Abstract
Purpose
To evaluate technical success and long-term outcome of CT-guided radiofrequency ablation (RFA) of spinal osteoid osteomas (OO) and osteoblastomas (OB) in six different European centres.
Methods
Eighty-seven patients with spinal OO (77) or OB (10) were treated with CT-guided RFA, after three-dimensional CT-guided access planning. Patient’s long-term outcome was assessed by clinical examination and questionnaire-based evaluation including 10-point visual analogue scales (VAS) regarding the effect of RFA on severity of pain and limitations of daily activities. Clinical success was defined as a reduction of > 30% in the VAS score and patient’s satisfaction.
Results
Overall, RFA was technically successful in 82/87 cases (94.3%) with no major complications; clinical success was achieved in 78/87 cases (89.7%). The OO/OB were localized in the cervical (n = 9/3), the thoracic (n = 27/1), the lumbar (n = 29/4), and the sacral spine (n = 12/2). A decrease in severity of pain after RFA was observed in 86/87 patients (98.9%) with a persistent mean reduction of overall pain score from 8.04 ± 0.96 to 1.46 ± 1.95 (p < 0.001) after a median follow-up time of 29.35 ± 35.59 months. VAS scores significantly decreased for limitations of both daily (5.70 ± 2.73 to 0.67 ± 1.61, p < 0.001) and sports activities (6.40 ± 2.58 to 0.67 ± 1.61, p < 0.001).
Conclusion
In a multicentric setting, this trial proves RFA to be a safe and efficient method to treat spinal OO/OB and should be regarded as first-line therapy after interdisciplinary case discussion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma (OO) is a common benign bone tumour that occurs usually in childhood and adolescence [1, 2] with a reported incidence of 2–3% among all bone primary tumours [3]. Ten percent of all osteoid osteomas are located in the spine, predominantly in posterior elements of the thoracic and lumbar spine [4]. OO larger than 15 mm and with non-aggressive behaviour are denominated giant OO or osteoblastoma (OB) [5]. OO usually presents with a characteristic focally lucent nidus and surrounding solid sclerotic reaction. OB is four times less frequent than OO, more expansive, has less sclerotic borders, and may have more aggressive imaging features than OO, which makes histologic sampling mandatory in all cases [6, 7].
Therapy is required both in OO and OB because of severe bone pain that is independent from physical strain and typically worsens at night. In the spine, OO/OB can result in painful scoliosis [4]. The pain is caused by the excessive production of prostaglandins within the tumour’s nidus, which explains that OO/OB respond well to non-steroidal anti-inflammatory drugs (NSAID) [8]. However, as NSAID is not a reasonable long-term treatment option in young patients due to undesirable side effects, open surgical excision of the nidus has been the preferred therapy for OB and also for spinal OO [4, 6, 9,10,11,12,13].
Computed tomography (CT)-guided radiofrequency ablation (RFA) is accepted as the gold standard treatment for OO in the extremities [2, 14, 15]. In the spine, however, RFA is limited because of the risk of thermal damage to adjacent neurovascular structures. Therefore, most authors have considered the spine and other critical locations, e.g. tumours close to neural structures, as a (relative) contraindication for RFA treatment [16,17,18]. Therefore, until a few years ago, successful RFA of spinal OO and OB had only been reported in several small case series [14, 19,20,21,22]. However, in order to prove safety and efficacy of RFA for OO/OB in the spine for different sites, interventionalists, and techniques, a multicentric approach is required.
Thus, the purpose of this study was to assess the clinical success of RFA of spinal OO and OB in a larger patient population in a multicentric setting based upon data of six different European institutions.
Materials and methods
Ethical policy, in- and exclusion criteria
The study was approved by the institutional review boards of Heidelberg and Rostock. It was performed according to the declaration of Helsinki in its present form of 2013. Written informed consent was obtained from all patients after the RFA procedure and possible complications (e.g. thermal skin burn, soft-tissue hematoma, nerve injury) had been fully explained. After individual interdisciplinary (interventional radiologist and orthopaedic spine surgeon) case discussion and by taking into account patients’ preferences, either RFA or open surgical resection of their spinal OO or OB was performed at the six institutions between 2004 and 2017.
Inclusion criteria for treatment of the spinal OO or OB were the typical clinical presentation to the interdisciplinary board, verification of a nidus using at least 16-row multidetector CT of the respective anatomic region of the spinal OO/OB. Exclusion criteria of this study were missing informed consent, missing clinical follow-up, and bone tumours of different entity. Thereafter, the local databases of the six participating centres were screened by the responsible interventional radiologists of each centre, and all available material including patients’ files and imaging data were reviewed. Two radiologists not involved in the conduct of spinal radiofrequency ablations have received data from all the six participating centres (image data, questionnaires, clinical examination protocols, etc.) and evaluated them in consensus. These included, for example, the determination of the nidus size and the complete statistical evaluation of the data obtained. This was performed to ensure that the most objective possible evaluation and processing of the data can be done by radiologists who were not involved in the actual procedure.
Patient population
All patients underwent a routine pre-procedural screening that included medical history, physical examination, and basic laboratory analysis (Table 1). Diagnosis of OO/OB was proven by the combination of typical clinical presentation and imaging studies, in particular multidetector CT that was performed in 87/87 patients (100%), magnetic resonance imaging (MRI) in 46/62 patients (74.2%, non-available data in 26 cases), conventional radiography in 46/61 patients (75.4%, non-available data in 26 cases), and scintigraphic bone scans in 24/61 patients (39.3%, non-available data in 26 cases).
Treatment protocol and RFA technique
The RFA procedure was performed by experienced and board-certified consultant interventional radiologists using at least 16-row multidetector CT under general anaesthesia. During the whole procedure, sterile conditions were ensured and the patients were prone positioned in the CT system. One or two grounding pads were placed to inhibit the transmission of current through the patient. At the level of the OO/OB, a spiral CT scan was performed, covering the nidus and the minimum caudal and cranial extent needed for planning safe access. Multiplanar reconstructions were used to guide the coaxial bone biopsy system and the RF-electrode placement. Image data sets were reconstructed using standard bone algorithms.
Skin access was identified using longitudinally placed cannulas as markers. Skin entry was made after a stab incision and the periosteum was additionally infiltrated with local anaesthetics (2–4 ml of bupivacaine hydrochloride 0.5% or lidocaine 1%). Procedural techniques included three-dimensional CT-guided access planning in all cases using multiplanar reconstructions in order to define the optimal access path and the number of needle positions needed to cover the whole nidus.
In all cases, the access into the nidus was made with a coaxial bone biopsy system including a coaxial manual drilling device and a penetration cannula. The nidus was then penetrated with the penetration cannula used as a placeholder. The Leiden centre has taken biopsy specimens with the compatible biopsy needle of the Bonopty® system in all of its 35 cases.Footnote 1 The radiofrequency electrode then was inserted through the cannula and the active tip was placed within the nidus. The ablation electrode with two different lengths of the active tip was used depending on the extent of the nidus. The following two equations predicted the maximum size of the treatment zone:
-
(1)
longitudinal axis of the treatment zone = 2 × lengths of the bare (i.e. active) tip
-
(2)
transverse axis = 2/3 long axis [23]
When the optimal needle position was achieved, the cannula was partially withdrawn to prevent heat propagation along the needle.
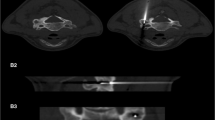
Thermal protection was used with osteoid osteomas in 26.7% of the cases of the Heidelberg centre (overall in 4 out of 15 cases) and in 27.3% of the cases of the Naples centre (overall 3 out of 11 cases). When used, thermal protection was achieved by epidural air insufflation, as has been reported before [14, 22], using one or two separate 22-gauge cannulas in order to yield distance between the nidus and the spinal cord or nerve and to profit from the insulating effect of the injected air within the adjacent epidural space. The 22-gauge cannulas were introduced through an interlaminar approach and an epidurogram was obtained after injection of 5 ml of air. In the neuroforamen, we injected 1–2 ml of air. Thermal protection has been used in two (Heidelberg and Naples centre) of the six participating centres in 7 patients (Fig. 1).
The RF-ablation was started without additional internal cooling using saline solution. The internal cooling has been used in some studies to increase the size of the ablation area but the ablation area is less predictable and may compromise adjacent structures [19]. The current was slowly increased until the target temperature of 90 °C was reached. The total ablation time was between 240 and 400 s for each single ablation (Table 2), regardless of the anatomic location and the total number of electrode positions [14, 15].
In general, patients were discharged within 24 h after a post-therapeutic ward round including a physical examination.
Outcome
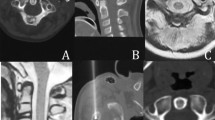
Technical success was defined as a completed RFA including placement of the active tip within the nidus (Fig. 2), with the nidus border not exceeding the active tip by more than 5 mm, as this distance is considered to be effective [24]. The patients were instructed to contact the institution where the RFA was carried out in case of pain recurrence or any other complications. Major complications were defined as prolonged hospitalization or additional treatment unrelated to the tumour [25].
The outcome was qualitatively and quantitatively assessed using a standardized questionnaire for all centres comprising 30 questions covering personal, social, and clinical issues [14]. The questionnaire was given to the patients or sent by email after being re-ordered for control after 6 to 12 weeks after RFA. The follow-up time results from the time at which the patient gave the last information about his condition after the RFA treatment. Long-term success was assessed using visual analogue scales (VAS) regarding the effect of RFA on severity of overall pain and limitations in daily activities as well as sports activities (0–10, with 0: no pain/limitations up to 10: maximum or most imaginable pain/limitations). Moreover, other key questions assessed the patient’s satisfaction (either “yes, satisfied” or “no, not satisfied”). We defined a reduction of > 30% in the VAS score, no pain medication, and a patient’s satisfaction rated at least “mostly satisfied” as clinical success [14, 15, 26]. Failure was defined as lack of significant pain reduction/persisting pain or patient’s dissatisfaction. Further questions quantitatively assessed the time to complete pain relief and the occurrence of any complications [22].
Data analysis and statistics
Data analysis and statistical evaluation were performed with SPSS, version 25 (SPSS Inc., Somers, NY). Descriptive statistics (mean, standard deviation, median, and range) was provided where appropriate, parametric data (e.g. changes in post-RFA VAS scores) were tested using the 2-tailed Student’s t test for OO/OB. Before applying the Student’s t test, normal distribution of data was tested and proved according to the Shapiro-Wilk test. In all tests, a p value of < 0.05 was considered to be statistically significant. p values were adjusted for multiple testing according to the method of Bonferroni.
Results
A total of 77 patients with spinal OO (27 female, 50 male; mean age 23.18 ± 10.46; range 4 to 60 years) and 10 patients with spinal OB (3 female, 7 male; mean age 18.40 ± 7.14; range 9 to 28 years) were treated with CT-guided RFA (Table 3). OO/OB were localized as follows: cervical (n = 9/3), thoracic (n = 27/1), lumbar (n = 29/4), sacral spine (n = 12/2). The morphology of the nidus was elliptical in 37 cases (60%) and spherical in 25 cases (40%; non-available data in 25 cases), with a mean nidus volume of 0.067 ± 0.104 ml in OO and 1.120 ± 0.770 ml in OB (p = 0.001), respectively. The nidus was located within the posterior elements in 78.6% of the cases (44 out of 56 patients; non-available data in 31 cases). Prior to statistical analysis, one patient was excluded from our study as the suspected OO turned out to be an osteosarcoma at histology, which subsequently was surgically resected.
Spinal RFA of an osteoblastoma (white arrow) in the first sacral vertebra of a 10-year-old boy. Thermal protection was used by epidural air insufflation in order to yield distance between the nidus and the spinal nerves and to profit from the insulating effect of the injected air within the adjacent epidural space. In addition, a thermistor-probe (black arrow) was inserted for temperature monitoring during the RF-ablation. Technical and clinical success was reached
Symptoms prior to intervention
The mean duration of symptoms prior to definitive diagnosis of OO/OB was 17.29 ± 14.30 months, in which 96.9% of the patients (62 out of 64; non-available data in 23 cases) needed medication for pain relief. The patient’s pain/limitations resulted in an average VAS score of 5.70 during daily activities and 6.40 during sports activities. The pain and limitations before treatment had negative effects on profession or education in 92.9% of the patients (26 out of 28; non-available data in 59 cases).
Spinal osteoid osteomas
The mean follow-up time of patients with OO was 28.49 ± 35.75 months (range 1–228 months) and the mean nidus volume measured 0.067 ± 0.104 ml (range 0.003–0.419 ml). In this study, we reached a technical success rate of 94.8% (73 out of 77 cases) with minor complications (temporary pain and limited mobility one case each) in 2.8% (2 out of 71 cases; non-available data in 6 cases) and relapses in 9.2% (7 out of 76 cases; non-available data in 1 case). There was an overall patient’s satisfaction with the RFA procedure in 90.09% (70 out of 77 cases) and clinical success rate of 89.6% (69 out of 77 cases). The median time interval until bone pain changed (decreased, resolved) after RFA was 8.28 ± 11.28 days, with a mean overall pain reduction of 6.62 ± 2.24 VAS score points, from a mean VAS score of 8.04 ± 0.99 before to a median VAS score of 1.42 ± 2.03 after the RFA procedure (p < 0.001). The mean VAS score for all spinal OO patients treated with RFA significantly decreased likewise for limitations in daily activities with a mean reduction of pain/limitations of 4.83 ± 2.94 VAS score points, from a mean VAS score of 5.54 ± 2.99 before to a median VAS score of 0.71 ± 1.71 after the RFA procedure (p < 0.001). In addition, for pain/limitations in sports activities, there was a significant amelioration with a mean reduction of pain/limitations of 5.50 ± 2.84 VAS score points, from a mean VAS score of 6.21 ± 2.81 before to a median VAS score of 0.71 ± 1.71 after the RFA procedure (p < 0.001). Bar diagrams illustrating the pain level at first presentation and after RFA are presented in Fig. 3.
Bar diagrams illustrating the decrease in pain and limitations after RFA. The mean values (error bars show standard deviations) of the visual analogue scale (VAS) are illustrated, which was assessed using a standardized questionnaire, regarding the effect of RFA on the severity of overall pain, and limitations in daily activities as well as sports activities of the patients with spinal osteoid osteomas (a) and osteoblastomas (b)
Spinal osteoblastomas
The mean follow-up time of patients with OB was 36.00 ± 35.45 months (range 1–99 months) and the mean nidus volume measured 1.120 ± 0.770 ml (range 0.186–2.143 ml). In this study, we reached a technical success rate of 90.0% (9 out of 10 cases) with minor complications (temporary pain and limited mobility one case each) in 22.2% (2 out of 9 cases; non-available data in 1 case) and relapses in 44.4% (4 out of 9 cases; non-available data in 1 case). There was an overall patient’s satisfaction with the RFA procedure in 90% (9 out of 10 cases) and clinical success rate of 90.0% (9 out of 10 cases). The median time interval until bone pain changed (decreased, resolved) after RFA was 7.67 ± 9.50 days, with a mean overall pain reduction of 6.21 ± 1.49 VAS points, from a mean VAS score of 8.05 ± 0.69 before to a mean VAS score of 1.84 ± 1.11 after the RFA procedure (p < 0.001). The mean VAS score for all spinal OB patients treated with RFA significantly decreased likewise for limitations in daily activities with a mean reduction of pain/limitations of 5.83 ± 1.84 VAS points, from a mean VAS score of 6.33 ± 1.21 before to a median VAS score of 0.50 ± 1.23 after the RFA procedure (p = 0.001). In addition, for pain/limitations in sports activities, there was a significant amelioration with a mean reduction of pain/limitations of 6.67 ± 2.34 VAS score points, from a mean VAS score of 7.17 ± 1.17 before to a median VAS score of 0.50 ± 1.23 after the RFA procedure (p = 0.001). Bar diagrams illustrating the pain level at first presentation and after RFA are given in Fig. 3.
Discussion
This is the first trans-European multicentre study with a large cohort to prove that RFA is a safe and efficient method to treat spinal OO and OB. RFA was technically successful in over 94% of the patients and there were no major complications. A decrease in severity of pain after RFA was observed in all but one patient with an overall significant VAS score reduction of 81.84% (t(86) = 28.39, p < 0.001). Both daily limitations and limitations of sports activities decreased significantly after RFA (OO/OB combined all with p < 0.001). We present technical and clinical success rates of 94.8%/89.6% for spinal OO and 90.0%/90.0% for spinal OB treated with RFA. Thus, the success rates of our study are comparable with figures (85–100%/89–100%) presented for RFA of OO [17,18,19, 22,23,24,25, 27,28,29,30,31,32,33,34,35]. However, in these studies, spinal [32] lesions or OB had been excluded [17, 18, 27, 30] and all of these studies were single-centre studies. There may be other threshold values applicable for the definition of clinical success apart from the 30% threshold used in our study, according to recent recommendations [14, 15, 22, 26]. However, when using a 50% threshold, the results do not change substantially and only one patient does not meet the criteria of clinical success, leading to an overall success rate of 88.5% (77/87). In view of the fact that not all patients in this study were completely free of pain after RFA, it may be noted that in OO/OB, the back pain may not entirely be due to the lesions themselves. In our population, some patients suffered from painful scoliosis secondary to the osteoid osteoma (n = 5), spondylarthrosis (n = 2), and spinal epidural fibrosis (n = 1). Even with a technically successful RFA, at least some degree of pain may persist due to such secondary/indirect causes in a small number of patients.
The treatment of spinal OO/OB depends on the exact localization of the tumour; the temperature during intervention decreases significantly beyond 1 cm distance from the active tip [28], and the cortical bone has an insulating effect [29]. Therefore, OO restricted to the vertebral body can be safely treated without specific protection techniques, when the needle positioning and ablation area are carefully planned. Thus, spinal CT-guided RFA can be performed similar to RFA in peripheral OO without specific protection techniques if the nidus is located within the posterior elements [22]. Prerequisite is an intact cortical bone as an insulator between the OO and the spinal nerves or a distance to critical neural structure of over 1 cm [28]. The distance to the thecal sac however could probably be chosen smaller; an assumption is that the flow of liquor in the thecal sac has a cooling effect for lesions close to it [36]. However, we recommend in case of missing cortical layer of the tumour with regard to the neuroforamen and less than 1 cm distance to neural elements that thermal protection should be performed prior to spinal RFA [19, 22, 31].
Our multicentric data substantiate the recommendation that for spinal OO and spinal OB RFA should also be regarded as first line therapy, as has been proposed by previous studies with smaller patient populations [22, 27, 30]. In the future, open surgical excision of spinal OO and of OB will be less necessary. Surgical treatment of spinal OO and OB has several drawbacks. Besides relapse rates of 4.5–25% [9, 13, 14], removal of large osseous parts necessitating augmentation, instability secondary to resection of posterior elements, spinal cord, or nerve injury has been reported [9, 37]. However, surgery will still be necessary in cases in which OO/OB are not accessible via RFA, or too close to neural structures so that thermal protection cannot not be performed safely. Spinal OO originating from the periosteum often cause nerve root compression and are as well not suitable for radiofrequency ablation and therefore represent an indication for surgery.
Limitations
First, the retrospective study design of this multicentre study is a limitation. Success rates were comparable with reports on other procedures, while a prospective comparison of RFA with other promising techniques like interstitial laser ablation [1], microwave ablation, or cryoablation as well as surgery would be desirable. Second, the decision towards RFA was made individually together with the patient after interdisciplinary case discussion at our institutions. A bias (i.e. assigning more complicated cases to surgery than to RFA) cannot be excluded. Third, histological tumour verification was not always possible when using RFA to treat OO, but we share the prevailing opinion that a histological confirmation is not necessary in the typical constellation of OO [22]. We recommend biopsy, however, in equivocal cases including expansive or aggressive looking OB. Also, the biological behaviour of OB might have the potential to influence the recurrence rate, which is an argument in favour of the additional effort of taking histological samples in all OB cases [38].
Conclusion
In summary, CT-guided RFA is an efficient method to treat spinal OO and OB, even if the tumour is adjacent to neural elements, and therefore, RFA should be regarded as first-line therapy after interdisciplinary individual case discussion.
Change history
19 June 2019
The original version of this paper contained an error: the Acknowledgements were dropped during early manuscript editing.
Notes
Of all the biopsies performed, 15 were consistent with the radiological diagnosis of osteoid osteoma. In three cases, a biopsy attempt was made but no material could be obtained. All other biopsies, performed at the Leiden centre, supplied insufficient material for classifying diagnosis.
References
Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C (2007) Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 242(1):293–301
Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, Menendez L (2009) Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics 29(7):2127–2141
De Filippo M, Russo U, Papapietro VR, Ceccarelli F, Pogliacomi F, Vaienti E, Miele V (2018) Radiofrequency ablation of osteoid osteoma. Acta Biomed 89(1-S):175–185
Pourfeizi HH, Tabrizi A, Bazavar M, Sales JG (2014) Clinical findings and results of surgical resection of thoracolumbar osteoid osteoma. Asian Spine J 8(2):150–155
Lucas DR (2010) Osteoblastoma. Arch Pathol Lab Med 134(10):1460–1466
Orguc S, Arkun R (2014) Primary tumors of the spine. Semin Musculoskelet Radiol 18(3):280–299
Papaioannou G, Sebire NJ, McHugh K (2009) Imaging of the unusual pediatric ‘blastomas’. Cancer Imaging 9:1–11
Mungo DV, Zhang X, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM (2002) COX-1 and COX-2 expression in osteoid osteomas. J Orthop Res 20(1):159–162
Boriani S, Amendola L, Bandiera S, Simoes CE, Alberghini M, Di Fiore M, Gasbarrini A (2012) Staging and treatment of osteoblastoma in the mobile spine: a review of 51 cases. Eur Spine J 21(10):2003–2010
Gasbarrini A, Cappuccio M, Bandiera S, Amendola L, van Urk P, Boriani S (2011) Osteoid osteoma of the mobile spine: surgical outcomes in 81 patients. Spine (Phila Pa 1976) 36(24):2089–2093
Li Z, Zhao Y, Hou S, Mao N, Yu S, Hou T (2013) Clinical features and surgical management of spinal osteoblastoma: a retrospective study in 18 cases. PLoS One 8(9):e74635
Omlor GW, Lehner B, Wiedenhöfer B, Deininger C, Weber MA, Rehnitz C (2012) [Radiofrequency ablation in spinal osteoid osteoma. Options and limits]. Orthopade 41(8):618–622
Zileli M, Cagli S, Basdemir G, Ersahin Y (2003) Osteoid osteomas and osteoblastomas of the spine. Neurosurg Focus 15(5):E5
Rehnitz C, Sprengel SD, Lehner B, Ludwig K, Omlor G, Merle C, Weber MA (2012) CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol 81(11):3426–3434
Rehnitz C, Sprengel SD, Lehner B, Ludwig K, Omlor G, Merle C, Weber MA (2013) CT-guided radiofrequency ablation of osteoid osteoma: correlation of clinical outcome and imaging features. Diagn Interv Radiol 19(4):330–339
Rimondi E, Mavrogenis AF, Rossi G, Ciminari R, Malaguti C, Tranfaglia C, Vanel D, Ruggieri P (2012) Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol 22(1):181–188
Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ (2003) Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 229(1):171–175
Schmidt D, Clasen S, Schaefer JF, Rempp H, Duda S, Trübenbach J, Pereira PL (2011) [CT-guided radiofrequency (RF) ablation of osteoid osteoma: clinical long-term results]. Rofo 183(4):381–387
Rybak LD, Gangi A, Buy X, La Rocca Vieira R, Wittig J (2010) Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. AJR Am J Roentgenol 195(4):W293–W298
Trotta B, Fox MG (2013) Benign osteoid-producing bone lesions: update on imaging and treatment. Semin Musculoskelet Radiol 17(2):116–122
Vanderschueren GM, Obermann WR, Dijkstra SP, Taminiau AH, Bloem JL, van Erkel AR (2009) Radiofrequency ablation of spinal osteoid osteoma: clinical outcome. Spine (Phila Pa 1976) 34(9):901–904
Weber MA, Sprengel SD, Omlor GW, Lehner B, Wiedenhöfer B, Kauczor HU, Rehnitz C (2015) Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skelet Radiol 44(7):981–993
Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR (2002) Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: tricks of the trade. AJR Am J Roentgenol 179(6):1633–1642
Albisinni U, Rimondi E, Malaguti MC, Ciminari R (2004) Radiofrequency thermoablation in the treatment of osteoid osteoma. Radiology 232(1):304; author reply 304-5–305
Jankharia B, Burute N (2009) Percutaneous radiofrequency ablation for osteoid osteoma: how we do it. Indian J Radiol Imaging 19(1):36–42
Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC (2008) Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 33(1):90–94
Albisinni U, Facchini G, Spinnato P, Gasbarrini A, Bazzocchi A (2017) Spinal osteoid osteoma: efficacy and safety of radiofrequency ablation. Skelet Radiol 46(8):1087–1094
Bitsch RG, Rupp R, Bernd L, Ludwig K (2006) Osteoid osteoma in an ex vivo animal model: temperature changes in surrounding soft tissue during CT-guided radiofrequency ablation. Radiology 238(1):107–112
Dupuy DE, Hong R, Oliver B, Goldberg SN (2000) Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol 175(5):1263–1266
Faddoul J, Faddoul Y, Kobaiter-Maarrawi S, Moussa R, Rizk T, Nohra G, Maarrawi J (2017) Radiofrequency ablation of spinal osteoid osteoma: a prospective study. J Neurosurg Spine 26(3):313–318
Klass D, Marshall T, Toms A (2009) CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol 19(9):2238–2243
Rimondi E, Bianchi G, Malaguti MC, Ciminari R, Del Baldo A, Mercuri M, Albisinni U (2005) Radiofrequency thermoablation of primary non-spinal osteoid osteoma: optimization of the procedure. Eur Radiol 15(7):1393–1399
Vanderschueren GM, Taminiau AH, Obermann WR, Bloem JL (2002) Osteoid osteoma: clinical results with thermocoagulation. Radiology 224(1):82–86
Wang B, Han SB, Jiang L, Yuan HS, Liu C, Zhu B, Liu ZJ, Liu XG (2017) Percutaneous radiofrequency ablation for spinal osteoid osteoma and osteoblastoma. Eur Spine J 26(7):1884–1892
Martel J, Bueno A, Nieto-Morales ML, Ortiz EJ (2009) Osteoid osteoma of the spine: CT-guided monopolar radiofrequency ablation. Eur J Radiol 71(3):564–569
Vidoni A, Grainger M, James S (2018) Experience of neuroprotective air injection during radiofrequency ablation (RFA) of spinal osteoid osteoma. Eur Radiol 28(10):4146–4150
Laus M, Albisinni U, Alfonso C, Zappoli FA (2007) Osteoid osteoma of the cervical spine: surgical treatment or percutaneous radiofrequency coagulation. Eur Spine J 16(12):2078–2082
Oliveira CR, Mendonça BB, Camargo OP, Pinto EM, Nascimento SA, Latorre MR, Zerbini MC (2007) Classical osteoblastoma, atypical osteoblastoma, and osteosarcoma: a comparative study based on clinical, histological, and biological parameters. Clinics (Sao Paulo) 62(2):167–174
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beyer, T., van Rijswijk, C.S.P., Villagrán, J.M. et al. European multicentre study on technical success and long-term clinical outcome of radiofrequency ablation for the treatment of spinal osteoid osteomas and osteoblastomas. Neuroradiology 61, 935–942 (2019). https://doi.org/10.1007/s00234-019-02226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02226-9