Abstract
The incidence of renal cell carcinoma is rising with the increased number of incidental detection of small tumours. During the past few years, percutaneous imaging-guided radiofrequency ablation has evolved as a minimally invasive treatment of small unresectable renal tumours offering reduced patient morbidity and overall health care costs. In radiofrequency ablation, thermal energy is deposited into a targeted tumour by means of a radiofrequency applicator. In recent studies, radiofrequency ablation was shown to be an effective and safe modality for local destruction of renal cell carcinoma. Radiofrequency applicator navigation can be performed via ultrasound, computed tomography or magnetic resonance guidance; however, ultrasound seems less favourable because of the absence of monitoring capabilities during ablation. On-line monitoring of treatment outcome can only be performed with magnetic resonance imaging giving the possibility of eventual applicator repositioning to ablate visible residual tumour tissue. Long-term follow-up is crucial to assess completeness of tumour ablation. New developments in ablation technology and radiological equipment will further increase the indication field for radiofrequency ablation of renal cell carcinoma. Altogether, radiofrequency ablation seems to be a promising new modality for the minimally invasive treatment of renal cell carcinoma, which was demonstrated to exhibit high short-term effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A worldwide incidence of 150,000 cases of renal cell carcinoma (RCC) is estimated constituting approximately 2% of the malignancies in adults [1]. More than 50% of asymptomatic cases are, meanwhile, incidentally discovered [2]. The incidence of RCC is increasing, which partly reflects the increasing incidental discovery of small localized RCC due to the more frequent use of imaging modalities such as ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI). Independent of the improved detection of RCC, a rise in incidence was registered in the Western industrialized states [3]. In parallel, several hereditary syndromes are related with an increased risk for the occurrence of RCC, of which von Hippel-Lindau (VHL) disease is the most characterized. VHL with 23–45% of afflicted patients developing one or more RCC during their lifetime [4] shows a tendency of the lesions to be bilateral and multifocal. In patients with sporadic renal cancers, nephron-sparing surgery (NSS) was shown to be equivalent at 5-year cancer control to total nephrectomy [5].
Many elderly patients with sporadic RCC are poor candidates for surgical resection due to comorbidities. RCC in these patients is often small in size due to incidental discovery [2]. In these patients, minimally invasive percutaneous ablation techniques can effectively be used for local tumour control preserving healthy renal parenchyma, reducing the overall morbidity of surgery, allowing for earlier hospital discharge and faster convalescence and, furthermore, decreasing overall health care costs. In percutaneous ablation techniques, a radiofrequency (RF) applicator is placed into the tumour via imaging guidance, and thermal energy is applied into the tumour leading to cell death. Two ablation techniques for the treatment of RCC have evolved as the most widely used: radiofrequency ablation (RFA) [6–13] and cryoablation [14], with RFA being the most extensively used technique for its ease of use and its efficacy. A summary of recent imaging-guided RFA studies is given in Table 1.
This review summarizes the technique of and indications for percutaneous RFA of RCC, factors influencing success of treatment and results of reported clinical trials. A further point of this article is focused on the comparison of different imaging guidance modalities, treatment complications during RFA and possible preventive techniques, as well as guidelines for follow-up imaging. A short discussion on upcoming developments in RFA and imaging is given that will possibly change the technique and improve the outcome of future RFA treatment of RCC.
Principles and technique of radiofrequency ablation
In thermal ablation techniques, tumour tissue is destroyed by creating target temperatures in excess of 48–50°C within the pathological tissue leading to coagulation necrosis and cellular death. The higher the temperature inducing irreversible damage to critical cell proteins, the shorter is the necessary treatment duration to secure cell death. The tissue thermal damage at high-temperature exposure may be predicted by means of biophysical relationships such as the Pennes bioheat equation [15], an Arrhenius analysis [16] or the Sapareto-Dewey isoeffect thermal dose relationship [17]. These relationships have been tested in several in vivo systems, which demonstrated that within defined temperature ranges, the thermal damage in tissue is approximately linearly dependent upon treatment time and exponentially dependent upon the increase in temperature. The sensitivity to heat exposure among different tissue types is variable [16].
In RFA, an alternating electrical current is used to create resistive heating within the targeted tissue. Frequencies of radiofrequency generators range between 375 and 480 kHz, and generator power of up to 250 W is available. The electrical energy is deposited in the tissue by means of a needle applicator of 1.6–2.5 mm in diameter that is insulated up to an active tip of 2–5 cm. In conventional monopolar RFA systems, the electrical circuit is established between the active applicator tip and one or several grounding pads attached to the patient’s thighs. The thermal heating is more intense close to the active needle tip, because of the narrow electrical streamlines and, therefore, the highest current density. The highest tissue temperatures are reached at only a few millimetres surrounding the applicator tip, and the resulting coagulation necrosis is formed by convective heating. The achievable ablation volumes are limited as charring and tissue boiling close to the active needle tip increase the electrical impedance with a drop in energy deposition. Several techniques have been developed to overcome this limitation: pulsed energy deployment [18, 19], expansion of electrode surface [20] and internal cooling [20] or perfusion [21]. These systems theoretically allow for almost spherical necrosis diameters in vivo of up to 3 cm in maximum diameter. Further increase of the coagulation diameter via augmenting the distance between the needles in the multi-applicator approach is not possible due to electrical shadowing in the centre of the applicators. For tumour diameters larger than 3 cm, several overlapping ablations with intermittent repositioning of the applicator have to be performed. New bipolar [22] and multipolar [23] devices are already available offering possible larger achievable coagulation diameters and better controlled energy deposition; however, they have not yet been evaluated for the treatment of RCC. Initial multipolar ablation protocols have been tested in ex vivo liver tissue suggesting ablation diameters of up to 7 cm in maximum diameter with the placement of three applicators in a triangular configuration [23].
Experimental studies and pathology
In several ablate and resect studies performed in animal models [24–29], pathological and histological examinations of the renal coagulation lesion induced via RFA were carried out. On gross examination, the coagulation necrosis is blanched, well-circumscribed and of yellowish-white colour. The light microscopic changes can be characterized by blurring of the nuclear chromatin, an increased eosinophilia of the cytoplasm, loss of cell border integrity and presence of interstitial haemorrhage. Electron microscopy revealed prominent cytoplasmic granularity and near-total loss of ultrastructural detail of the cytoplasmic organelles. The loss of cellular integrity with lyses of nuclei, degeneration of cell membranes and cellular fragmentation may increase during the following 2–4 weeks leading to complete necrosis within the coagulated area. A sharp junction between the affected tissue and the surrounding normal renal parenchyma is formed. Marked inflammation can be found surrounding the area of coagulation with tissue oedema, inflammatory infiltrates, neovascularisation and haemorrhage. Four zones can be distinguished from the centre to the periphery of the lesion: (1) carbonization in the direct vicinity of the applicator, (2) complete coagulation necrosis, (3) a surrounding rim of inflammation and haemorrhage and (4) normal renal parenchyma. The coagulation lesion shows a slow shrinkage due to absorption and fibrotic conversion during the following months.
Technique of percutaneous treatment
Several different approaches are possible in renal RFA including intraoperative open surgical, laparoscopic and imaging-guided percutaneous treatment. In this manuscript, the focus is set on percutaneous imaging-guided treatment, which may be regarded as the minimally invasive modality. Imaging guidance may be performed with US, CT or MRI. Advantages and disadvantages of each modality will be discussed in detail in the next section.
Before the intervention, a physical examination and a check of coagulation laboratory parameters has to be carried out, as well as written informed consent to the treatment has to be obtained from the patient. Most of the percutaneous ablation treatments can be performed in conscious sedation using, for example, pethidine and midazolam, although a general anaesthesia may be required in selected patients. Patients are typically placed in supine or semi-supine position to allow for a posterior puncture tract. After preoperative imaging for tumour localization, the puncture site and the needle trajectory are planned. After disinfection and sterile covering, skin and adjacent subcutaneous regions are anaesthetized with 1% Xylocaine solution. After diagnostic imaging to ensure that the applicator is correctly positioned in all spatial directions, the ablation procedure can be started with typically 10–15 min of energy deposition. Subsequently, the applicator may be repositioned if necessary, and another ablation cycle can be performed to coagulate residual tumour tissue. With overlapping ablations, RCC of up to 5.5 cm in maximum diameter were reported to be successfully treated [12].
Several histological studies have demonstrated frequent microscopic tumour penetration of the pseudocapsule that surrounds most of the RCC emphasizing the need for a safety margin [30]. The often reported multicentricity in sporadic RCC may be regarded in most cases as intrarenal metastases [31]. To avoid local tumour relapse, the coagulation zone should encompass a 5- to 10-mm layer of peritumoural margin in addition to the complete tumour. Therefore, it is advisable to use cluster electrodes or expandable multiprong electrodes for all tumour sizes as these systems achieve the largest possible ablation volume within a reasonable time [18–20]. After complete tumour destruction, the applicator is retracted under constant heating using 25–30 W power (without cooling) for needle tract coagulation, which prevents haemorrhage and tumour seeding. Patients may be treated on an outpatient basis after a short observation time. Prophylactic antibiotics before or immediately after the treatment are not recommended. A standard abdominal US examination should be performed to exclude ablation-related complications before dismissal from the hospital.
Indication for RFA of renal cell carcinomas and predictors of outcome
The indication for percutaneous interventional thermoablation of RCC has to be discussed in interdisciplinary collaboration of urology and interventional radiology. The treatment strategy has to be defined in the context of the overall clinical situation including age and life expectancy, laboratory parameters, radiological assessment of local and general extension of disease, and patient compliance. Radical nephrectomy is the gold standard for treatment of renal cell carcinoma against which all other forms of treatment including NSS and RFA have to be measured.
The indication for NSS is given if radical nephrectomy would render the patient anephric, with a need for dialysis or renal replacement therapy [30], which involves all cases of anatomically or functionally solitary kidneys due to unilateral kidney agenesis, previous contralateral nephrectomy or irreversible impairment of contralateral kidney function. For low-stage disease (single, small tumour ≤4 cm) and a normal contralateral kidney, NSS was shown to provide equally effective curative treatment as compared to radical nephrectomy with a long-term renal functional advantage [30, 31]. However, NSS is technically more challenging as compared to radical nephrectomy with higher intraoperative blood loss. In elderly patients with significant comorbidities and reduced life expectancy (e.g. due to chronic congestive heart failure, chronic obstructive pulmonary disease or other primary malignancy), partial nephrectomy with general anaesthesia bears a significant operational risk. As the tumour growth is low in RCC with increase in tumour diameter of 0.2–1.2 cm/year, and as RCC smaller than 4 cm seldom metastasize, “watchful waiting” may be considered an appropriate option in the elderly patient, especially with a life expectancy of less than 1 year [32, 33].
Percutaneous RFA may offer a minimally invasive treatment in the elderly patient not requiring general anaesthesia and may potentially preserve renal function without significant blood loss. RFA may preserve a larger amount of healthy renal parenchyma as compared to NSS, and therefore, may additionally be an alternative to NSS in patients with reduced renal function or multiple RCC, as these patients might require dialysis following NSS. However, no clinical trials have yet been published comparing long-term renal function after RFA versus NSS, which could corroborate this hypothesis.
The tumour size is a strong predictor of ablation outcome. Small tumours with diameters up to 4 cm seem to be the most appropriate indication for RFA. These tumours are technically successfully treated in 92–100% of cases with one or multiple sessions using the commercially available RF ablation systems [6–13, 34] under CT guidance. With tumours smaller than 3 cm in size, complete ablation will typically be performed within one single treatment session [34]. However, complete ablation was reported for tumours up to 5.5 cm in diameter [12], which requires many applicator repositionings and subsequently longer treatment times. To increase ablation effectiveness for large tumour sizes, pre-ablation embolization to reduce perfusion-mediated cooling was suggested by some authors [8, 9].
A further predictor of technical successfulness seems to be the tumour location [6]. Exophytic tumours, which according to the definition invade the perirenal fat, are easier to puncture compared to more central tumours. The insulating effect of the fat capsule allows for higher ablation temperatures, whereas blood flow in the large hilar blood vessels causes a cooling “heat-sink” effect. Additionally, the risk of bleeding seems to be increased for centrally located tumours [6]. However, it was reported that 61–78% of tumours with central components can completely be ablated within one or multiple sessions. In another study, however, no significant differences in ablation outcome for central and exophytic tumours were found [34]. Problems may arise in RCC tumours exhibiting large cystic components as RFA is less effective for cystic lesions. However, first reports have been published showing successful treatment of benign cysts via RFA [35, 36]. No reports have yet been published on the effectiveness of RFA for RCC exhibiting large cystic components.
NSS or RFA in patients with advanced or metastatic disease is primarily indicated to avoid rendering the patient anephric [31] and necessitating renal replacement therapy or dialysis. By resection or ablation of the primary tumour, symptomatic relief may be possible while maintaining acceptable renal function. No benefit in life expectancy has yet been demonstrated for such an indication; however, several studies recently showed that the combination of immunotherapy and radical nephrectomy leads to an increase in life expectancy [37]. The same may be assumed for complete tumour ablation or NSS. Altogether, RFA seems to be a treatment alternative especially for patients who are poor candidates for surgery or suffering from multifocal RCC such as VHL patients and patients with small exophytic tumours of less than 4 cm in size, which are not close to vessels potentially causing a heat-sink effect.
Imaging guidance and ablation monitoring
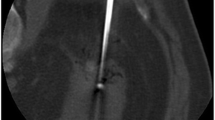
Imaging guidance in RFA may be performed using US, CT or MRI. A MR-guided RFA of a renal mass is shown in Fig. 1. Of the mentioned modalities, US seems to be the least favourable for RFA as steam bubbles produced by the vaporization during the procedure significantly disturb the image quality due to their hyperechogenicity. Therefore, reliable ablation monitoring is not feasible with US. In CT, no significant changes in the imaging pattern of the ablated renal masses can be found [26] (Fig. 2). Residual tumour tissue can be detected at CT by administration of contrast medium because the tumour tissue generally exhibits signal enhancement, whereas the coagulation necrosis does not. However, injection of contrast medium can only be performed once per ablation session as the contrast medium clearance may need several hours. The necessity of up to four treatment sessions has been described for CT guidance to achieve complete tumour necrosis [6]. In certain RCC not showing contrast enhancement, administration of contrast media does not allow ablation monitoring in CT guidance [6].
MR-guided RFA of a 3.1×2.0×3.1 cm mixed RCC (white arrowheads, tumour volume 10.1 cm3) performed in a female 82-year-old patient. Pre-interventional T1w (a) and T2w (b) spin echo and fast spin echo images are displayed, which were acquired at an interventional low-field scanner at 0.2 tesla. In T2w imaging recorded after one ablation cycle (c), the coagulation zone surrounding the applicator exhibits signal loss. Ventral to the applicator, residual tumour tissue (white arrow) is visible showing hyperintense signal characteristics. After repositioning of the applicator and another ablation cycle (d), complete ablation of the RCC is achieved
RFA of a 3.3×3.2×3.3 cm RCC in a 72-year-old male patient carried out with CT guidance. Pre-interventional native (a) and contrast-enhanced (b) images are displayed. Only slight changes in the signal pattern are seen after the 12-min ablation cycle in an image acquired immediately after the ablation (c) and an image recorded after the removal of the applicator and administration of another contrast medium bolus (d)
In several studies, MR imaging for applicator navigation and treatment monitoring was suggested [38–40]. MRI is optimally suited for imaging guidance in RFA due to its intrinsically high soft tissue contrast, the capability of true multiplanar imaging due to the free choice of the applied imaging gradients and the possibility of applicator targeting with sequences of MR fluoroscopy (Fig. 3). Rapid T1w gradient echo sequences (GRE) and T2w steady-state free precession sequences (SSFP) with acquisition times of approximately 2 s allow for safe and fast applicator placement. MR imaging is the only modality providing near on-line monitoring of the ablation procedure. Clear post-ablation changes are visible in T2w MR imaging. Complete tumour ablation is achieved in areas of signal loss in T2w imaging replacing the isointense or hyperintense signal of the RCC (Fig. 1c,d). In the acute phase after ablation, the hypointense area of complete necrosis is typically surrounded by a small hyperintense rim, which corresponds to the zone of inflammation and haemorrhage. Residual tumour tissue can be detected due to its persisting isointense or hyperintense signal. The applicator may be repositioned to the area of incomplete treatment, and a further ablation cycle may be performed with the patient still in the same position on the MR table. As RFA allows for an indefinite number of subsequent ablation cycles, the repeated assessment of ablation outcome without new injection of contrast media provides a major advantage over other imaging modalities. In cases of central tumour location close to hilar vessels, immediate control of ablation outcome allows for assessment of a possible heat-sink effect. For MR guidance, a success rate of complete tumour ablation in 92–100% of cases within one single session was reported [38–40].
In MR-guided RFA, fast gradient echo sequences can be used for applicator positioning. In a, the site of applicator insertion is chosen by skin impression with the fingertip (arrowhead). Rapid MR sequences with 2-s acquisition time for each image allow for fast and safe applicator positioning within the tumour
Complications
According to the recent literature, complication rates in RFA of RCC seem lower as compared to partial or radical nephrectomy. Only minimal morbidity and no mortality are associated with RFA. Complication rates for partial or total nephrectomy range between 14 and 26% [41, 42], whereas complication rates for CT-guided RFA are reported between 0 and 11% [6–12].
The most common complication is haemorrhage, which seldom requires blood transfusion. A possible complication of thermal ablation therapy is the unwanted damaging of healthy bordering tissue by heat conductance. In central tumours, the ureter may be injured by the local heating. Thermal damage to the ureter may result in ureteral stricture or urine leakage [6, 40], which in rare cases requires nephrostomy or ureteral stent placement. More frequently, affection of the lumbar plexus or the genitofemoral nerve is reported, which leads to pain and insensitiveness in the inguinal skin area possibly persisting over many months [6, 7, 10, 11, 39, 40]. No cases of bowel perforation and only one case of tract seeding have been described [11]. Infection and skin burn at the grounding pad site are rare occurrences.
Recently, several techniques have been proposed for separating heat-sensible organ structures from the ablation area: positional manoeuvres of the patient, paranephric instillation of fluid or air/CO2, or balloon displacement [43, 44]. To obviate injury to the lumbar plexus and the genitofemoral nerve, displacement of the kidney from the major psoas muscle in cases of critical tumour location has been recommended by using the applicator as a lever to move the kidney away [45]. For safe treatment of renal masses critically close to susceptible organ structures, MR guidance is the best imaging modality due to the multiplanar capabilities, the availability of near real-time MR fluoroscopy and the immediate visibility of thermally affected organ structures in T2w imaging.
Follow-up
One of the potential disadvantages of RFA is that the coagulated tissue remains in situ, which requires short-interval follow-up examinations for assessment of residual tumour or relapse. As the growth rate of RCC is low, this has to be performed at regular intervals over several years. Most studies applied a scheme with follow-up imaging every 3 months in the 1st year and every 6 months in the 2nd year after RFA [6–12, 38–40]. No trials have so far been published as to whether CT or MR imaging is more reliable for assessment of tumour relapse in follow-up examinations. In CT, any area exhibiting contrast enhancement within the ablation zone is conventionally regarded as residual tumour tissue or tumour relapse.
For iodinated contrast media, an estimated nephrotoxicity of 20–30% was reported if administered in patients with pre-existing renal disease [46]. Therefore, contrast-enhanced CT should be avoided in the post-RFA patient cohort, and MR imaging follow-up should be preferred. Only a few studies have been published so far describing the MRI findings in renal RFA follow-up [47, 48].
In MRI, the hypointense coagulation zone retains its signal behaviour in T2w imaging, whereas a change in the signal pattern of T1w imaging from hypointense to hyperintense is found. This signal change may be due to the fibrotic conversion of the coagulation necrosis. The thin hyperintense rim, which can be seen in T2w imaging and contrast-enhanced imaging surrounding the coagulation zone in the acute stage after RFA, is barely detectable in follow-up after several weeks. After an increase in size in the first 2 weeks, the coagulation necrosis shows a slow involution during subsequent follow-up examinations [38]. Contrast-enhanced perfusion imaging was found to exhibit higher concordance with definite coagulation volume as compared to T2w imaging, which underestimates the true ablation size [48]. In the assessment of residual tumour tissue or tumour relapse, T2w imaging and contrast-enhanced perfusion imaging complement each other [48]. As the applied gadolinium chelates are potentially nephrotoxic in patients with renal disease [49], perfusion imaging could be performed by means of arterial spin labelling. Arterial spin labelling uses magnetically marked blood water as an endogenous tracer. The feasibility of renal RFA follow-up using spin labelling in diagnostic imaging quality was recently reported [48].
Conclusion and future prospects
Imaging-guided RFA of renal masses has been proven to be a reliable and safe treatment modality, which may be applied as minimally invasive therapy in patients with significant comorbidities rendering them poor candidates for surgical resection. MR-guided RFA is the most recent development providing many advantages over CT or US guidance, among which is the immediate visibility of thermally affected tissue. With the new generation of interventional open-bore high-field scanners, significant improvement of the treatment protocols and a further increase in the rate of complete coagulation within one session seems possible. More studies are necessary in the field of follow-up imaging assessing the different imaging modalities for early visualisation of residual tumour tissue or tumour relapse after RFA. New thermoablative techniques such as multipolar RFA or microwave ablation will possibly allow for more consistent and successful ablation of larger NCC with an improved convection profile and reduced heat-sink effect.
References
Godley P, Kim SW (2002) Renal cell carcinoma. Curr Opin Oncol 14:280–285
Konnak JW, Grossman HB (1985) Renal cell carcinoma as an incidental finding. J Urol 134:1094–1096
Chow WH, Devesa SS, Warren JL et al (1999) Rising incidence of renal cell cancer in the United States. JAMA 281:1628–1631
Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM (2001) Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10–year experience. J Urol 165:777–781
Novick AC (2002) Nephron-sparing surgery for renal cell carcinoma. Annu Rev Med 53:393–407
Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR (2005) Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185:64–71
Gervais DA, Arellano RS, Mueller PR (2005) Percutaneous radiofrequency ablation of renal cell carcinoma. Eur Radiol 15(5):960–967
Mahnken AH, Gunther RW, Tacke J (2004) Radiofrequency ablation of renal tumors. Eur Radiol 14(8):1449–1455
Mahnken AH, Rohde D, Brkovic D, Gunther RW, Tacke JA (2005) Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol 46(2):208–214
Farrell MA, Charboneau WJ, DiMarco DS, Chow GK, Zincke H, Callstrom MR, Lewis BD, Lee RA, Reading CC (2003) Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol 18:1509–1513
Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ (2003) Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol 180(6):1503–1508
Pavlovich CP, Walther MM, Choyke PL, Pautler SE, Chang R, Linehan WM, Wood BJ (2002) Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol 167:10–15
Anderson JK, Matsumoto E, Cadeddu JA (2005) Renal radiofrequency ablation: technique and results. Urol Oncol 23:355–360
Silverman SG, Tuncali K, vanSonnenberg E, Morrison PR, Shankar S, Ramaiya N, Richie JP (2005) Renal tumors: MR imaging-guided percutaneous cryotherapy—initial experience in 23 patients. Radiology 236(2):716–724
HH Pennes (1948) Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1:93–122
Dewey WC (1994) Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 10(4):457–483
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10:787–800
Goldberg SN, Gazelle GS (2001) Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology 48:359–367
Mulier S, Miao Y, Mulier P, Dupas B, Pereira P, de Baere T, Lencioni R, Leveillee R, Marchal G, Michel L, Ni Y (2005) Electrodes and multiple electrode systems for radiofrequency ablation: a proposal for updated terminology. Eur Radiol 15:798–808
Pereira PL, Trubenbach J, Schenk M, Subke J, Kroeber S, Schaefer I, Remy CT, Schmidt D, Brieger J, Claussen CD (2004) Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology 232:482–490
Frich L, Hol PK, Roy S, Mala T, Edwin B, Clausen OP, Gladhaug IP (2006) Experimental hepatic radiofrequency ablation using wet electrodes: electrode-to-vessel distance is a significant predictor for delayed portal vein thrombosis. Eur Radiol 16:1990–1999. 16 Mar DOI 10.1007/s00330-006-0177-6
Burdio F, Navarro A, Sousa R, Burdio JM, Guemes A, Gonzalez A, Cruz I, Castiella T, Lozano R, Berjano E, Figueras J, de Gregorio MA (2006) Evolving technology in bipolar perfused radiofrequency ablation: assessment of efficacy, predictability and safety in a pig liver model. Eur Radiol 16:1826–1824. 16 Mar DOI 10.1007/s00330-005-0131-z
Clasen S, Schmidt D, Boss A, Dietz K, Krober SM, Claussen CD, Pereira PL (2006) Multipolar radiofrequency ablation with internally cooled electrodes: experimental study in ex vivo bovine liver with mathematic modeling. Radiology 238(3):881–890
Miao Y, Ni Y, Bosmans H et al (2001) Radiofrequency ablation for eradication of renal tumor in a rabbit model by using a cooled-tip electrode technique. Ann Surg Oncol 8:651–657
Hsu TH, Fidler ME, Gill IS (2000) Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology 56:872–875
Crowley JD, Shelton J, Iverson AJ, Burton MP, Dalrymple NC, Bishoff JT (2001) Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology 57:976–980
Lee JM, Kim SW, Chung GH, Lee SY, Han YM, Kim CS (2003) Open radio-frequency thermal ablation of renal VX2 tumors in a rabbit model using a cooled-tip electrode: feasibility, safety, and effectiveness. Eur Radiol 13:1324–1332
Aschoff AJ, Sulman A, Martinez M, Duerk JL, Resnick MI, MacLennan GT, Lewin JS (2001) Perfusion-modulated MR imaging-guided radiofrequency ablation of the kidney in a porcine model. AJR Am J Roentgenol 177:151–158
Eichel L, Kim IY, Uribe C, Khonsari S, Basillote J, Steward E, Coad J, Bischof J, Rudie E, Kluge S, McDougall EM, Clayman RV (2005) Third Prize: Comparison of radical nephrectomy, laparoscopic microwave thermotherapy, cryotherapy, and radiofrequency ablation for destruction of experimental VX-2 renal tumors in rabbits. J Endourol 19:1082–1087
Novick AC (2002) Nephron-sparing surgery for renal cell carcinoma. Annu Rev Med 53:393–407
Uzzo RG, Novick AC (2001) Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 166:6–18
Sowery RD, Siemens DR (2004) Growth characteristics of renal cortical tumors in patients managed by watchful waiting. Can J Urol 11:2407–2410
Wehle MJ, Thiel DD, Petrou SP, Young PR, Frank I, Karsteadt N (2004) Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology 64:49–52
Zagoria RJ, Hawkins AD, Clark PE, Hall MC, Matlaga BR, Dyer RB, Chen MY (2004) Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol 183:201–207
Ryo E, Takeshita S, Shiba M, Ayabe T (2006) Radiofrequency ablation for cystic adenomyosis: a case report. J Reprod Med 51:427–430
Sahin M, Kartal A, Haykir R, Cakir M (2006) RF-assisted cystectomy and pericystectomy: a new technique in the treatment of liver hydatid disease. Eur Surg Res 38:90–93
Mickisch GH, Mattes RH (2005) Combination of surgery and immunotherapy in metastatic renal cell carcinoma. World J Urol 23:191–195
Lewin JS, Nour SG, Connell CF, Sulman A, Duerk JL, Resnick MI, Haaga JR (2004) Phase II clinical trial of interactive MR imaging-guided interstitial radiofrequency thermal ablation of primary kidney tumors: initial experience. Radiology 232:835–845
Boss A, Clasen S, Kuczyk M, Anastasiadis A, Schmidt D, Claussen CD, Schick F, Pereira PL (2005) Radiofrequency ablation of renal cell carcinomas using MR imaging: initial results. Rofo 177:1139–1145
Boss A, Clasen S, Kuczyk M, Anastasiadis A, Schmidt D, Graf H, Schick F, Claussen CD, Pereira PL (2005) Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol 40(9):583–590
Beisland C, Medby PC, Sander S, Beisland HO (2000) Nephrectomy—indications, complications and postoperative mortality in 646 consecutive patients. Eur Urol 37:58–64
Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR (1995) Management of small unilateral renal cell carcinomas: radical versus nephron-sparing surgery. Urology 45:34–40
Kam AW, Littrup PJ, Walther MM, Hvizda J, Wood BJ (2004) Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 15:753–758
Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol 181:1315–1317
Boss A, Clasen S, Kuczyk M, Anastasiadis A, Schmidt D, Claussen CD, Schick F, Pereira PL (2005) Thermal damage of the genitofemoral nerve due to radiofrequency ablation of renal cell carcinoma: a potentially avoidable complication. AJR Am J Roentgenol 185:1627–1631
Morcos SK, Thomsen HS, Webb JA (1999) Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 9:1602–1613
Merkle EM, Nour SG, Lewin JS (2005) MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology 235:1065–1071
Boss A, Martirosian P, Schraml C, Clasen S, Fenchel M, Anastasiadis A, Claussen CD, Pereira PL, Schick F (2006) Morphological, contrast-enhanced and spin labeling perfusion imaging for monitoring of relapse after RF ablation of renal cell carcinomas. Eur Radiol 16:1-11. DOI 10.1007/s00330-005-0098-9
Thomsen HS (2004) Gadolinium-based contrast media may be nephrotoxic even at approved doses. Eur Radiol 14:1654–1656
Zlotta AR, Wildschutz T, Wood BJ et al (1997) Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol 11:251–258
McGovern FJ, Wood BJ, Goldberg SN et al (1999) Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol 161:599–600
Hall WH, McGahan JP, Link DP, deVere White RW (2000) Combined embolization and percutaneous radiofrequency ablation of a solid renal tumor. AJR Am J Roentgenol 174:1592–1594
Ogan K, Jacomides L, Dolmatch BL et al (2002) Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology 60:954–958
Sabharwal R, Vladica P (2006) Renal tumors: technical success and early clinical experience with radiofrequency ablation of 18 tumors. Cardiovasc Intervent Radiol 29:202–209
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boss, A., Clasen, S., Kuczyk, M. et al. Image-guided radiofrequency ablation of renal cell carcinoma. Eur Radiol 17, 725–733 (2007). https://doi.org/10.1007/s00330-006-0415-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0415-y