Abstract
Purpose
The purpose of this study was to evaluate the diagnostic efficacies of CT fistulography and MRI, in the diagnostic work-up of perianal fistula patients.
Materials and methods
All 41 patients who were included in the study (36 males and 5 females, with an average age of 41 years) underwent CT fistulography and MRI examinations prior to surgery. The fistula characteristics obtained from these examinations were compared with the surgical findings. The comparative results were evaluated by means of the Kappa analysis method.
Results
CT fistulography predicted the correct perianal fistula classification in 30 (73.1%) of the 41 patients, whereas MRI correctly defined fistula classification in 38 (92.7%) of these patients (the K values were 0.621 and 0.896, respectively; with p < 0.001). CT fistulography depicted 29 secondary extensions in 16 patients, whereas MR imaging revealed 28 secondary extensions in 15 patients. A substantial agreement was found between surgical findings and two modalities (K value was 0.789 and 0.793 for CT fistulography and MRI, respectively, with a p value < 0.001). In terms of locations of internal openings, CT fistulography was able to detect the locations in 28 patients (68.2%), whereas MRI was more successful in this aspect, with a number of 35 patients (85.3%). Granulation tissues, inflammation and edema around the fistula, abscesses, and fistular wall fibrosis were also evaluated.
Conclusion
CT fistulography and MRI have different advantages in the diagnosis of perianal fistulas. A good command of knowledge concerning the issue may be a key factor in modality decision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Perianal fistula is thought to develop secondary to perianal abscess. The disease has a rather high rate of recurrence [1, 2]. Recurrence is usually due to secondary extensions which are missed during the operation and thus are not properly treated [1]. It is of vital importance to diagnose correctly the fistula tract, secondary extensions, abscesses, and the extensions to the pelvic structures, in terms of prevention of recurrences and the sustainment of fecal continence [3–5]. Anal endosonography, CT fistulography, and MRI may be used in the diagnosis of the disease. Anal endosonography is a fast and inexpensive method, but it is highly operator dependent, it bears the risk of infectious spread, and its reliability decreases especially in complex fistulas [6, 7]. CT is advantageous in the detection of inflammation and abscess in the intersphincteric region and the low-density fatty tissue compartment in the ischioanal fossa; besides, CT fistulography may correctly define the internal opening of the perianal fistula [7, 8]. MRI can depict the relation between the fistula tract and the anal sphincter better due to its multiplanar imaging capability and superior soft tissue resolution [9–11]. But MRI is a relatively expensive and not-easily-available modality. MRI has been shown in previous studies to be of superior value in the detection of perianal fistulas, over anal endosonography [5, 12, 13]. Highly sophisticated CT systems which utilize isotropic voxel and multiplanar imaging techniques have already entered the market; but prior to our study, we were not able to find an original article in the English literature about the comparison of CT fistulography and MRI. In this study, the imaging findings of CT fistulography and MRI have been studied in comparison to surgical results, and the superior characteristics of each modality have been investigated. The purpose of this study was to evaluate the diagnostic efficacies of CT fistulography, and MRI, in the diagnostic work-up of perianal fistula patients.
Materials and methods
This prospective study was approved by our institutional review board and ethical committee and it was carried out in accordance with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines. CT fistulography and MRI examinations were performed in 62 consecutive patients who had the complaints of perianal discharge, swelling, and pain, and whose physical examination findings were compatible with perianal fistulae between July 2013 and December 2015. These 62 patients comprised 52 males and 10 females. Those patients with perianal fistula orifices detected at physical examination underwent a CT fistulography procedure. Full informed consents were obtained from all patients prior to the study. Those with known or probable inflammatory bowel diseases were excluded from the study. All histories and complaints of all of the patients were recorded by a radiologist prior to the examinations. The CT fistulography and MRI examinations were evaluated by two radiologists who had no idea about the other modality and the patient’s history. CT fistulography examination was evaluated by two radiologists. In case of discordance between the two radiologist’s readings, a consensus reading was made. MRI examinations were evaluated by two radiologists. In case of discordance between the two radiologists’ readings, a consensus reading was made. CT fistulography and MRI revealed an anal sinus in 4 and a pilonidal sinus in 7 patients. These patients were excluded from the study. Ten patients were also excluded from the study, due to lack of information about their surgical results. A total of 21 patients were excluded from the study. Forty-one patients underwent surgery. The mean age of the 41 patients who were included in the study was 41 years [14–60 years, standard deviation (SD): 11]. Thirty-six (87.8%) patients were male, and 5 (12.2%) were female. The CT fistulography and MRI examinations were evaluated on the basis of the presence of perianal fistulas, fistula classifications, the number of secondary extensions, the presence of inflammation in the periphery of the fistulas, associating abscesses, and the extension of the disease processes to the pelvic structures. The fistulas were recorded as active and chronic, according to the presence of scar tissue at the fistula wall and contrast enhancement. The internal openings were defined with regard to the anal clock. The internal opening locations, whenever detected, were recorded according to their localizations on this segmented map. In case nonvisualization of the internal openings, the location of maximum intersphincteric sepsis was recorded as the internal opening location. There was a time gap of a maximum of 3 days between the CT fistulography and MRI examinations. Following these imaging procedures, the patients were operated within 10 days. Findings at surgery were recorded.
CT fistulography technique
CT and CT fistulography examinations were performed with a 16-channel multidetector CT unit (Optima 520, GE Healthcare, Milwaukee, Wisconsin). The imaging parameters were as follows: KvP: 120, mAs: 10, collimation: 128 × 0.6 mm2, pitch factor: 1.375, helical thickness: 2.5 mm, and rotation time: 0.8 s. The location of the external orifice was found on the prone position and the region was cleaned with alcohol and povidone-iodine solution. Then, a mixture of 1 mL of nonionic iodinated contrast material (Iomeprol, Iomeron, Bracco Diagnostics, Milan, Italy) and 10 mL of saline was injected without a needle into the orifice, following a local anesthetic application of xylocaine gel. The injection was aborted in case the contrast material could not be administered because of internal pressure or because the patient had pain. In all cases, an intravenous (iv) contrast material injection was made, utilizing Iomeprol, with a dose of 0.5 cc/kg. The injection was performed with a speed of 3 cc/s, and serial images were obtained. Axial and 3-dimensional (3-D) reconstructed images of all patients were evaluated on a separate workstation.
MRI technique
MRI examinations were performed with a 1.5 T scanner (Siemens, Avanto Syngo, Erlangen, Germany) using phased array coil. The procedures were performed without a prior bowel preparation. Oblique axial T1W TSE, oblique axial T2W TSE, oblique coronal fat-suppressed T2W TSE, oblique axial fat-suppressed T1W TSE, oblique coronal fat-suppressed T1W TSE, sagittal fat-suppressed T2W TSE, and oblique axial DWI sequences were performed during MR imaging. In all patients, oblique coronal T1W TSE and oblique axial T1W TSE sequences were performed, following iv injections of gadolinium contrast (0.1 mmol/kg body weight, Gadodiamide, Omniscan, Nycomed Imaging, Oslo, Norway). All of the parameters administered in the sequences used in MR imaging are listed in Table 1.
Systematic evaluation of the CT fistulography and MRI findings
Enhancing soft tissue densities with irregular border obliterating the ischiorectal/ischioanal fat tissue compartment were defined as granulation tissue [14]. Those soft tissue densities localized in the vicinity of the fistula tract, obliterating the adipose tissues, were defined as inflammation and edema [15, 16]. Lesions over 10 mm in diameter with hypodense centers and peripheral contrast enhancement were defined as abscesses [7, 17]. Fistula tracts with enhancing walls were evaluated as active, and those with non-enhancing walls as chronic fistula tract [18]. In nine patients with fistular adhesions and infectious discharge, contrast material could not be passed through the fistulous tract. In three patients, contrast media could not be injected into the fistula due to the absence of an external opening. In these patients, numbering of extensions and fistula grading were performed by means of the evaluation of linear soft tissue densities with hypodense centers, located in the low-density fatty tissue compartments. In these patients, it was impossible to determine the internal openings. Focal areas located in the ischiorectal/ischioanal fossa, demonstrating high T2 signal intensities and contrast enhancement, were recorded as granulation tissue [1]. Focal areas with high T2 signals, located in the vicinity of the fistula tract, were recorded as inflammation and edema [1]. Lesions larger than 10 mm, which demonstrated T1 hypointensity and T2 hyperintensity, and which showed peripheral contrast enhancement, were evaluated as abscesses, whereas tubular lesions smaller than 10 mm were recorded as fistula tracts [17, 19]. Linear signal pathologies with T1 hypointensity and T2 hyperintensity that showed contrast enhancement were noted as active fistula tracts [20]. Non-enhancing linear signal regions with hypointense characters both on T1- and T2-weighted sequences were recorded as chronic fistula tracts [4, 21]. The perianal fistulas were classified according to the “St. James’s University Hospital MR Imaging Classification of Perianal Fistulae” protocol, proposed by Morris et al. [22] (Table 2). Fistula tracts showing no relation with the anal canal and having no internal openings were recorded as sinus tracts [20].
Statistical analysis
Statistical analysis was made by the utilization of the SPSS 20.0 program. Descriptive statistics were shown as the mean, standard deviation, and percentage values. The Kappa analysis method was used in the determination of the congruence between the findings from surgery, which was the reference standard, and those from CT and MRI examinations. The numerical values obtained by means of the Kappa analysis method were evaluated as follows [23]: ≤0 as indicating no agreement, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement [23].
Results
Concerning perianal fistulas, surgery revealed grade 1 in 19 (46.3%), grade 2 in 7 (17%), grade 3 in 5 (12.2%), grade 4 in 3 (7.3%), and grade 5 in 6 (14.6%) patients. In 1 patient, CT fistulography and MRI revealed a grade 1 perianal fistula, whereas surgical exploration showed an anal sinus that did not reach the anal mucosa.
When CT fistulography and MRI results were compared with surgical findings, CT fistulography predicted the correct perianal fistula classification in 30 (73.1%) of 41 patients, whereas MRI defined correct classification in 38 (92.7%) of the 41 patients (Table 3). Eighteen (94.7%) of 19 patients with surgically proven grade 1 perianal fistulas were correctly identified at MRI, whereas CT fistulography correctly classified 15 (78.9%) of 19 patients with grade 1 perianal fistula. All seven patients with grade 2 perianal fistulas were correctly classified with MRI and CT fistulography predicted correct classification in 5 (71.4%) of seven patients with grade 2 perianal fistula. There were five patients with surgically proven grade 3 perianal fistula. MRI was able to classify 4 (80%) of 5 patients correctly in this group. CT fistulography established the correct classification in 2 (40%) of 5 patients with grade 3 perianal fistula. All the 3 patients with grade 4 perianal fistulas were correctly classified at MRI, whereas CT fistulography was able to predict the correct grade in 2 (66.6%) of 3 patients in this group. All the 6 patients with grade 5 perianal fistulas were correctly classified at CT fistulography and MRI. The results of CT fistulography and MRI findings correlated with surgical results in terms of perianal fistula grading are shown in Table 4.
When surgical findings were compared with CT fistulography and MRI findings, kappa values for agreement were found to be 0.621 and 0.893, respectively (p > 0.001).
CT depicted 29 secondary extensions, whereas MRI revealed 28 secondary extensions. CT detected at least one secondary extension in 16 patients, whereas MRI showed at least one secondary extension in 15 patients. In 36 (87.8%) of 41 patients, MR imaging findings were in concordance with surgical findings in terms of the number of secondary extensions. In 35 (%85.3) of 41 patients, CT fistulography findings were in concordance with surgical findings in terms of the number of secondary extensions. A comparison was made between the number of secondary extensions detected by CT fistulography and MRI, with surgery. A substantial agreement was found between surgical findings and two modalities (K value was 0.789 and 0.793 for CT fistulography and MRI, respectively, with a p value < 0.001).
The internal opening quadrants of 28 (68.2%) of 41 patients reported in CT fistulography were congruent with surgical results (K 0.52; p < 0.001). The internal openings could not be identified in 3 (7.3%) patients, due to the absence of an external opening, and in 9 (22%) patients because of internal adhesions. Excluding these patients, the exact location of the internal openings could be defined in 28 (96.5%) of the 29 patients at CT fistulography (K 0.877, p < 0.001). In 35 (85.3%) of 41 patients, the exact location of the internal openings was in concordance with the surgical findings at MRI (K 0.767, p < 0.001).
All abscesses in three cases were detected by CT, whereas MRI showed only two abscesses. The remaining 1 abscess was mistakenly reported as a wide and active fistula (K 1 and 0.844 consecutively, p < 0.001). The sensitivity and specificity of CT fistulography in abscess detection were found to be 100%. These values were 100% and 97.37% for MRI, respectively.
As compared to surgical findings, CT fistulography correctly identified all but 2 patients with granulation tissue, whereas MRI missed only 1 patient with a granulation tissue. When the agreement rates of CT fistulography and MRI, with surgery, were evaluated, the Kappa values were found to be 0.888 and 0.945, respectively (p < 0.001). The sensitivity and specificity values of CT fistulography in detecting granulation tissue were 85.71% and 100%, while these were found to be 92.86% and 100% for MRI, respectively.
When the inflammation and edema around the fistulas were evaluated, it was found that CT fistulography and MRI results were incongruent with surgical findings in 3 patients. In the remaining patients, the results were in congruence. When the CT fistulography and MRI results were compared with surgical findings, the Kappa value for agreement was found to be 0.827 for both modalities (p < 0.001). The sensitivity and specificity values for CT fistulography and MRI, in terms of detecting edema and inflammation around the fistula, were 96.43% and 84.62%, respectively (Table 5).
In terms of defining active and chronic fistula formations, a substantial agreement was found between CT fistulography and surgical results (K 0.643). On the other hand, MRI showed an almost perfect agreement with surgical findings (K 0.861).
Discussion
Classification of perianal fistulas
Our study shows that MRI performs better than CT fistulography in the classification of perianal fistulas.
It has been shown in many studies that preoperative radiologic evaluation of perianal fistulae provides additional information about the disease and has positive effects on prognosis [10, 24–29]. In a study performed by Buchanan et al., MRI was able to define the primary fistular tract properly in 97 of the 104 patients included in the study, who had perianal fistulas [26]. It has been reported that the sensitivity and specificity of MRI in the detection of perianal fistulas were 95–97% and 80–100%, respectively [26, 30–32]. In accordance with previous results, we were able to identify fistula classification in 38 of 41 patients (92.7%) with MRI. Many different MRI sequences have been reported in the literature, with varying degrees of success in these terms [22, 27, 32–38]. It has been reported that the T1-weighted contrast-enhanced and T2-weighted sequences are the most useful ones in this aspect [21, 27] (Fig. 1). It is also well known that diffusion-weighted sequences increase the radiologists’ level of confidence [37]. As was done in our study, the usage of the benefits of all sequences suggested in the literature will increase the rate of success in the task of detecting and classifying the primary fistula tract.
There are only a few research studies on the usefulness of CT fistulography in the diagnosis of perianal fistulas [7]. In a previous study, Liang et al. reported that a correct diagnosis could be made by CT fistulography in 18 (82%) of the 22 patients [7]. In the present study, CT fistulography predicted the correct perianal fistula classification in 30 (73.1%) of 41 patients. The limited contrast resolution of CT might have decreased the correct classification of perianal fistulas especially those that were located close to the external sphincter and levator ani muscle. The fistula tract in the ischiorectal fossa may be easily recognized as a soft tissue density, and the classification may easily be made. But in tracts lying near and parallel to the muscle tissues, filling the fistulous tracts with iodinated contrast material may be helpful in terms of a correct classification (Fig. 2).
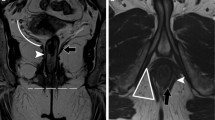
A 57-year-old man with grade 4 perianal fistula. Axial CT and axial fat-saturated T2-weighted images show extrasphincteric perianal fistula (horizontal arrows). Axial fat-saturated T2-weighted image shows secondary extension (vertical arrow) which is not seen clearly in axial CT image (contrast media could not be injected into the fistula due to the absence of an external opening). At CT, perianal fistula was mistakenly evaluated as grade 3
Secondary extensions
Our study reveals that there is no significant difference between these two imaging modalities in terms of detecting the number of secondary fistular extensions.
Recurrence is usually due to the presence of secondary extensions not recognized during surgery [7, 24]. It was shown in previous studies that MRI was successful in determining secondary extensions [32, 39]. In a study conducted by Singh et al., 15 of the 16 secondary extensions detected during surgery in 16 of the 45 patients were also defined by MRI [32]. In our study, we defined the number of secondary extensions in each patient and made a comparison with the surgical results. In our study, CT fistulography detected 29 secondary extensions in 16 patients, whereas MR imaging revealed 28 secondary extensions in 15 patients. Surgical evaluation detected 35 secondary extensions in 16 patients. In 36 (%87.8) of 41 patients, MR imaging findings were in concordance with surgical findings in terms of the number of secondary extensions. In 35 (85.3%) of 41 patients, CT fistulography findings were in concordance with surgical findings in terms of the number of secondary extensions (Figs. 3, 4). At CT fistulography, there may not be contrast in some secondary extensions. Still, secondary extensions located in the homogenous low-density fatty tissues may be recognized as linear soft tissue densities. It has been reported that in MRI, the high-spatial-resolution endoanal coil is superior in defining the primary fistular tract, whereas external phased array coil with large field of view has a superiority in defining the secondary extensions [40–43]. An external phased array coil was utilized in our study. The usage of both coil types together may enhance the visualization of anatomic detail.
Internal openings
According to our study, MRI can depict the internal opening locations with high accuracy. The problem with CT fistulography, in this aspect, is that contrast material sometimes cannot be administered through the fistula due to adhesions and pus. In cases with no intrafistular adhesions and in which the contrast material can be administered properly through the fistulas, the success of CT in delineating the internal openings is better than that of MRI.
A correct definition of the location of internal openings plays a crucial role in reducing the incidence of recurrence and defining surgical options [26, 30, 44, 45]. In a previous study, Holzer et al. reported that MRI defined the exact location of the internal openings in 10 (35.7%) of 28 patients [30]. In another study, Singh et al. reported that the sensitivity of MRI in the detection of internal openings was 95.83% [32]. In the present study, the exact location of internal openings was in concordance with surgical findings in 35 (85.3%) of 41 patients. It is rather easy to define the location of the internal opening when the tract can be traced up to the anal mucosa, but unfortunately this tracing may not be possible in many patients (Fig. 5). Halligan et al. reported that the internal opening may be located at the area nearest to the maximal intersphincteric sepsis location [1]. Our study was conducted in accordance to this suggestion. In 28 (68.2%) of 41 patients, CT fistulography revealed the exact location of the internal opening, whereas MRI depicted the internal opening in 35 (85.3%) of 41 patients. Both in our study and in a study by Singh et al., MRI predicted more internal openings than literature. This fact confirms the suggestion that internal openings must be searched at locations nearest to the maximal intersphincteric sepsis (Fig. 6).
It has been reported that CT fistulography is an inexpensive and fast technique in determining internal openings [7]. In a previous study, Liang et al. reported that contrast material could not be injected into the fistula in 2 (8.3%) of the 24 patients, due to the absence of an external opening. In another 5 (20.7%) patients, the researchers were unable to administer the contrast material inside the fistulas; thus, they could not identify the internal openings [7]. In our study, on the other hand, the internal openings could not be demonstrated in 3 (7.3%) patients, due to the absence of an external opening, and in 9 (22%) patients because of internal adhesions. But in 28 (96.5%) of the 29 patients in which the contrast material could be administered up to the intestinal lumen, the exact locations of internal openings could be predicted correctly. The main problem in this issue seems to be the lack of contrast agent filling inside the fistula. This problem may be overcome by the insertion of a metal probe into the fistula, as has been used by surgeons conventionally. This process will extract the pus out of the fistula and may improve visualization of the fistula tract at CT fistulography. This approach may especially be helpful when access to MRI is limited (Fig. 7).
Inflammation around fistula, abscess, and fistular wall fibrosis
Our study reveals that CT is able to differentiate inflammation, edema, and abscess, as good as MRI. But MRI is better than CT fistulography in the discrimination of active and chronic fistulas.
The therapeutic process of perianal fistulas is strongly related with associated complications and abscess formations. MRI can detect perianal abscesses with a high accuracy, and it can depict the anatomical relation between the abscess and the anal canal [46]. Maruyama et al. have found a 95% sensitivity rate for MRI in their study, in the diagnosis of ischiorectal abscesses [47]. In a recent study performed by Caliste et al., 113 (77%) of 130 patients with a proven perirectal abscess were correctly diagnosed with CT [48]. Three of the 41 patients of our study had abscesses. All of these three abscesses were diagnosed correctly by CT fistulography. But MRI was able to diagnose only two; one case was diagnosed as a wide fistula. The 10-day gap between the operation and MRI examination may be responsible for this situation. CT can diagnose granulation tissue with an accuracy rate similar to that of MRI. In our study, the sensitivity and specificity rates for CT fistulography in the diagnosis of granulation tissue were found as 85.71% and 100%, whereas these values were found to be 92.86% and 100% for MRI, respectively. The sensitivity and specificity rates were the same for both modalities, in terms of detecting inflammation and edema around the fistula, with the values being 96.43% and 84.62%, respectively. In our study, MRI was found to be superior in discriminating active fistulas from chronic fistulas. With CT, the absence of contrast enhancement at the fistula wall was validated in our study as a finding of a chronic fistulous tract, whereas MRI had an additional advantage to this, because MRI can also detect fibrosis at the fistular wall (Fig. 8).
Limitations of the study
Surgery was accepted as the gold standard in our study. But, as is also reported in the literature, secondary extensions may sometimes be missed at surgery. This seems to be the limitation of this study. Inability to inject into the fistula is also a limitation. Thus, in a patient with a difficulty to inject into the fistula, MRI may perform better in terms of delineation of the fistula tract and internal opening.
Conclusion
CT fistulography and MRI both possess different advantages in perianal fistula diagnosis. A thorough knowledge of these advantages will provide an additional advantage in modality detection. CT fistulography may be the first choice, especially in noncomplicated cases, due to its rather inexpensiveness and vast availability. In cases with negative or obscure CT findings, MRI must be performed.
References
Halligan S, Stoker J (2006) Imaging of fistula in ano. Radiology 239:18–33
Rickard MJ (2005) Anal abscesses and fistulas. ANZ J Surg 75:64–72
Kumar N, Agarwal Y, Chawla AS, Jain R, Thukral BB (2015) MRI Perianal Fistulae . doi:10.1016/j.crad.2015.09.001
Ziech M, Felt-Bersma R, Stoker J (2009) Imaging of perianal fistulas. Clin Gastroenterol Hepatol 7:1037–1045
Chowbey PK, Khullar R, Sharma A, et al. (2015) Minimally invasive anal fistula treatment (MAFT)—an appraisal of early results in 416 patients. Indian J Surg 77:716–721
Morren GL, Beets-Tan RGH, van Engelshoven JMA (2001) Anatomy of the anal canal and perianal structures as defined by phased-array magnetic resonance imaging. Br J Surg 88:1506–1512
Liang C, Jiang W, Zhao B, et al. (2013) CT imaging with fistulography for perianal fistula: does it really help the surgeon? Clin Imaging 37:1069–1076. doi:10.1016/j.clinimag.2013.04.014
Sahni VA, Ahmad R, Burling D (2008) Which method is best for imaging of perianal fistula? Abdom Imaging 33:26–30
Liang C, Lu Y, Zhao B, et al. (2014) Imaging of anal fistulas: comparison of computed tomographic fistulography and magnetic resonance imaging. Korean J Radiol 15:712–723. doi:10.3348/kjr.2014.15.6.712
Beets-Tan RGH, Beets GL, van der Hoop AG, et al. (2001) Preoperative MR imaging of anal fistulas: does it really help the surgeon? Radiology 218:75–84
Gourtsoyianni S, Goh V (2014) MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging 39:2–17. doi:10.1007/s00261-013-0032-6
Sudoł-Szopińska I, Kucharczyk A, Kołodziejczak M, et al. (2014) Endosonography and magnetic resonance imaging in the diagnosis of high anal fistulae—a comparison. J Ultrason 14:142–151
Felt-Bersma RJ (2006) Endoanal ultrasound in perianal fistulas and abscesses. Dig Liver Dis 38:537–543
Khati NJ, Lewis NS, Fraizer AA, et al. (2015) CT of acute perianal abscesses and infected fistulae: a pictorial essay. Emerg Radiol 22:329–335. doi:10.1007/s10140-014-1284-3
Barral M, Boudiaf M, Dohan A, et al. (2015) MDCT of acute colitis in adults: an update in current imaging features. Diagn İnterv Imaging 96:133–149. doi:10.1016/j.diii.2014.04.008
Lee MH, Moon MH, Sung CK, Woo H, Oh S (2014) CT findings of acute pelvic inflammatory disease. Abdom Imaging 39:1350–1355. doi:10.1007/s00261-014-0158-1
Mendoza LR, Borobia AR, Gonzalez CZ, Pena T, Ros PR (2004) MR imaging in anal fistulae. Rev Argent Radiol 68:237–244
Bartram C, Buchanan G (2003) Imaging anal fistula. Radiol Clin N Am 41:443–457
Tonolini M, Campari A, Bianco R (2011) Ileal pouch and related complications: spectrum of imaging findings with emphasis on MRI. Abdom Imaging 36:698–706
O’Malley RB, Al-Hawary MM, Kaza RK, et al. (2012) Rectal imaging: part 2, perianal fistula evaluation on pelvic MRI–what the radiologist needs to know. Am J Roentgenol 199:W43–W53
de Miguel Criado J, del Salto LG, Rivas PF, et al. (2012) MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics 32:175–194. doi:10.1148/rg.321115040
Morris J, Spencer JA, Ambrose NS (2000) MR imaging classification of perianal fistulas and its implications for patient management. Radiographics 20:623–635
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363–374
Buchanan G, Halligan S, Williams A, et al. (2002) Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet 360:1661–1662
Buchanan GN, Halligan S, Williams AB, et al. (2003) Magnetic resonance imaging for primary fistula in ano. Br J Surg 90:877–881
Buchanan GN, Halligan S, Bartram CI, et al. (2004) Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology 233:674–681
Gage KL, Deshmukh S, Macura KJ, Kamel IR, Zaheer A (2013) MRI of perianal fistulas: bridging the radiological-surgical divide. Abdom Imaging 38:1033–1042. doi:10.1007/s00261-012-9965-4
Spencer JA, Chapple K, Wilson D, et al. (1998) Outcome after surgery for perianal fistula: predictive value of MR imaging. Am J Roentgenol 171:403–406
Chapple KS, Spencer JA, Windsor AC, et al. (2000) Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum 43:511–516
Holzer B, Rosen HR, Urban M, et al. (2000) Magnetic resonance imaging of perianal fistulas: predictive value for Parks classification and identification of the inner opening. Colorectal Dis 2:340–345
Beckingham IJ, Spencer JA, Ward J, et al. (1996) Prospective evaluation of dynamic contrast enhanced magnetic resonance imaging in the evaluation of fistula in ano. Br J Surg 83:1396–1398
Singh K, Singh N, Thukral C, Singh KP, Bhalla V (2014) Magnetic resonance imaging (MRI) evaluation of perianal fistulae with surgical correlation. J Clin Diagn Res 8:1–4
Halligan S, Healy JC, Bartram CI (1998) Magnetic resonance imaging of fistula-in-ano: STIR or SPIR? Br J Radiol 71:141–145
Sabir N, Sungurtekin U, Erdem E, Nessar M (2000) Magnetic resonance imaging with rectal Gd-DTPA: new tool for the diagnosis of perianal fistula. Int J Colorectal Dis 15:317–322
Hori M, Oto A, Orrin S, Suzuki K, Baron RL (2009) Diffusion-weighted MRI: a new tool for the diagnosis of fistula in ano. J Magn Reson Imaging 30:1021–1026
Yildirim N, Gökalp G, Öztürk E, et al. (2012) Ideal combination of MRI sequences for perianal fistula classification and the evaluation of additional findings for readers with varying levels of experience. Diagn Interv Radiol 18:11–19
Kinner S, Blex S, Maderwald S, et al. (2014) Addition of diffusion-weighted imaging can improve diagnostic confidence in bowel MRI. Clin Radiol 69:372–377
Burdan F, Sudol-Szopinska I, Staroslawska E, et al. (2015) Magnetic resonance imaging and endorectal ultrasound for diagnosis of rectal lesions. Eur J Med Res 20:4. doi:10.1186/s40001-014-0078-0
Mahjoubi B, Haizadch Kharazi H, Mirzaei R, Moghimi A, Changizi A (2006) Diagnostic accuracy of body coil MRI in describing the characteristics of perianal fistulas. Colorectal Dis 8:202–207
deSouza NM, Gilderdale DJ, Coutts GA, Puni R, Steiner RE (1998) MRI of fistula-in-ano: a comparison of endoanal coil with external phased array coil techniques. J Comput Assist Tomogr 22:357–363
Stoker J, Hussain SM, van Kempen D, Elevelt AJ, Laméris JS (1996) Endoanal coil in MR imaging of anal fistulas. Am J Roentgenol 166:360–362
deSouza NM, Gilderdale DJ, MacIver DK, Ward HC (1997) High-resolution MR imaging of the anal sphincter in children: a pilot study using endoanal receiver coils. Am J Roentgenol 169:201–206
Stoker J, Rociu E, Schouten WR, Laméris JS (2002) Anovaginal and rectovaginal fistulas: endoluminal sonography versus endoluminal MR imaging. Am J Roentgenol 178:737–741
Robertson WG, Mangione JS (1998) Cutaneous advancement flap closure: alternative method for treatment of complicated anal fistulas. Dis Colon Rectum 41:886–887
Tang CL, Chew SP, Seow-Choen F (1996) Prospective randomized trial of drainage alone vs. drainage and fistulotomy for acute perianal abscesses with proven internal opening. Dis Colon Rectum 39:1415–1417
Li X, Qiang JW, He C, Ji XS, Zhang B (2011) Magnetic resonance imaging study of perianal abscess. Zhonghua Wei Chang Wai Ke Za Zhi 14:868–870
Maruyama R, Noguchi T, Takano M, et al. (2000) Usefulness of magnetic resonance imaging for diagnosing deep anorectal abscesses. Dis Colon Rectum 43:2–5
Caliste X, Nazir S, Goode T, et al. (2011) Sensitivity of computed tomography in detection of perirectal abscess. Am Surg 77:166–168
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Soker, G., Gulek, B., Yilmaz, C. et al. The comparison of CT fistulography and MR imaging of perianal fistulae with surgical findings: a case–control study. Abdom Radiol 41, 1474–1483 (2016). https://doi.org/10.1007/s00261-016-0722-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0722-y