasound in the evaluation of renal masses in patients with renal impairment. Med J Malaysia 71:193–198
Narasimhan N, Golper TA, Wolfson M, et al (1986) Clinical characteristics and diagnostic considerations in acquired renal cystic disease. Kidney Int 30:748–752. https://doi.org/10.1038/ki.1986.251
Hofmann JN, Corley DA, Zhao WK, et al (2015) Chronic kidney disease and risk of renal cell carcinoma: differences by race. Epidemiology 26:59–67. https://doi.org/10.1097/EDE.0000000000000205
Christensson A, Savage C, Sjoberg DD, et al (2013) Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 133:1452–1458. https://doi.org/10.1002/ijc.28144
Lowrance WT, Ordoñez J, Udaltsova N, et al (2014) CKD and the risk of incident cancer. J Am Soc Nephrol 25:2327–2334. https://doi.org/10.1681/ASN.2013060604
Chang EH, Chong WK, Kasoji SK, et al (2017) Diagnostic accuracy of contrast-enhanced ultrasound for characterization of kidney lesions in patients with and without chronic kidney disease. BMC Nephrol 18:266. https://doi.org/10.1186/s12882-017-0681-8
Barr RG, Peterson C, Hindi A (2014) Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology 271:133–142. https://doi.org/10.1148/radiol.13130161
Bertolotto M, Bucci S, Valentino M, et al (2018) Contrast-enhanced ultrasound for characterizing renal masses. Eur J Radiol 105:41–48. https://doi.org/10.1016/j.ejrad.2018.05.015
Reuter VE, Presti JC (2000) Contemporary approach to the classification of renal epithelial tumors. Semin Oncol 27:124–137
Pan K-H, Jian L, Chen W-J, et al (2020) Diagnostic Performance of Contrast-Enhanced Ultrasound in Renal Cancer: A Meta-Analysis. Front Oncol 10:586949. https://doi.org/10.3389/fonc.2020.586949
Wang C, Yu C, Yang F, Yang G (2014) Diagnostic accuracy of contrast-enhanced ultrasound for renal cell carcinoma: a meta-analysis. Tumour Biol 35:6343–6350. https://doi.org/10.1007/s13277-014-1815-2
Geyer T, Schwarze V, Marschner C, et al (2020) Diagnostic Performance of Contrast-Enhanced Ultrasound (CEUS) in the Evaluation of Solid Renal Masses. Medicina (Kaunas) 56:E624. https://doi.org/10.3390/medicina56110624
Gulati M, King KG, Gill IS, et al (2015) Contrast-enhanced ultrasound (CEUS) of cystic and solid renal lesions: a review. Abdom Imaging 40:1982–1996. https://doi.org/10.1007/s00261-015-0348-5
Bertolotto M, Cicero C, Catalano O, et al (2018) Solid Renal Tumors Isoenhancing to Kidneys on Contrast-Enhanced Sonography: Differentiation From Pseudomasses. Journal of Ultrasound in Medicine 37:233–242. https://doi.org/10.1002/jum.14335
Tamai H, Takiguchi Y, Oka M, et al (2005) Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. J Ultrasound Med 24:1635–1640. https://doi.org/10.7863/jum.2005.24.12.1635
Liu H, Cao H, Chen L, et al (2022) The quantitative evaluation of contrast-enhanced ultrasound in the differentiation of small renal cell carcinoma subtypes and angiomyolipoma. Quantitative Imaging in Medicine and Surgery 12:10618–10118. https://doi.org/10.21037/qims-21-248
Xu Z-F, Xu H-X, Xie X-Y, et al (2010) Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med 29:709–717. https://doi.org/10.7863/jum.2010.29.5.709
Lu Q, Li C, Huang B, et al (2015) Triphasic and epithelioid minimal fat renal angiomyolipoma and clear cell renal cell carcinoma: qualitative and quantitative CEUS characteristics and distinguishing features. Abdom Imaging 40:333–342. https://doi.org/10.1007/s00261-014-0221-y
Wu Y, Du L, Li F, et al (2013) Renal oncocytoma: contrast-enhanced sonographic features. J Ultrasound Med 32:441–448. https://doi.org/10.7863/jum.2013.32.3.441
Mazziotti S, Zimbaro F, Pandolfo A, et al (2010) Usefulness of contrast-enhanced ultrasonography in the diagnosis of renal pseudotumors. Abdom Imaging 35:241–245. https://doi.org/10.1007/s00261-008-9499-y
Granata A, Zanoli L, Insalaco M, et al (2015) Contrast-enhanced ultrasound (CEUS) in nephrology: Has the time come for its widespread use? Clin Exp Nephrol 19:606–615. https://doi.org/10.1007/s10157-014-1040-8
Bertolotto M, Martegani A, Aiani L, et al (2008) Value of contrast-enhanced ultrasonography for detecting renal infarcts proven by contrast enhanced CT. A feasibility study. Eur Radiol 18:376–383. https://doi.org/10.1007/s00330-007-0747-2
Yusuf GT, Sellars ME, Huang DY, et al (2014) Cortical necrosis secondary to trauma in a child: contrast-enhanced ultrasound comparable to magnetic resonance imaging. Pediatr Radiol 44:484–487. https://doi.org/10.1007/s00247-013-2818-7
Fontanilla T, Minaya J, Cortés C, et al (2012) Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging 37:639–646. https://doi.org/10.1007/s00261-011-9781-2
Jung HJ, Choi MH, Pai KS, Kim HG (2020) Diagnostic performance of contrast-enhanced ultrasound for acute pyelonephritis in children. Sci Rep 10:10715. https://doi.org/10.1038/s41598-020-67713-z
Goyal A, Hemachandran N, Kumar A, et al (2020) Evaluation of the Graft Kidney in the Early Postoperative Period: Performance of Contrast-Enhanced Ultrasound and Additional Ultrasound Parameters. J Ultrasound Med. https://doi.org/10.1002/jum.15557
Cantisani V, Bertolotto M, Weskott HP, et al (2015) Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol 84:1675–1684. https://doi.org/10.1016/j.ejrad.2015.05.008
Stacul F, Sachs C, Giudici F, et al (2021) Cryoablation of renal tumors: long-term follow-up from a multicenter experience. Abdom Radiol 46:4476–4488. https://doi.org/10.1007/s00261-021-03082-z
Lackey L, Peterson C, Barr RG (2012) Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. Ultrasound Q 28:269–274. https://doi.org/10.1097/RUQ.0b013e318274de66
Xu L, Rong Y, Wang W, et al (2016) Percutaneous radiofrequency ablation with contrast-enhanced ultrasonography for solitary and sporadic renal cell carcinoma in patients with autosomal dominant polycystic kidney disease. World J Surg Oncol 14:193. https://doi.org/10.1186/s12957-016-0916-3
Johnson DB, Duchene DA, Taylor GD, et al (2005) Contrast-enhanced ultrasound evaluation of radiofrequency ablation of the kidney: reliable imaging of the thermolesion. J Endourol 19:248–252. https://doi.org/10.1089/end.2005.19.248
Cokkinos DD, Antypa EG, Skilakaki M, et al (2013) Contrast Enhanced Ultrasound of the Kidneys: What Is It Capable of? BioMed Research International 2013:e595873. https://doi.org/10.1155/2013/595873
Boss A, Clasen S, Kuczyk M, et al (2007) Image-guided radiofrequency ablation of renal cell carcinoma. Eur Radiol 17:725–733. https://doi.org/10.1007/s00330-006-0415-y
O’Neal D, Cohen T, Peterson C, Barr RG (2018) Contrast-Enhanced Ultrasound-Guided Radiofrequency Ablation of Renal Tumors. J Kidney Cancer VHL 5:7–14. https://doi.org/10.15586/jkcvhl.2018.100
Hoeffel C, Pousset M, Timsit M-O, et al (2010) Radiofrequency ablation of renal tumours: diagnostic accuracy of contrast-enhanced ultrasound for early detection of residual tumour. Eur Radiol 20:1812–1821. https://doi.org/10.1007/s00330-010-1742-6
Bertolotto M, Campo I, Sachs C, et al (2021) Contrast-enhanced ultrasound after successful cryoablation of benign and malignant renal tumours: how long does tumour enhancement persist? J Med Imaging Radiat Oncol 65:272–278. https://doi.org/10.1111/1754-9485.13149
Sessa B, Trinci M, Ianniello S, et al (2015) Blunt abdominal trauma: role of contrast-enhanced ultrasound (CEUS) in the detection and staging of abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med 120:180–189. https://doi.org/10.1007/s11547-014-0425-9
Paajanen H, Lahti P, Nordback I (1999) Sensitivity of transabdominal ultrasonography in detection of intraperitoneal fluid in humans. Eur Radiol 9:1423–1425. https://doi.org/10.1007/s003300050861
Chiu WC, Cushing BM, Rodriguez A, et al (1997) Abdominal injuries without hemoperitoneum: a potential limitation of focused abdominal sonography for trauma (FAST). J Trauma 42:617–623; discussion 623-625. https://doi.org/10.1097/00005373-199704000-00006
Morey AF, Brandes S, Dugi DD, et al (2014) Urotrauma: AUA guideline. J Urol 192:327–335. https://doi.org/10.1016/j.juro.2014.05.004
Bowen DK, Back SJ, Van Batavia JP, et al (2020) Does contrast-enhanced ultrasound have a role in evaluation and management of pediatric renal trauma? A preliminary experience. J Pediatr Surg 55:2740–2745. https://doi.org/10.1016/j.jpedsurg.2020.06.010
Piscaglia F, Nolsøe C, Dietrich CF, et al (2012) The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33:33–59. https://doi.org/10.1055/s-0031-1281676
Cagini L, Gravante S, Malaspina CM, et al (2013) Contrast enhanced ultrasound (CEUS) in blunt abdominal trauma. Crit Ultrasound J 5 Suppl 1:S9. https://doi.org/10.1186/2036-7902-5-S1-S9
Regine G, Atzori M, Miele V, et al (2007) Second-generation sonographic contrast agents in the evaluation of renal trauma. Radiol Med 112:581–587. https://doi.org/10.1007/s11547-007-0164-2
Ntoulia A, Aguirre Pascual E, Back SJ, et al (2021) Contrast-enhanced voiding urosonography, part 1: vesicoureteral reflux evaluation. Pediatr Radiol 51:2351–2367. https://doi.org/10.1007/s00247-020-04906-8
Drudi FM, Angelini F, Bertolotto M, et al (2021) Role of Contrast-Enhanced Voiding Urosonography in the Evaluation of Renal Transplant Reflux - Comparison with Voiding Cystourethrography and a New Classification. Ultraschall Med. https://doi.org/10.1055/a-1288-0075
Huang DY, Yusuf GT, Daneshi M, et al (2018) Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol (NY) 43:960–976. https://doi.org/10.1007/s00261-018-1473-8
Guo X, Zhang Z, Liu Z, et al (2021) Assessment of the Contrast-Enhanced Ultrasound in Percutaneous Nephrolithotomy for the Treatment of Patients with Nondilated Collecting System. Journal of Endourology 35:436–443. https://doi.org/10.1089/end.2020.0564
Shen C, Zhang B, Han WK, et al (2017) [Percutaneous renal access for percutaneous nephrolithotomy guided by contrast enhanced ultrasound: a single-center preliminary experience in China]. Beijing Da Xue Xue Bao Yi Xue Ban 49:1071–1075
Como G, Da Re J, Adani GL, et al (2020) Role for contrast-enhanced ultrasound in assessing complications after kidney transplant. World J Radiol 12:156–171. https://doi.org/10.4329/wjr.v12.i8.156
Yoon HE, Kim DW, Kim D, et al (2020) A pilot trial to evaluate the clinical usefulness of contrast-enhanced ultrasound in predicting renal outcomes in patients with acute kidney injury. PLOS ONE 15:e0235130. https://doi.org/10.1371/journal.pone.0235130
Funding
No funding received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aggarwal, A., Goswami, S. & Das, C.J. Contrast-enhanced ultrasound of the kidneys: principles and potential applications. Abdom Radiol 47, 1369–1384 (2022). https://doi.org/10.1007/s00261-022-03438-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03438-z