Abstract
Anal cancer is an uncommon malignancy of the gastrointestinal tract but has a relatively good prognosis with an 80% 5-year overall survival. In this article, we review the role of MRI for assessing treatment response in anal cancer after completion of definitive chemoradiotherapy. New generation MRI scanners with optimal-phased array body coils, resulting in better signal to noise and improved contrast and spatial resolution, have contributed to high-resolution imaging in clinical practice enabling visualization of relevant anatomy including the sphincter complex, adjacent structures, mesorectal and pelvic lymph nodes with a diameter down to 2 mm. Multiplanar, high-resolution T2-weighted and diffusion-weighted sequences have a role in initial locoregional staging of anal SCC, assisting radiotherapy planning, as well as in assessing response to treatment and treatment-related complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Squamous cell carcinoma (SCC) of the anal canal is a rare malignancy in the general population; however, it is increasingly diagnosed in patients with human immunodeficiency virus (HIV), immunosuppressed patients after transplantation, as well as in patients with chronic inflammatory bowel disease. It accounts for less than 5% of anorectal tumors. An established association exists with human papillomavirus (HPV) infection and premalignant anal intra-epithelial dysplasia (AIN) [1, 2]. 85% of anal SCCs are encountered in the anal canal, with only 15% originating in the anal margin. These predominantly affect women and tend to be poorly differentiated with a poorer prognosis, compared to anal margin tumors.
The anatomical midpoint of the anal canal, the dentate line, marks the transition from the squamous epithelium to the intestinal mucosa. Anal canal SCCs can be either keratinizing or nonkeratinizing according to their origin below or above the dentate line, without any difference in their biological behavior and prognosis [3]. Acknowledging the distance of primary lesion site from the dentate line is of importance as it defines regional lymph node drainage. Anal cancers lying distal to the dentate line predominantly drain to the inguinal and femoral lymph nodes, while tumors arising above the dentate line involve the inguinal, internal iliac, and mesorectal lymph nodes [4–6]. External, common iliac, and para-aortic nodes are considered non-regional [6].
Accurate staging of anal SCCs at presentation provides prognostic information and allows for correct therapeutic planning. This is performed according to the UICC/AJCC tumor-node-metastasis (TNM) system (Table 1). Specifically the longest diameter of the primary tumor rather than extramural extension, and the site of involved lymph nodes rather than the number are important prognosticators.
Anal cancers typically spread upwards into the rectum, and large anorectal tumors may be difficult to distinguish from rectal cancers. The same applies in cases of rectal cancer with significant caudal spread that might be mistaken on clinical examination for a primary anal carcinoma. Imaging is not particularly helpful in the distinction of low-lying rectal adenocarcinomas from anal canal SCC that may extend upwards involving the rectum as the imaging characteristics of the two cancer types are quite similar, as will be described later on. It is of paramount importance that staging is performed after histological confirmation has been obtained and that radiologists involved in the specialist multidisciplinary teams meetings are aware of the underlying histology in order to provide accurate staging information.
Current standard of care treatment of anal canal SCCs is definitive chemoradiation (CRT), including radiation therapy with a dose ranging between 45 and 50 Gy, combined with mitomycin-C and infusional 5-FU chemotherapy. This yields an 80% 5-year survival rate with preservation of sphincter function. In 35% of cases, usually patients presenting with advanced T3/T4 tumors, locoregional and/or metastatic relapse may occur [7] and for this reason further neoadjuvant and adjuvant treatments are being investigated in advanced disease settings. Salvage surgery with abdomino-perineal resection (APR) is reserved only for non-responsive patients with persistent or recurrent tumors post CRT [4, 8–10].
Clinical response is usually assessed at 6–8 weeks after completion of CRT, and 60–85% of patients demonstrate complete clinical response at this time. Clinical evaluation relies on digital rectal examination as well as careful examination of the inguinal regions. Partial regression is managed by close follow-up to confirm that complete regression takes place as this may take up to 6 months. Unnecessary biopsies post CRT are avoided, where possible, as there is an associated risk of infection and necrosis of the anal wall. However, residual tumor must be confirmed histologically before considering proceeding onto surgery.
Imaging is thought to serve as a useful complement to clinical evaluation and provides a more comprehensive assessment of therapeutic response after CRT.
Current imaging status for anal cancer
MRI
The latest European Society for Medical Oncology (ESMO) recommendations published in 2010 [4] have proposed phased-array MRI as the primary imaging modality of choice for accurate locoregional staging. MRI may provide information of local disease extent and nodal involvement, which may also assist radiotherapy planning and contouring.
High-resolution MRI of the anorectal region without an endoluminal coil has been successfully performed for more than 15 years [11]. Use of external phased array coils allows detailed imaging of the anal sphincter, the rectum, and the surrounding pelvic structures. MRI is considered as the imaging method of choice by providing information regarding maximum tumor size, local extent and spread, and invasion of adjacent organs and nodal involvement [3, 4].
MRI of the anal canal may be performed on ≥1.5 T magnetic-field scanners. Acquisition protocols include high resolution T2-weighted sequences along three planes, with coronal and axial scans planned parallel and perpendicular to the long axis of the anal canal [12] (Table 2). The same protocol used to stage anal cancer at baseline should be used for therapy response assessment. Despite the increased tumor conspicuity provided by background fat suppression, short-tau inversion recovery (STIR) sequences may be less useful because of limited spatial detail resulting in difficulty in delineating anatomic landmarks. However, these are very helpful for identifying fistula tracts [5] present at initial staging or that developed during treatment. In some centers, T1-weighted sequences with fat suppression in at least one plane are routinely acquired following standard dose intravenous gadolinium contrast, to allow detection of lesion enhancement. Although contrast-enhanced images are not considered to offer additional information to the high soft tissue contrast intrinsic to T2-weighted imaging [3, 12–14], these could be considered useful in view of recently published data on ability of dynamic contrast enhanced MRI to increase accuracy for nodal staging in rectal cancer by combining morphological criteria, namely presence of T2-weighted heterogeneity in nodes, with early incomplete arterial enhancement (rim enhancement) [15]. Diffusion-weighted imaging appears to have an emerging role [5], especially for differentiating suspected small residual/recurrent tumor from treatment-related changes; however, no dedicated studies have been published so far.
Role of MRI after definitive CRT
The use of imaging in patients following CRT is still debatable. Traditionally, response to CRT has been assessed clinically. Treatment-related changes such as fibrosis and presence of reactive lymph nodes pose difficulties in interpretation, especially on EAUS. The high contrast resolution and anatomical resolution of pelvic MRI makes it an ideal modality for locoregional response assessment, yet there is little published data on anal cancer. MRI serves as a useful baseline following treatment as anal cancer patients tend to undergo long-term follow-up and surveillance, and MRI may assist in the early detection of disease relapse. MRI has undoubtedly a significant role in the preoperative evaluation and surgical planning of cases of non-responders to definitive CRT demonstrating significant residual tumor volume following treatment [3] or even progression.
Findings indicative of a positive response include reduction in tumor size, in T2-weighted signal intensity of the treated-tumor and in associated lymphadenopathy. The appearance of T2-weighted-hypointense signal at the site of primary tumor is consistent with fibrosis, a morphological sign of response; however, MRI cannot exclude residual neoplastic foci within dense fibrosis, the same problem encountered with CRT-treated locally advanced rectal cancers.
Consensus criteria for non-invasive, MRI assessment of disease response have not been described. There are only a few, single center, small series, studies published to date that have reported in changes of different MR imaging findings before and after CRT (Table 3). From these studies and current everyday practice an impression is given that MRI for assessing anal cancer response to CRT is requested by clinicians after 6–8 weeks post definitive therapy completion, the same time frame used for rectal cancer undergoing long scheme CRT. However, published studies have raised the issue of appropriate timing for imaging complete response (CR) [16], stating that size involution is most evident at 6 months post-treatment compared with the immediate post-treatment stage where inflammation is superimposed on treated disease. Due to the rarity of this tumor and lack of bigger series, the optimal timing for therapy assessment with MRI has not been reported to date.
At MRI assessment within 6–8 weeks post CRT, it is not anticipated that all tumors will have achieved CR; CR has been reported to occur later on, at around 26 weeks [17]. As 50% of local recurrences occurring within the first 2 years post treatment are located around the primary site of disease or as pelvic/inguinal lymph nodes, it is important to be able to assess treatment response non-invasively.
Imaging features and tumor staging post CRT
MRI provides a detailed visualization of the anal canal and adjacent anatomical structures. Although the dentate line is not directly recognizable with MRI, its position can be inferred as it corresponds approximately to the upper portion of external sphincter muscles, at 2.5–3 cm from anal verge [5, 18]. The exact distance of the tumor from the anal verge, if residual present and visible, is again measured. Sensitivity of MRI for the identification of anal SCCs has been reported to approach 90–100%, with high concordance regarding tumor size [19]. Tumor MR imaging characteristics after CRT have been described in small cohorts of patients [3, 5, 12, 16, 18–21]. In cases of residual tumor identified at post treatment MRI, this does not present with significantly different signal intensity than that on initial staging. Post treatment the same staging parameters as at baseline MRI scan are followed.
SCCs of the anal canal present with low-to-intermediate T1 signal intensity and demonstrate a degree of enhancement after intravenous gadolinium contrast [18]. On T2-weighted images, tumors display intermediate signal intensity, lower signal compared to normal ischioanal fat and higher signal than of anal sphincters and gluteal muscles.
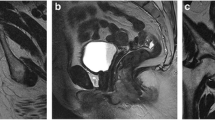
Following CRT there is usually a reduction in tumor signal intensity, with treated tumor appearing as homogeneously low signal intensity on T2-weighted images. Extrapolating from observations made in rectal cancer following chemoradiation, the appearance of low signal intensity within the treated anal canal on T2-weighted MR imaging is likely to represent fibrosis. However, it is still not possible for MR to detect the foci of microscopic disease within dense fibrotic tissues, and follow-up imaging is thus important for ensuring stability of appearance, and for the detection of early relapse. In addition, improvement in the extent of infiltration is noted in the majority of cases. The adjacent mucosa often shows high signal and at times focal thickening, giving a pseudotumor appearance. These appearances are in keeping with post-treatment effect due to mucosal edema and should not be mistaken for residual tumor (Figs. 1, 2). Occasionally, there will be a radiologic CR, in which there is no residual tumor discernible (Fig. 3) and only a small volume of low-signal fibrosis remains (Figs. 1, 4).
67-year-old female with a moderately differentiated anal canal SCC. High resolution T2-weighted MRI images (axial oblique (A, C) and coronal (B, D) orientation) at baseline (A, B) and 8 weeks post completion of definitive CRT (C, D) are shown. This 4.6 cm T2N1 tumor was located 1.8 cm from the anal verge, and involved the right internal sphincter (B, arrowhead). There was an involved right obturator node measuring 6 mm (A, arrow). At restaging, the right obturator node showed almost complete resolution measuring 2 mm (C, arrow). The high signal intensity area identified in the mucosa at the level of the right puborectalis sling is most likely a post-treatment focal area of edema (D, arrowhead). An area of low T2-weighted signal intensity at the level of primary tumor site (E, arrow) is attributed to fibrosis; this presents with degree of restricted diffusion on the corresponding ADC map image (F, arrow). MRI images obtained at the same level at 2 years after completion of definitive CRT show stable appearances of fibrotic area (arrows G, H).
56-year-old female with poorly differentiated SCC. This 4 cm intermediate signal tumor 1.6 cm from the anal verge showed lymphovascular invasion prior to treatment on T2-weighted images (A, B). Baseline MRI demonstrated at least five suspicious mesorectal nodes with a diameter of 5–9 mm, (A, arrow) more conspicuous on DWI images (C, only two shown at chosen level, arrow). Nodes and tumor-demonstrated restriction of diffusion (C, E b800 images, D, F corresponding ADC images, nodes pointed out with arrow, tumor with asterisk), while nodes presented with variable ADC values, slightly higher than that of the primary tumor. MRI performed at 9 weeks after completion of definitive CRT did not demonstrate discernible residual tumor (G). However, pronounced post therapy changes were noted including presence of presacral fluid, submucosal edema of anorectal wall, and marked inflammatory changes within the mesorectal fat (H).
55-year-old female with a poorly differentiated SCC. This 3.7 cm tumor located 0.8 cm from the anal verge involved the right anal sphincter complex (A, B, arrowhead), right and left obturator nodes with a short axis of 1.3 cm (arrow A) and right and left inguinal nodes (C, D arrow) which demonstrated restricted diffusion, similar to the primary tumor (C, D, arrowhead). At MRI 8 weeks after CRT completion, only inguinal lymph nodes are seen with a short axis of 7 mm (F, arrow), significantly reduced in size from baseline, with no evidence of a residual primary (E, F, arrowhead).
72-year-old female with a moderately differentiated SCC. High resolution T2-weighted image at baseline demonstrated an intermediate T2-weighted signal intensity tumor mesorectal deposit (A, arrow), sharing the same signal characteristics as the primary tumor (not shown in these images) as well as a suspicious mesorectal node with heterogeneous T2 signal intensity, adjacent to it. MRI performed 9 weeks post completion of definitive CRT showed resolution of the mesorectal tumor deposit and adjacent mesorectal node. Only a small area of low T2-weighted signal is identified in the area in keeping with fibrosis (B, arrow).
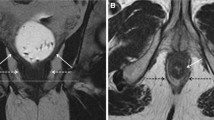
The longest residual lesion diameter on T2-weighted images is measured, as this is important for providing T restaging [3, 5, 12, 18]. The reduction in the size of tumor appears to parallel the reduction in signal intensity on T2-weighted MR imaging. In the remote case scenario that there is no/limited response to treatment, persistent extramural neoplastic spread involving the anal sphincter complex (external sphincter, levator ani, and puborectalis) or the anterior urogenital triangle with possible vaginal, urethral or bladder involvement is documented. Tumor may also extend laterally invading the ischioanal fossa, superiorly to the rectum and mesorectal fat, or inferiorly to the skin and subcutaneous planes of the perianal region (Fig. 5). If this is the case, T2-weighted intermediate signal intensity solid tissue is seen infiltrating above listed structures. Direct invasion of the rectal wall, perianal skin, subcutaneous tissue, or the sphincter muscle does not render tumor stage as T4.
57-year-old female patient with an extensive poorly differentiated SCC of the anal canal measuring 10 cm in craniocaudal extent, involving the distal rectum, the right side of the vagina, and the urethral meatus (A, asterisk), without any pelvic or inguinal nodes identified, demonstrating restricted diffusion (B, C, asterisk), and staged as T4N0 at baseline. At 9 weeks post CRT completion, MRI demonstrated little response with tumor progression in the left ischioanal fossa, and development of a malignant fistula draining in the left buttock (D–F, asterisk), in addition to an infected deep grade 4 ulcer present in the sacrum.
Incidence of involved regional lymph nodes increases with primary tumor size. Lymph node metastases may be present in 25% of cases even with superficial, ≤T2 stage tumors, at initial staging [20]. Nodal staging relies on the distance of nodes from the primary tumor site rather than on the number of involved nodes. MRI, especially with the implementation of DWI, is helpful in identifying pelvic lymph nodes. As with rectal adenocarcinoma, it has been found that almost half of all involved lymph nodes have a diameter <5 mm. Short-axis threshold values of 8, 5 and 10 mm have been suggested for pelvic, mesorectal, and inguinal lymph nodes, respectively [18]. Involved lymph nodes have been reported as having signal intensity similar to or the same as that of the primary tumor [3] and may demonstrate same changes post therapy as primary tumor (Fig. 6). Application of morphological criteria such as signal heterogeneity and inhomogeneous enhancement increase specificity [3, 15]. Morphological criteria used for lymph node characterization have been reported to work better post neoadjuvant treatment for rectal cancer [22], recognizing MRI as a useful tool for assessment of treatment. In addition, introduction of DWI for lymph detection and characterization has so far succeeded in improving identification of small pelvic lymph nodes, as is the case of mesorectal lymph nodes especially when morphological T2-weighted images are fused with highest b value obtained DWI images [23]. Involved pelvic lymph nodes and tumor deposits demonstrate a range of responses from complete resolution post treatment to progression of disease (Figs. 7, 8).
62-year-old female with a moderately differentiated keratinized anal SCC (A, coronal T2-weighted image, arrow) and involved enlarged left mesorectal lymph node (arrow) demonstrating heterogenous T2-weighted signal (B, axial oblique T2-weighted image, arrow) and restricted diffusion (C, D, arrow). At 8 weeks post CRT, small volume residual tumor demonstrating fibrosis was identified, more conspicuous on the DWI images as well as a decrease in size in the left mesorectal lymph node (E, arrow), with a higher ADC value and signal intensity compared to baseline (F, arrow).
37-year-old male with small volume well differentiated T2N0 disease. The left mesorectal node and left inguinal node were not considered to be involved at baseline (A, left inguinal node indicated with arrow). At 8 weeks post CRT, the left inguinal node has increased in size (B, arrow), while the primary tumor was no longer visible. FNA biopsy of the node confirmed a metastasis.
74-year-old male with poorly differentiated SCC (A). In addition to the 8 cm tumor (A, arrow) there were numerous pelvic lymph nodes, loss of periprostatic fat plane as well as a tumor deposit in the sigmoid mesentery (B, arrow). Early post CRT MRI at 5 weeks demonstrated a significant decrease in size of primary tumor (C, arrow); however, the satellite lesion in the sigmoid mesentery had progressed as this was outside the radiotherapy field (D, arrow).
It is not infrequent that anal tumors coexist with inflammatory conditions such as proctitis and abscesses. MRI allows detection of perirectal inflammatory changes and purulent collections that are differentiated from solid neoplastic tissue, allowing for appropriate therapeutic management (Fig. 9). Resolution of associated inflammatory changes during treatment is also easily monitored by MRI [13, 24]. Koh et al. [16] was the first to report the range of post CRT MRI appearances of anal cancers using T2-weighted and STIR imaging before and after chemoradiation. Tumor response was assessed by recording change in tumor size, signal intensity, distortion of anal canal/sphincter complex, infiltration of adjacent structures and nodal disease immediately after chemoradiation, every 6 months for the first year and then yearly. Tumors appeared mildly hyperintense at baseline T2-weighted and STIR imaging. Responders with long disease remission demonstrated the greatest size involution of MR signal abnormality in the tumor area at 6 months after treatment. Stabilization of signal intensity abnormality 1 year after CRT completion was found to be suggestive of treatment success. In addition, in the same small series, regression of all involved nodes visualized at baseline MRI with no suspicious nodes detected after CRT was reported. In a series of 35 patients, Goh et al. [21] applied RECIST criteria for categorizing patients into responders and non-responders at 6–8 weeks post-CRT and showed no difference between disease-free and relapsed patients between the two groups, supporting that RECIST response based on MRI at 6–8 weeks is not a relevant end point to explore Phase II trials novel treatments and CRT combinations.
63 year old male with a poorly differentiated SCC. This 4.5 cm anal canal tumor demonstrated intermediate T2-weighted signal intensity on high resolution T2-weighted axial and coronal images (A, B, arrow) and no involved lymph nodes at baseline. At 8 weeks post CRT completion, MRI demonstrated no residual tumor on T2-weighted images but a cavity containing fluid and air (C, D, arrow) in keeping with a localized treatment related perforation.
There is only one study investigating the concordance between EAUS and MRI for primary anal cancer staging so far in an initial cohort of 45 patients [19], in which six patients demonstrated tumor progression on follow up despite definitive chemoradiotherapy and where surgery was performed and histology available. The two methods showed agreement in four of the six cases. Both deviations were errors in MRI: an undetected T1 tumor and a lymph node falsely diagnosed as invaded. However, lymph nodes were staged as being metastatic based solely on size criteria (>1 cm), and it is widely accepted that EAUS is superior for small lesions especially when functional MRI techniques such as DWI are not included in the protocol (Fig. 10).
Other imaging techniques
18 F-FDG PET/CT
Most anal carcinomas are FDG-avid. Several studies have shown that 18 F-FDG PET/CT compared with standard imaging can alter staging in approximately 20% of cases, with a distinct trend toward upstaging, and treatment intent in approximately 3–5% of cases [25–27].The main impact of 18 F-FDG PET/CT on therapy stems from its high sensitivity in identifying involved lymph nodes, and high specificity in immunocompetent patients. 18 F-FDG PET/CT can also be used for accurate radiation therapy planning by clearly defining sites of metabolically active tumor [25, 26, 28]. To date, there have been few 18 F-FDG PET/CT studies that have assessed treatment response. In one study of 53 patients, Schwarz et al. [29] demonstrated that FDG uptake decreased with treatment with a complete metabolic response in 83%. Mistrangelo et al. [30] suggested that 18 F-FDG PET/CT may be better at 3 months than 1 month in determining response.
Endoanal ultrasound
Endoanal ultrasound (EAUS) allows for accurate assessment of tumor size and depth of mural invasion [5, 12, 31], but is best reserved for small lesions. Unfortunately, in patients with anal lesions, positioning of endoanal sonography probes is hampered by pain and stricture. In addition, although trans-anal imaging allows for excellent spatial detail with a limited field-of-view, it does not allow adequate assessment of the entire ischiorectal spaces and of regional lymph nodes. EAUS in the follow-up of treated anal carcinoma is still controversial. Edema and scar tissue may be difficult to distinguish from persistent tumor.
Conclusion
It is important to follow patients non-invasively in the remote case scenario that there is no response and salvage surgery (APR) is required. MRI can be useful as a screening test to demonstrate a high suspicion of residual disease and therefore can provoke examination under anesthesia and biopsy in due time. Good response to treatment is perceived as a reduction in the tumor size accompanied by signal intensity change at T2-weighted MR imaging. Extrapolating from observations made in rectal cancer following chemoradiation, the appearance of low signal intensity within the treated anal canal on T2-weighted MR imaging is likely to represent fibrosis. However, it is still not possible for MR to detect foci of microscopic disease within dense fibrotic tissues, and follow-up imaging is thus important for ensuring the stability of appearance, and for the detection of early relapse.
References
Uronis HE, Bendell JC (2007) Anal cancer: an overview. Oncologist 12:524–534
Palefsky J (2008) Human papillomavirus and anal neoplasia. Curr HIV/AIDS Rep 5:78–85
Roach SC, Hulse PA, Moulding FJ, et al. (2005) Magnetic resonance imaging of anal cancer. Clin Radiol 60:1111–1119
Glynne-Jones R, Northover JM, Cervantes A (2010) Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v87–v92
Kochhar R, Plumb AA, Carrington BM, et al. (2012) Imaging of anal carcinoma. AJR Am J Roentgenol 199:W335–W344
McMahon CJ, Rofsky NM, Pedrosa I (2010) Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 254:31–46
Renehan AG, Saunders MP, Schofield PF, O’Dwyer ST (2005) Patterns of local disease failure and outcome after salvage surgery in patients with anal cancer. Br J Surg 92:605–612
Meyer J, Willett C, Czito B (2010) Current and emerging treatment strategies for anal cancer. Curr Oncol Rep 12:168–174
Martin FT, Kavanagh D, Waldron R (2009) Squamous cell carcinoma of the anal canal. Surgeon 7:232–237
Lim F, Glynne-Jones R (2011) Chemotherapy/chemoradiation in anal cancer: a systematic review. Cancer Treat Rev 37:520–532
Beets-Tan RG, Beets GL, van der Hoop AG, et al. (1999) High-resolution magnetic resonance imaging of the anorectal region without an endocoil. Abdom Imaging 24(6):576–581
Parikh J, Shaw A, Grant LA, et al. (2011) Anal carcinomas: the role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur Radiol 21:776–785
Tappouni RF, Sarwani NI, Tice JG, et al. (2011) Imaging of unusual perineal masses. AJR Am J Roentgenol 196:W412–W420
Hoeffel CC, Azizi L, Mourra N, et al. (2006) MRI of rectal disorders. AJR Am J Roentgenol 187:W275–W284
Alberda WJ, Dassen HP, Dwarkasing RS, et al. (2013) Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 28(4):573–580
Koh DM, Dzik-Jurasz A, O’Neill B, et al. (2008) Pelvic phased array MR imaging of anal carcinoma before and after chemoradiation. Br J Radiol 81:91–98
Glynne-Jones R, James R, Meadows H, ACT II Study Group et al. (2012) Optimum time to assess complete clinical response (CR) following chemoradiation (CRT) using mitomycin (MMC) or cisplatin (CisP), with or without maintenance CisP/5FU in squamous cell carcinoma of the anus: Results of ACT II. J Clin Oncol 30 (suppl: abst 4004)
Tonolini M, Bianco R (2012) MRI and CT of anal carcinoma: a pictorial review. Insights Imaging 4(1):53–62
Otto SD, Lee L, Buhr HJ, et al. (2009) Staging anal cancer: prospective comparison of transanal endoscopic ultrasound and magnetic resonance imaging. J Gastrointest Surg 13:1292–1298
Raghunathan G, Mortele KJ (2009) Magnetic resonance imaging of anorectal neoplasms. Clin Gastroenterol Hepatol 7:379–388
Goh V, Gollub FK, Liaw J, et al. (2010) Magnetic resonance imaging assessment of squamous cell carcinoma of the anal canal before and after chemoradiation: can MRI predict for eventual clinical outcome? Int J Radiat Oncol Biol Phys 78(3):715–721
Koh DM, Chau I, Tait D, et al. (2008) Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys 71(2):456–461
Mir N, Sohaib SA, Collins D, Koh DM (2010) Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol 54(4):358–364
Nadal SR, Manzione CR, Galvao VM, et al. (1999) Perianal diseases in HIV-positive patients compared with a seronegative population. Dis Colon Rectum 42:649–654
Krengli M, Milia ME, Turri L, et al. (2010) FDG-PET/CT imaging for staging and target volume delineation in conformal radiotherapy of anal carcinoma. Radiat Oncol 5:10
de Winton E, Heriot AG, Ng M, et al. (2009) The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer 100:693–700
Bhuva NJ, Glynne-Jones R, Sonoda L, Wong WL, Harrison MK (2012) To PET or not to PET? That is the question. Staging in anal cancer. Ann Oncol 23(8):2078–2082
Nguyen BT, Joon DL, Khoo V, et al. (2008) Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol 87:376–382
Schwarz JK, Siegel BA, Dehdashti F, et al. (2008) Tumor response and survival predicted by post-therapy FDG-PET/CT in anal cancer. Int J Radiat Oncol Biol Phys 71(1):180–186
Mistrangelo M, Pelosi E, Bellò M, et al. (2012) Role of positron emission tomography-computed tomography in the management of anal cancer. Int J Radiat Oncol Biol Phys 84(1):66–72
Drudi FM, Raffetto N, De Rubeis M, et al. (2003) TRUS staging and follow-up in patients with anal canal cancer. Radiol Med 106:329–337
Acknowledgments
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London and by the Comprehensive Cancer Imaging Centre, funded by the Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gourtsoyianni, S., Goh, V. MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging 39, 2–17 (2014). https://doi.org/10.1007/s00261-013-0032-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-013-0032-6