Abstract
The iliotibial tract, also known as Maissiat’s band or the iliotibial band, and its associated muscles function to extend, abduct, and laterally rotate the hip, as well as aid in the stabilization of the knee. A select group of associated injuries and pathologies of the iliotibial tract are seen as sequela of repetitive stress and direct trauma. This article intends to educate the radiologist, orthopedist, and other clinicians about iliotibial tract anatomy and function and the clinical presentation, pathophysiology, and imaging findings of associated pathologies. Specifically, this article will review proximal iliotibial band syndrome, Morel-Lavallée lesions, external snapping hip syndrome, iliotibial band syndrome and bursitis, traumatic tears, iliotibial insertional tendinosis and peritendonitis, avulsion fractures at Gerdy’s tubercle, and Segond fractures. The clinical management of these pathologies will also be discussed in brief.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The iliotibial (IT) tract is a portion of the fascia lata, the deep fascia of the thigh investing the muscles of the hip and lower extremity in this region. Specifically, the IT tract is a strong band of the fascia lata located in the lateral thigh [1]. The IT tract receives contributions from the tensor fascia lata (TFL) and gluteus maximus muscles in the proximal thigh and inserts distally about the knee, including onto the proximal tibia (Fig. 1) [2]. It transmits forces from the hip to the knee and functions as one of the lateral stabilizers of the knee joint [3, 4]. Traumatic or overuse injuries of the IT tract can cause significant pain and impairment of physical activity and occur relatively commonly. A recent epidemiological study in the USA concluded that the incidence of acute knee injuries presenting to emergency departments is 2.29 per 1000 population [5], and evidence suggests that perhaps roughly half of all patients with acute knee injuries will demonstrate injury to the IT tract on magnetic resonance imaging (MRI) [6]. Iliotibial band friction syndrome, caused by repetitive physical activity, is believed to be the most common running injury to the lateral knee and accounts for 15–24% of overuse injuries in cycling [7]. Imaging, particularly MRI, can aide in the diagnosis of both acute and chronic injury to the IT tract. As such, it is important for the radiologist to be familiar with the normal anatomy of the IT tract as well as the clinical presentation and imaging findings of its various pathologies.
Anatomy of the iliotibial tract
The fascia lata is the deep investing fascia of the thigh, encasing the muscles of the hip and lower extremity in this region [1]. The iliotibial (IT) tract is a strong longitudinal band of this deep fascia in the lateral thigh [1]. Many investigators have sought to characterize the anatomy of the IT tract, but reports of its exact anatomy are still varied and inconsistent. The anatomist Vesalius described the fascia lata as “one of the muscles of the tibia” in 1543 [2, 8, 9]. In 1855, Gerdy described the insertion of the fascia lata on the tibia [2, 9, 10]. Segond later termed this insertion point “Gerdy’s tubercle” [2, 9, 11].
In 1958, Kaplan conducted a careful review of the historical literature and comparative anatomical dissections to further elucidate the IT tract as a structure unique to humans, likely representing adaptation to erect posture and bipedal gait [2, 12, 13]. Kaplan characterized the IT tract as a ligament connecting the ilium with the tibia [12]. He described the IT tract as arising intimately from the tensor fascia lata and the gluteus maximus in the region below the greater trochanter and extending to its insertions on the lateral femoral condyle and the lateral tibial tubercle (Gerdy’s tubercle) [12].
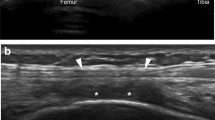
Descriptions of the proximal anatomy of the IT tract have been varied [13, 14]. The IT tract is generally understood to be comprised of a coalescence of the aponeurotic coverings of the tensor fascia lata and gluteus maximus muscles, as well as the fascia lata (Fig. 2) [2, 9, 12–16]. Along the lateral thigh, the fascia lata receives expansions from gluteus maximus and gluteal aponeurosis (i.e., aponeurotic fascia) posteriorly and tensor fascia lata (TFL) anteriorly [13, 15, 16]. This lateral, thickest region of the fascia lata is the IT tract [1, 13, 15, 16].
Illustration (a) and axial oblique PDFS MR image (b) of the cross-sectional anatomy of the thigh demonstrating the iliotibial tract/band made up of contributions from the aponeurotic fascia of the tensor fascia lata anteriorly, gluteus maximus posteriorly, and the lateral portion of the deep investing fascia (fascia lata)
Huang et al. recently described the proximal anatomy of the IT tract, including its bony and muscular origins (Fig. 3) [16]. The IT tract consists of three layers: the superficial layer, intermediate layer, and deep layer [9, 16]. These layers fuse in the region of the greater trochanter to form the proximal IT tract [14, 16]. The superficial layer arises from the ilium superficial to the TFL, while the intermediate layer arises from the ilium slightly below the origin of the TFL and lies deep to the muscle [14, 16]. The superficial and intermediate layers of the IT tract merge at the distal end of the TFL and essentially serve as the tendon for the TFL [14, 16]. Huang et al. describe the deep layer as a constant structure arising from the supraacetabular fossa between the hip capsule and the tendon of the reflected head of the rectus femoris [16]. This deep layer merges just distal to where the superficial and intermediate layers fuse [16].
Illustration of the sites of the proximal origins of the iliotibial tract and adjacent muscles: pink = superficial and deep (*) layers of the iliotibial tract; yellow = gluteus minimus; light blue = gluteus medius; green = gluteus maximus; dark blue = tensor fascia lata; red = gluteal aponeurotic fascia
Posteriorly, the IT tract receives contributions of tendinous fibers from the gluteus maximus muscle and gluteal aponeurotic fascia. The fibers of the superior gluteus maximus and the superficial fibers of the inferior gluteus maximus insert into the posterior IT tract [16]. The deep fibers of the inferior gluteus maximus continue toward the femur to insert onto the gluteal tuberosity of the linea aspera [16]. The gluteal aponeurosis, arising from the iliac crest, also contributes to the IT tract posteriorly [2, 16]. The gluteal aponeurosis arises from the posterior iliac crest and extends distally, covering the anterior two-thirds of the gluteus medius, and inserts into the posterior IT tract and onto the gluteal tuberosity [16].
While the layers of the IT tract merge over the area of the great trochanter, the IT tract does not insert onto the greater trochanter [14, 16]. However, the IT tract attaches to the linea aspera of the femur via the lateral intermuscular septum, which arises from the IT tract [2, 9, 14, 16]. The lateral intermuscular septum also receives tendinous contributions from the gluteus maximus [16].
At least five distal insertions of the IT tract about the knee have been described (Fig. 4) [2, 14]. These insertions extend to an extensive periarticular area in the lateral knee, including the distal femur, patella, proximal tibia, and joint capsule [2].
Illustration of the distal iliotibial band insertions. 1 = Direct: wide ribbon-shaped insertion at Gerdy’s tubercle; 2 = capsular-osseous: posterior slip inserting on the tibia posterolateral to Gerdy’s tubercle (lateral femorotibial ligament); 3 = lateral epicondyle: strong ligament inserting at the upper edge of the lateral epicondyle near the lateral collateral ligament; 4 = linea aspera: deepest portion inserts on lateral portion of femoral diaphysis through the lateral intermuscular septum; 5 = patellar: wide fusiform insertion into the lateral patellofemoral ligament/retinacular complex
The IT tract courses over the vastus lateralis to terminate as a ribbon-shaped insertion onto the infracondylar tubercle of the tibia (Gerdy’s tubercle) [2, 14]. This direct insertion occurs widely at and around Gerdy’s tubercle [2]. Birnbaum et al. also describe this portion of the IT tract as giving rise to insertions on the fibular head [14].
The IT tract inserts onto the lateral portion of the femoral diaphysis along the linea aspera through fibrous bundles connecting the deep portion of the IT tract to the lateral intermuscular septum [2, 14]. The IT tract also inserts onto the distal femur at the lateral epicondyle [2, 14]. This insertion is through a strong ligament at the superior aspect of the lateral epicondyle near the lateral collateral ligament [2].
The IT tract has a patellar insertion that is wide and fuses into the lateral transverse and longitudinal patellar retinaculum [2, 14]. Through this insertion, the IT tract contributes to the lateral patellofemoral ligament complex [2].
The capsular-osseous insertion of the IT tract is a posterior slip inserting onto the tibia posterolateral to Gerdy’s tubercle [2, 9]. This insertion is also known as the lateral femorotibial ligament [2]. Some authors argue that this capsular-osseous insertion of the IT tract is the same structure as the anterolateral ligament, only with different dissection protocols, and it is discussed more thoroughly in the following section [17].
Function of the iliotibial tract
The IT tract and its associated muscles help extend, abduct, and laterally rotate the hip [18]. The IT tract also serves an important postural function, allowing for asymmetrical standing (pelvic slouch), with the upward pull of the lower attachment of the IT tract locking the knee in hyperextension and creating a rigid support pillar [19].
The IT tract is also a key structure contributing to the lateral stability of the knee [3]. However, given the many components and insertions of the IT tract, it is perhaps unsurprising that the specific role of the IT tract in lateral knee stability has been the subject of much investigation and debate. Recently, the “re-discovery” of the so-called anterolateral ligament of the knee has prompted renewed interest in the anatomy and function of the lateral stabilizers of the knee, including that of the IT tract [20].
The IT tract has been relatively well defined as contributing to anterolateral knee stability. Its fibers attaching to the lateral femoral condyle divert forces of the tensor fascia lata and gluteus maximus muscles. The distal IT tract is a dynamic structure in its proximity to the knee; its posterior fibers are isometric between 0° and 50° of knee flexion and increase in length between 50° and 90° of flexion while its anterior fibers increase in length between 0° and 40° of flexion and then are essentially isometric from 40 to 90° [3].
Based on his studies of the IT tract, Kaplan advanced the hypothesis that it provides anterolateral stability to the knee [12]. This view was reinforced by subsequent biomechanical studies that determined the distal IT tract was strong enough to act as a ligamentous stabilizer and demonstrated the mechanism by which this stabilizing action would occur [9, 21–23]. However, Hughston et al. questioned the importance of the IT tract in lateral stability based on their observations of patients with posterolateral corner and lateral capsular injury with 3+ instability, but no concurrent lesion of the IT tract [24].
Terry et al. agreed with Kaplan’s assessment of the IT tract as a lateral stabilizer of the knee [9]. Based on their cadaveric study of 17 knee specimens, Terry et al. described the IT tract as forming a sling posterior to the lateral femoral epicondyle and acting to prevent posterior subluxation of the femur on a fixed tibia. The layer of the IT tract they name the “capsular-osseous layer” was taken to act synergistically with the anterior cruciate ligament (ACL); when the knee extends, the distance between the femoral and tibial fixations of the IT tract increases, tensing this segment of the IT tract, which restricts anterolateral subluxation and anterior translation of the tibia. Terry et al. also saw the IT tract as providing adduction stability by preventing varus joint lengthening [2, 9].
Subsequently, Hughston and Terry et al. produced a series of 82 patients with anterolateral and anteromedial knee instability, which supported the hypothesis that the IT tract is vital for anterolateral knee stability. In this study, 98% of patients had an ACL lesion and 93% of patients had an IT tract lesion of any kind. The authors posited that the number of possible IT tract lesions accounts for the varied clinical presentation of ACL rupture with regards to anterior tibial displacement [25].
Terry et al. also advanced the hypothesis that the combined layers of the IT tract functionally constituted an anterolateral ligament of the knee [9]. In a similar cadaveric study, Vieira et al. largely supported this notion [2]. The authors found the capsular-osseous layer of the IT tract to be a well-defined anatomical structure that has a location and thickness to appropriately constitute an anterolateral ligament of the knee [2]. In earlier work, Hughston et al. suggested a capsuloligamentous thickening of the anterolateral joint capsule was the true anterolateral ligament (ALL), distinct from the IT tract [3, 24].
The ALL had previously been described, albeit inconsistently, since at least 1879 by Segond [3, 20], but Claes et al. sought to define its anatomy through a rigorous cadaveric study in 2013 [20]. The authors found the origin of the ALL to be at the prominence of the lateral femoral epicondyle, anterior to the origin of the lateral collateral ligament (LCL) [20]. The ALL coursed toward the anterolateral proximal tibia, attaching to the lateral meniscus and the tibia between Gerdy’s tubercle and the fibular head [20]. The authors asserted their dissections demonstrated the ALL as definitively distinct from the IT tract and hypothesized the function of the ALL as being to control internal rotation of the tibia [20].
Subsequent qualitative and quantitative studies of the ALL found similar but not wholly consistent anatomy, particularly in regards to its origin being anterior, posterior, or at the lateral femoral epicondyle [26–30]. Additionally, the ALL has been variously implicated in resisting varus joint lengthening and anteroposterior translation of the tibia in addition to internal rotation of the tibia. Currently, the anatomy of the ALL and its functional contribution to knee stability remain controversial [31, 32]. Thus, it is possible that the ALL in fact accounts, to some degree, for the anterolateral stability previously attributed to the IT tract by Terry et al. [9, 26].
Other major structures contributing to the lateral stability of the knee are the biceps femoris and the other posterolateral corner structures of the knee [4]. The biceps femoris is a muscle of the posterior thigh composed of two parts: the long head of the biceps and the short head of the biceps. Both the long and short heads function act as knee flexors. The long head arises from the posterior surface of ischial tuberosity. The short head arises from middle third of linea aspera and the lateral supracondylar ridge of femur. The main insertion site of the biceps femoris is at the head and styloid process of the fibula. In addition, it has several tendinous and fascial insertional components, including one that inserts at the posterior edge of the IT tract.
The additional structures usually described as being part of the posterolateral corner structures of the knee are the fibular (or lateral) collateral ligament (FCL), popliteus tendon, popliteofibular ligament, lateral capsule, arcuate ligament, and fabellofibular ligament [33]. These structures stabilize the knee from posterolateral rotatory instability. The FCL is the primary restraint to varus stress in early knee flexion. The arcuate ligament fibers contribute to the lateral joint capsule. The lateral aspect of the gastrocnemius muscle also contributes fibers to the joint capsule [4].
Imaging of the iliotibial tract
Proximally, the IT tract can be seen forming in the anterolateral thigh with contributions from the deep fascia of the thigh, gluteus maximus, and tensor fascia lata [16]. Distally, the IT tract can be visualized as part of the soft tissue layers of the lateral knee [4, 6]. The soft tissue of the lateral knee is generally understood to be comprised of three layers; the IT tract and biceps femoris tendon form the superficial layer, the patellar retinaculum forms the intermediate layer, and the LCL and joint capsule form the deep layer (Fig. 5).
Illustration (a) and axial PDFS MR image (b) of the cross-sectional layer anatomy of the lateral support system at the knee. Layer 1: superficial layer: anteriorly: IT band, posteriorly: superficial portion of the biceps; layer 2: middle layer: anteriorly: lateral patellofemoral ligament/retinacular complex; layer 3: deep layer: superficial: fibular collateral ligament (FCL) and the fabellofibular ligament, intermediate: lateral geniculate artery, deep: arcuate ligament, popliteus tendon, popliteofibular ligament, and capsule
The IT tract is typically not well visualized on plan radiographs but can sometimes be identified on anterior-posterior (AP) radiograph of the lower extremity as a vertical linear soft-tissue opacity [16]. On CT imaging, the IT tract will be a hyperdense structure relative to adjacent muscular structures.
The IT tract is best visualized on MRI [6, 16, 34]. On MRI, the IT tract is a hypointense, flat, linear structure in the lateral hip, thigh, and knee [6]. In the absence of pathology, there should be no adjacent edema or significant intrasubstance signal changes. Axial images help to differentiate intra-articular fluid in the lateral joint space from signal changes associated with IT tract pathology [34, 35]. As with other ligamentous structures, thickening and intrasubstance signal changes of the IT tract on fat-suppressed fast spin-echo T2- or proton density (PD)-weighted imaging suggest a sprain [4].
On MRI, the normal IT tract will typically measure about 1 to 3 mm thick at the level of the lateral femoral epicondyle [36, 37]. Sonographic measurement of IT tract thickness is generally consistent with MR measurement, but studies using ultrasound (US) have reported slightly lower average thicknesses [38, 39]. Two sonographic investigations have attempted to determine whether age, height, and or weight influence IT tract thickness in normal individuals; the studies differed as to whether aging causes a baseline decrease in IT tract thickness, but neither study found a significant correlation between IT tract thickness and height or weight [39, 40].
Ultrasound is well regarded as an excellent imaging modality for superficial soft tissues, including the IT tract [41]. In general, US offers an alternative imaging modality for the IT tract that is more expedient and cost-effective than MRI; it also allows for dynamic assessment of the IT tract [39, 41–45]. On sonographic examination, the IT tract is a relatively hyperechoic linear structure that has a fibrillar pattern and is susceptible to anisotropy. Proximally, the IT tract can be easily visualized in the axial plane as a stripe over the greater trochanter [44]. Around the knee, the IT tract can readily be seen in the coronal plane, with the distal insertion at Gerdy’s tubercle, and in the axial plane over the lateral formal epicondyle [41, 45]. The use of dynamic US examination at the greater trochanter and lateral femoral epicondyle can be used to demonstrate abrupt movement of the IT tract over these bony landmarks as the cause of snapping hip or snapping knee syndrome [43–46].
Proximal iliotibial band syndrome
Proximal iliotibial band syndrome (PITBS) is essentially an overuse enthesopathy of the IT tract at its origin on the iliac tubercle. Sher et al. first coined the term PITBS in 2011 in their discussion of a cohort of patients presenting with pain and tenderness at the iliac tubercle. The authors described MR findings in seven patients that were consistent with a strain or tear of the proximal IT tract, positing that these findings represented strain injury of the IT tract iliac tubercle enthesis [13].
All of the patients Sher et al. described were female; four were athletes who reported gradual onset pain localized to the iliac tubercle, and three were older, non-athletes. None recalled an inciting trauma. The authors hypothesized this female predilection may be due to the greater hip width to femoral length ratio leading to increased hip adduction torque and/or greater peak hip adduction and internal rotation in all walking and running conditions in women [13].
Huang et al. described similar presentations and imaging findings in their patients with PITBS and agreed with Sher et al. as to the pathophysiology of the condition [16].
Imaging
MR findings in PITBS include increased signal intensity with fluid-sensitive sequences both superficial and deep to the IT tract origin from the iliac crest, representing edema about the IT tract enthesis (Fig. 6). MR can also demonstrate thickening of the proximal IT tract attachments and partial tearing (intrinsic hyperintense signal) [13, 16].
In contrast to Sher et al., Huang et al. described patients with PITBS as infrequently demonstrating marrow edema in the iliac tubercle. They hypothesize marrow edema in the iliac tubercle to be a manifestation of the enthesopathy, similar to marrow edema in the calcaneus of plantar fasciitis patients [16]. Sher et al. noted that patients with PITBS often are misdiagnosed or treated for presumed hip pathology, perhaps because the field of view (FOV) for routine hip MRI does not always include the proximal IT tract enthesis [13].
Treatment
As with other symptomatic fascial injuries, PITBS is usually treated with rest, anti-inflammatory medications, and physical therapy [16].
Morel-Lavallée Lesion
The Morel-Lavallée lesion (MLL) is a closed internal degloving soft tissue injury caused by a shearing separation of the subcutaneous layer from the deep fascia and secondary formation of a seroma, hematoma, or fat necrosis. This lesion was first described by Maurice Morel-Lavallée in the proximal thigh, but the term has since been used to describe similar lesions at other sites. However, the soft tissue of the proximal thigh lateral to the greater trochanter remains the most common location for the MLL [47, 48].
The abrupt separation of skin and subcutaneous fatty tissue from the underlying fascia severs the lymphatics and vasculature of the subdermal plexus, leading to a hemolymphatic collection in the potential space created between the two layers. The accumulation of this heterogeneous material can be slow or rapid. If left untreated, an inflammatory reaction leads to the formation of a peripheral fibrous capsule. A superimposed infection of the lesion may ensue [47, 48].
Lateral to the greater trochanter, the IT tract lies deep to the dermis, subcutaneous fat, and superficial fascia. The dermis and subcutaneous fat are more mobile than the firmer IT tract, putting these superficial soft tissues at risk for a shearing injury and subsequent MLL formation [47].
MLLs are usually due to severe pelvic or thigh trauma; motor vehicle collisions are the most common mechanism. MLLs can also be caused by low-velocity crush injuries, for which the thigh is the most common site. Contact sports have also been associated with MLLs [47].
MLLs may present acutely or remote to the trauma depending on the rate and extent of hemolymphatic accumulation. A patient’s body habitus or the presence of distracting polytrauma may delay the clinical diagnosis of MLLs, especially smaller lesions. Fractures of the proximal femur, pelvis, and acetabulum may occur simultaneously [47, 48].
Patients with MLLs typically report painful swelling. On physical examination, MLLs can demonstrate ecchymosis, soft tissue swelling, fluctuance, or skin hypermobility. As the fibrous capsule forms, the area may become firm. The overlying skin may also experience necrosis [47, 48].
Imaging
MLLs are classified into six types based on the chronicity and tissue composition of the collections and their appearance on MRI. Specifically, this classification is based on lesion shape, presence or absence of a capsule, overall T1 and T2 signal characteristics, and enhancement features [47, 49].
MLLs are described as either a seroma (type I), subacute hematoma (type II), chronic organizing hematoma (type III), perifascial dissection with closed fatty laceration and no capsule (type IV), perifascial pseudonodular lesion (type V), or having a superimposed infection with variable sinus tract formation, internal septations, and a thick enhancing capsule (type VI) [47, 49].
A type I MLL appears as a homogeneous and hyperintense collection on T2 and homogeneously hypointense on T1 without evidence of outer capsule formation [47, 49].
Type II MLLs are usually homogeneously hyperintense on both T1 and T2. The T1 hyperintensity is due to the presence of the methemoglobin characteristic of subacute hematomas. Type II lesions can demonstrate intra-lesional heterogeneity due to trapped fat globules, fluid-fluid levels from evolving blood products, and/or internal septations (Fig. 7). If new or residual capillaries are present within the lesion, patchy internal enhancement may be seen [47, 49, 50].
Morel-Lavallée lesions in three separate patients with histories of trauma. Coronal STIR MR image (a) demonstrates a thin hyperintense serous fluid collection superficial to the iliotibial tract (curved arrows), consistent with a type I Morel-Lavallée lesion. Coronal T1 MR image (b) demonstrates a large heterogeneous collection superficial to the iliotibial tract (curved arrow) with internal hypointense foci (arrow) corresponding to blood products and hyperintense foci that followed fat signal intensity on all pulse sequences (arrowhead) corresponding to fat globules, consistent with a type II Morel-Lavallée lesion. Coronal T1 MR image (c) demonstrates a hypointense organized hematoma superficial to the iliotibial tract (curved arrows), representing a type III Morel-Lavallée lesion
Type III MLLs demonstrate hypointensity on T1 and heterogeneous hypointensity/isointensity on fluid-sensitive images due to hemosiderin deposition, granulation tissue, necrotic debris, fibrin, and blood clots. Type III lesions may show internal enhancement secondary to neovascularization and organizing granulation tissue. A hypointense peripheral ring may be seen, which represents a hemosiderin-laden fibrous capsule with inflammatory infiltrate [47, 49, 50].
Type IV MLLs demonstrate T1 hypointensity, T2 hyperintensity, variable enhancement, and the absence of a capsule, while type V MLLs will demonstrate variable T1 and T2 intensity as well as areas of both internal and peripheral enhancement. Type V lesions have a small, rounded, pseudonodular appearance. A type VI lesion will have thick enhancing capsule with or without an associated sinus tract [47, 49].
While MRI is the best modality to characterize a MLL, patients are usually emergently evaluated with CT first. CT can be helpful to localize the lesion to the interfascial plane [47, 51].
US also offers the ability to evaluate a suspected MLL expediently, and it is also the modality of choice for imaged-guided interventions [47]. MLLs will appear as nonspecific fluid collections with heterogeneous echogenicity; they will be compressible and fail to demonstrate flow on color Doppler [47, 52, 53]. The chronicity of the lesion will influence the US findings. Acute and subacute (less than 1 month) lesions tend to have a heterogeneous appearance with irregular margins and a lobular shape, while chronic lesions (greater than 18 months) are more often homogeneous with smooth margins and a flat or fusiform shape [47, 52].
Treatment
Management of MLLs depends on the stage of the lesion as well as its size and location. Treatment options include compression banding, aspiration, or debridement and irrigation, with or without injection of sclerosing agents. Smaller, more acute lesions may be more amendable to nonsurgical management (compression banding with or without sclerosants followed by percutaneous drainage if the lesion fails to resolve). Percutaneous drainage with sclerotherapy can be used initially for chronic lesions, but these may require subsequent surgical debridement. Similarly, larger, symptomatic lesions may benefit most from debridement and irrigation. Infected lesions necessitate open drainage and secondary closure [47, 48].
External snapping hip syndrome
External snapping hip syndrome (ESHS) refers to a snapping, popping, or clicking sensation over the greater trochanter with hip motion [54]. ESHS itself is a form of snapping hip syndrome, which is defined by a sensation of snapping during hip motion (“snapping hip” or “coxa saltans”) with or without associated pain [43, 55, 56]. For clarity, some have advocated for reserving the term snapping hip syndrome only for those cases where this snapping sensation is accompanied by pain [46].
Snapping hip syndrome is typically classified as intra-articular or extra-articular, depending on where the site of pathology is located. Intra-articular snapping hip is caused by any intra-articular derangement, such as acetabular labral or ligamentum teres tears, or intra-articular bodies. Extra-articular snapping hip is further divided into two forms: external and internal [43, 55, 56]. The internal form of extra-articular snapping hip has been attributed to the iliopsoas tendon moving over the iliopectineal eminence, producing an audible snap emanating from the anterior hip [43, 46]. However, some dynamic US studies suggest the actual source of this snap to be the psoas tendon rolling laterally and anteriorly over the medial fibers of the iliacus muscle [57, 58].
In the external form of snapping hip, the snap occurs in the lateral hip as a result of abrupt forward movement of the proximal IT tract or, less often, the distal gluteus maximus over the greater trochanter during hip flexion [44, 54, 59, 60]. Passive movement from an adducted and internally rotated hip into flexion and external rotation may also elicit this snap. Pain, which may reflect bursitis or tendonitis from repeated rubbing, often occurs at the moment of the sudden IT tract translocation/snapping sound.
ESHS is typically associated with repetitive physical activity/chronic overuse or activities that involve extremes of hip motion [43]. Thickening of the posterior IT band or anterior gluteus maximus is also associated with the development of ESHS [43, 54, 59]. Variant anatomy, such as a smaller femoral neck angle, narrower bi-iliac width, increased distance between the greater trochanters, or prominent greater trochanters, may predispose patients to ESHS as well [55, 61]. Other rare causes of ESHS include anatomical and/or biomechanical derangements resulting from surgery or other pathological conditions [62–65].
ESHS is common among athletes and dancers. Patients are typically young and physically active. Women may be more affected than men, but this finding may be confounded by the greater rates of intra-articular pathologies such as acetabular labral tears and hip dysplasia in women. In addition to pain and snapping, patients often report the feeling of hip subluxation or dislocation. Patients may report difficulty with activities such as running, climbing stairs, or heavy lifting. On examination, reproduction of the audible or palpable snap should be possible with a provocative test of femoral flexion and/or rotation [43].
Imaging
In practice, the diagnosis of ESHS is usually made by history and physical examination, but imaging can be used to rule out other diagnoses or confirm the diagnosis/involved structures. In particular, dynamic US examination is increasingly used to make the definitive diagnosis of ESHS. Plain radiographs are usually normal in ESHS but are potentially useful in helping exclude other pathologies [43].
More often, MRI will demonstrate findings suggestive of ESHS. T1 imaging may demonstrate hypointense thickening of the proximal IT tract, while T2 imaging may show thickening and hyperintensity of the proximal IT tract. Hyperintense greater trochanteric bursal inflammation or a fluid collection may also be visualized on T2 (Fig. 8); however, this finding is non-specific and should only be considered pathologic when the appropriate clinical signs and symptoms are also present. Post-contrast imaging can show peritendinous enhancement of involved structures [66].
A case series of three patients suggested that atrophy of the gluteus maximus muscle on MRI could be a secondary sign of ESHS. The authors hypothesized that this finding could be due to gait changes made by patients to avoid pain [59].
Similar to MRI, a conventional sonographic examination may reveal IT tract tendinopathy (increased thickness, heterogeneous echogenicity) or greater trochanteric bursitis [46, 54, 59]. A dynamic US examination is usually successful in demonstrating the translation of the IT tract/gluteus maximus over the greater trochanter in ESHS; this movement can be correlated in real time to the audible or palpable snap perceived by the examiner or pain reported by the patient, allowing for the definitive diagnosis of ESHS [44, 46, 54, 59, 60]. Dynamic US can also visualize movement of the iliopsoas tendon over the iliopectineal eminence (or the psoas tendon rolling over the iliacus muscle), thus differentiating between internal and external snapping hip when a physical examination is inconclusive [46, 57, 58].
In evaluating for ESHS, the US examination is performed with the transducer transverse to the greater trochanter [57]. The patient can be supine or standing, but normal gluteus maximus contraction with weight bearing may be necessary to reproduce the snap/abrupt translation of the IT tract or gluteus maximus [54, 59]. Common maneuvers to elicit the pain/snap include flexion of an adducted, extended hip and external rotation of an adducted, internally rotated hip [54, 57]. It is recommended that both active and passive movements be used when evaluating for ESHS [57].
Treatment
ESHS is usually first treated conservatively with rest, avoidance of aggravating activities, stretching of the IT tract, and NSAIDs. Unresponsive patients can undergo injection of local anesthetic and corticosteroids into the trochanteric bursa. Between one-third and two-thirds of patients see symptom response or resolution with conservative management. Refractory cases are sometimes treated with surgical lengthening of the IT tract with Z-plasty or endoscopic IT tract release [43].
Iliotibial band syndrome and iliotibial bursitis
Iliotibial band syndrome (ITBS), sometimes referred to as iliotibial band friction syndrome, refers to pain around the lateral femoral epicondyle that is associated with lower limb activity. Typically, this activity involves repetitive motion of the lower limb, such as that encountered in running or cycling. ITBS is usually due to chronic overuse, so that the classic presentation of a patient with ITBS is an active young person with lateral knee pain that increases throughout an episode of physical activity.
ITBS is thought to be the most common running injury of the lateral knee, and it is commonly diagnosed in other active populations such as cyclists, soccer players, and basketball players [67]. In an epidemiological study of military recruits undergoing basic training, Linenger and West found ITBS to account for 22% of all lower extremity injuries [68].
Renne first described ITBS in 1975 and thought it was due to the IT tract rubbing back and forth across the lateral femoral epicondyle during repetitive knee extension and flexion, causing friction and resultant irritation of the IT tract and periosteum or a possible bursa of the lateral epicondyle [69]. Renne offered this etiology based on Kaplan [12], who concluded the IT tract passed over the lateral epicondyle free of bony attachments to insert on the tibia and moved anterior and posterior with knee extension and flexion [69]. Thus, irritation of the IT tract caused by friction of its movement against the lateral epicondyle has been the historical understanding of the etiology of ITBS.
This understanding has since been challenged [18, 36, 67, 70–73]. First, there is debate as to what direction and to what extent the IT tract moves as the knee ranges. Fairclough et al. considered the anatomy of the IT tract to preclude any anterior-posterior movement about the lateral epicondyle since the IT tract is simply a thickening of the lateral fascia and is fixed to the linea aspera through the lateral intermuscular septum [18]. On MR imaging, their study demonstrated medial-lateral rather than anterior-posterior movement of the IT tract about the lateral epicondyle with knee flexion and extension. More recently, however, a sonographic evaluation of the IT tract during knee flexion and extension did demonstrate anterior-posterior movement of the IT tract relative to the lateral epicondyle [74].
Second, several studies suggest that irritation of a cyst, bursa, or synovial recess deep to the IT tract, rather than inflammation of the IT tract itself, is the true etiology of ITBS [70–72]. In addition, one histological examination of tissue from the lateral joint capsule in patients with chronic ITBS demonstrated synovial tissue, which was inflamed and thickened [70]. Further, documented successes with treating refractory ITBS with surgical resection of the lateral synovial recess, surgical resection of the sometimes identified sub-IT tract bursa, or surgical release of the IT tract attachment to the lateral epicondyle have been used to support this etiology [72, 75–79].
However, other studies based on MRI findings in patients with ITBS and cadavers show soft tissue signal changes in the sub-IT tract space without demonstrating a distinct cyst, bursa, or synovial recess [36, 80]. It is possible that compression of a vascularized fat pad in the sub-IT tract space is the cause of ITBS pain [18, 81]. Ultimately, ITBS may have multiple etiologic subtypes [67] or at least be more accurately understood as an impingement syndrome rather than a frictional one, given the relative lack of evidence for pathological changes occurring within the IT tract itself, and the possibility that the IT tract may not always move anterior-posterior relative to the lateral epicondyle.
With these anatomical considerations concerning the etiology of ITBS in mind, investigators have examined physical and biomechanical risk factors for the development of ITBS [67, 82–85]. Factors thought to increase the compression of structures underneath the IT tract, such as increased IT tract tightness, have generated conflicting evidence [67, 82]. However, the physical factors with the most robust association with ITBS include limb length discrepancy, genu varum, foot overpronation, hip adductor weakness, and myofascial restriction [35, 82, 85–87].
Regardless of the etiology of ITBS, patients usually have a consistent clinical presentation. Pain in the region of the distal IT tract between the lateral femoral epicondyle and Gerdy’s tubercle of the tibia is a consistent complaint, usually with a history of regular athletic activity. Patients often notice the onset of lateral knee pain late in or after completing an activity, and as the syndrome progresses, pain begins earlier in the course of activity. Running downhill is a typical and reproducible aggravating factor. Physical examination reveals tenderness to palpation at the lateral epicondyle, approximately 2–3 cm proximal to the joint line [86].
Iliotibial bursitis is a rare sequela of chronic ITBS. As discussed previously, MRI studies have demonstrated focal fluid collections between the IT tract and the lateral femoral epicondyle in patients with ITBS, but anatomical investigations of normal knees have failed to consistently identify the gross and/or histological presence of a sub-IT tract bursa [81]. However, the focal fluid collections often identified on MRI in ITBS patients may represent the formation of an adventitial bursa due to chronic inflammation [36]. Continued irritation of this adventitial bursa can lead to chronic ITBS symptoms, which can be understood as iliotibial bursitis [72].
Surgical and histological case reports support the existence of pathological bursa in patients who suffer from refractory ITBS [72, 88]. Hariri et al. describe consistently identifying an apparent inflamed bursa underneath a benign-appearing IT tract in a case series of 12 patients undergoing surgery for recalcitrant ITBS. They also describe high rates of clinical relief in these patients after surgical IT tract bursectomy [72].
The presentation of iliotibial bursitis is the same as ITBS. Patients are typically physically active long distance runners or cyclists with lateral knee pain localized to the lateral femoral condyle that is exacerbated by activity. They may have a history of being treated for ITBS. Physical examination usually reveals tenderness at the lateral femoral epicondyle that is worst with 30° of flexion [72, 88].
Imaging
The diagnosis of ITBS can typically be made by history and physical examination, with imaging reserved for recurrent or refractory cases [86]. In patients with ITBS, routine radiographs, including anterior-posterior (AP), lateral, and sunrise views, are typically normal, but can be used to help exclude other knee pathology [69, 86, 89]. In contrast, characteristic MRI findings associated with ITBS have been described [36, 86].
Most consistently, MR imaging demonstrates increased fluid signal in the fat between the ITB and the lateral femoral condyle (usually superolateral to the lateral joint recess) (Fig. 9) [34, 36, 37, 89, 90]. However, mild hyperintensity on fluid-sensitive sequences in this region is not infrequently seen in older patients and should only be considered pathologic when the appropriate clinical signs and symptoms are also present. This area of edema can be up to several centimeters in size. A discrete fluid collection may be visualized in this space [34, 36, 37]. The IT tract may be thickened [37], but some studies have questioned this finding, suggesting it only occurs in a subset of chronic ITBS cases [36, 89, 90]. Additionally, reactive edema in the lateral epicondyle may be present. Less frequently, reactive bursitis can occur [36].
A 28-year-old female with left knee pain after running a marathon. Coronal STIR MR image demonstrates thickening of the iliotibial band with increased intrasubstance signal intensity (arrowhead) with edema both deep (arrow) and superficial (curved arrow) at the level of the lateral femoral epicondyle, consistent with iliotibial band syndrome
It should be noted that relatively few published studies have sought to investigate what MR findings are associated with ITBS. In 1992, Murphy et al. evaluated six patients with ITBS by clinical history and physical examination. MR imaging demonstrated ill-defined, decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images deep to the IT tract adjacent to the lateral femoral epicondyle; there were no signal intensity abnormalities in the IT tract itself [34].
In a case series comparing MR imaging of ITBS patients and age-and-sex matched controls, Ekman et al. similarly demonstrated increased fluid signal over the lateral epicondyle of ITBS patients, but determined this signal to represent discrete, localized fluid collections. The study also showed thickening of the distal IT tract in most of the ITBS cases [37, 86].
A subsequent case series of four ITBS patients by Nishimura et al. suggested soft tissue inflammation and/or edema rather than focal fluid collection in the space between the IT tract and the lateral epicondyle. The ITB itself did not show any signal alteration or increased thickness [89].
In 1999, Muhle et al. attempted to define MRI findings in ITBS patients and to correlate these findings with anatomic features defined by MR arthrography in cadavers. This study supported the findings of Murphy et al. and Nishimura et al., demonstrating ill-defined signal intensity alterations in the fatty tissue deep to the IT tract in the majority of ITBS patients. A minority of ITBS patients had well-defined fluid collections in the same distribution as this soft-tissue edema. The authors concluded these well-defined fluid collections likely represented secondary bursa formation as a result of chronic inflammation. The study did not find a significant difference in the thickness of the IT tract in ITBS patients versus control patients [36].
While MRI is considered the gold-standard for diagnosing ITBS in patients with unclear presentations and physical examinations, US has also been used successfully to diagnose some cases. The use of US in diagnosing ITBS is not well documented in the literature, but it has been proposed that US findings in ITBS can correlate well with MRI findings. US has been shown to demonstrate edematous swelling of the soft tissues between the IT tract and the femoral epicondyle in ITBS, similar to what is seen on MRI, although the sensitivity is likely lower [42, 91].
In iliotibial bursitis, MRI will demonstrate a well-defined fluid collection between the IT tract and the lateral femoral condyle (Fig. 10), although some sources suggest the adventitial bursa may be between the IT tract and the tibia, close to its insertion onto the tibia [72, 92, 93]. This fluid collection is best seen on short tau inversion recovery (STIR) or T2 fat-suppressed (T2FS) imaging. Signal abnormalities within the IT tract should be minimal or not visualized at all [92, 93].
Treatment
ITBS is almost always managed non-surgically. Rest from the inciting activity followed by gradual return to activity is a mainstay of treatment [86]. Physical therapy and manual therapy also play important roles [85, 86]. Oral non-steroidal anti-inflammatory drugs (NSAIDs), local corticosteroid injections, or both can be used to control acute inflammation, although corticosteroid injections have been shown to alleviate symptoms more effectively [86, 94]. Response to corticosteroid injection can be used to help diagnose ITBS as well [86, 95].
Recalcitrant cases of ITBS with functional impairment are sometimes treated with surgery [86]. As previously discussed, favorable results have been reported with several surgical treatments. These include surgical release of the IT tract, IT tract bursectomy, and surgical resection of the lateral synovial recess [72, 75–79].
Similar to ITBS, iliotibial bursitis can initially be treated conservatively with rest and NSAIDs to reduce inflammation and potentially allow the bursa to heal. Truly refractory cases in patients with poor symptom response despite adherence to nonoperative measures can be treated with surgery. IT tract bursectomy, as previously mentioned, has been used in these patients as well as surgical IT tract release [72, 88].
Iliotibial band injury in acute knee trauma
IT tract injury is common in the setting of acute knee trauma. However, perhaps because the IT tract is rarely injured in isolation, there is relatively little consideration of discrete IT tract injury as a topic of inquiry in the literature. In addition, while the IT tract presumably acts synergistically with the other lateral stabilizers of the knee, the clinical significant of its injury is unclear.
IT tract injury may occur in as many as 58% of cases of acute knee trauma [6]. In patients undergoing surgery for functional knee instability due to complete tears of a posterolateral structure, the deep layer of the IT tract may be the most commonly damaged structure [96]. However, isolated injury to the IT tract is rare as this is thought to require purely varus force, which rarely occurs [97, 98]. In acute knee trauma, the incidence may be as low as 2% [6].
In up to two-thirds of cases of IT tract injury the ACL will also be torn. In up to 20% of cases, patellar dislocation will also have occurred. Additionally, when an ACL tear or patellar dislocation is identified, injury to the IT tract is common (∼70 and ∼74% of cases, respectively). As determined by MRI, most IT tract injuries are sprains. Complete tears are rare [6].
Imaging
MRI is well established as the first-line imaging modality in evaluating traumatic internal derangement of the knee [99], but discussion of IT injury, particularly sprains, as a discrete imaging findings is lacking [6]. Mansour et al. classified IT tract injuries as grade 0–3 [6], similar to grading for other ligamentous injuries [100].
The IT tract is classified as grade 0 if it is normal (thin, intact, low signal intensity). Grade 1 injuries (minor sprains) demonstrate edema superficial and deep to an otherwise intact IT tract. Grade 2 injuries (severe sprains) show edema adjacent to a partial tear of the IT tract, with some intact fibers. Grade 3 injuries (tears) demonstrate a grossly torn, discontinuous IT band with waviness of the torn fibers (Fig. 11) [6].
Treatment
The clinical significance of IT tract injury in acute knee trauma is unclear, but it likely contributes to knee instability, especially in cases with concomitant ACL tear [6, 25]. If the IT tract injury is part of a broader injury pattern contributing to knee instability, surgical repair is generally indicated [33].
Iliotibial tract insertional tendinosis and peritendonitis
Most descriptions of IT “tendinitis,” “tendinosis,” or “tendinopathy” in the literature are equated with ITBS. However, this should occur only at or near the level of the lateral femoral epicondyle. Unlike ITSB, insertional tendinosis and peritendonitis are not related to chronic compression or friction. The pathogenesis of insertional tendinosis usually involves aging and overuse, particularly in unstable joints, due to osteoarthrosis [101].
The IT tract lacks a tendon sheath, but can have inflammation of its paratenon, similar to the Achilles, quadriceps, and patellar tendons. Presumably, its distal insertion may be affected by any disorder that causes enthesopathy, e.g., chronic repetitive stress, spondyloarthropathies, diffuse idiopathic skeletal hyperostosis (DISH), calcium pyrophosphate deposition disease (CPPD), or endocrine (hyperparathyroidism, acromegaly) or metabolic (fluorosis) disorders. The incidence of iliotibial insertional tendinosis/peritendonitis has not been described in the literature, but is probably under-recognized and under-reported.
Imaging
There are few reports describing the imaging findings associated with iliotibial insertional tendinosis/peritendonitis; however, the findings are the same as those reported with tendinosis of other tendons, such as the Achilles tendon. MRI may demonstrate thickening and increased intrasubstance intermediate signal intensity, most prominent on fluid-sensitive sequences (Fig. 12). In cases with associated peritendonitis, there will be adjacent edema. On ultrasound, the site of highest pain sensitivity will correspond to a region of thickening and hypoechogenicity of the iliotibial band [101].
Treatment
In general, treatment is conservative, consisting of rest, ice, physical therapy, and NSAIDs. In refractory cases, ultrasound-guided tenotomy or prolotherapy may improve patient symptoms [102].
Avulsion fracture at Gerdy’s Tubercle
Avulsion fractures of the IT tract’s insertion at Gerdy’s tubercle are rare [33, 97, 98]. The mechanism of injury is thought to be most commonly a direct blow to the anteromedial proximal tibia, directed posterolaterally, with the knee near full extension [97, 98]. However, case reports exist of isolated avulsions with jumping [103].
When encountered, avulsion fractures of Gerdy’s tubercle are usually associated with injuries to the other posterolateral corner structures of the knee. Isolated tears of the IT tract are thought to require a pure varus force, which is rarely seen. Patients will have severe pain with weight bearing and point tenderness over Gerdy’s tubercle. Concomitant ACL injury is a common finding in these patients [97, 98].
Imaging
Plain radiographs, CT, and MRI have potential roles in the diagnosis and treatment of avulsion fractures at Gerdy’s tubercle. A plain radiograph can demonstrate the avulsed osseous fragment at the IT band insertion site on Gerdy’s tubercle. Associated findings may include a lipohemarthrosis, suggestive of an intra-articular fracture, and/or widening of the lateral compartment, suggestive of a ligamentous injury [98]. CT can facilitate recognition of the extent of the fractured fragment as well as aide in determining the surgical approach and methods for internal fixation [103].
On MRI, there will be a linear T2 hyperintense/T1 hypointense fracture line with adjacent bone marrow edema at Gerdy’s tubercle for non-displaced fractures (Fig. 13) [97]. There will be a gap between the ossific fragments for displaced fractures with waviness of the retracted distal IT band fibers.
A 33-year-old male with right knee pain after a fall. AP radiograph (a) and coronal STIR MR image (b) demonstrate a minimally displaced avulsion fracture of Gerdy’s tubercle at the iliotibial band insertion (arrows) with associated bone marrow edema on the MRI. There is also a medial tibial plateau fracture (curved arrows)
Treatment
Conservative treatment for avulsion fractures at Gerdy’s tubercle is usually forgone in favor of open reduction with cancellous screw fixation of the fracture fragment to the tibia using a lateral parapatellar approach [98, 103].
Segond fracture
The Segond fracture is an avulsion fracture involving the proximal tibia immediately distal to the lateral plateau as a result of varus stress with internal rotation of the knee. It is well established that Segond fractures are associated with ACL tears, meniscal tears, and damage to the other structures of posterolateral corner of the knee. Clinical diagnosis of Segond fractures in the acute stage can be difficult because of pain, muscle spasm, and/or swelling, so radiologic recognition of this bony injury is important as it could reflect ligamentous derangement with significant instability of the knee [104].
The mechanism of injury in Segond fractures is generally understood to be varus stress applied to an internally rotated knee, which causes abnormal tension on the lateral joint capsule at its midpoint [97, 104]. Historically, this tension was thought to produce an avulsion facture of the lateral tibial plateau at the insertion of the lateral capsular ligament. However, the precise anatomic structure causing the avulsion has been extensively debated, and the pathogenesis is likely more complex than the historical understanding. In the past, this avulsion has been described as being at the insertion of the capsular-osseous layer (posterior slip) of the IT tract, lateral capsular ligament, and/or anterior oblique band of fibular collateral ligament, but recent literature suggests that this primary occurs at the insertion of the anterolateral ligament [97, 104–106]. Although there is likely overlap of the anterolateral ligament with some of these other previously described structures based on differences in terminology and dissection techniques, the course of this ligament from the lateral femoral epicondyle to the lateral tibial plateau correlates with the Segond fracture site, and there is general consensus in the current literature that the anterolateral ligament is the cause of the Segond fracture [107–111].
Patients with Segond fractures often present with pain at the lateral joint line, swelling, and anterolateral rotational instability [97, 104, 112]. In 75–100% of cases, patients will have an associated ACL tear, and an associated medial meniscal tear will be present in 66–75% of cases [104]. Segond fractures may also be associated with avulsion of the fibular attachment of the long head of the biceps femoris tendon and the fibular collateral ligament [97, 112]. More rarely, patients will also have an avulsion of the ACL from its insertion anterior to the tibial eminence [97, 112].
Imaging
Plain radiographs may reveal an elliptical osseous fragment parallel to the tibia, just distal to the lateral tibial plateau; this finding has been referred to as the “lateral capsular sign” [97]. However, the avulsed fragment may not be visualized because of its small size and/or projection inadequacies [112]. Proper AP views may help visualize the fragment, but CT imaging may be needed to demonstrate it best [47]. Additionally, case reports exist of US revealing a chronic Segond fracture after plain radiographs failed to demonstrate a fragment in a patient presenting with persistent knee pain 6 months after a trauma [113].
MRI should show abnormal signal intensity (low signal on T1, high signal on T2) in the marrow along the lateral tibial rim, representing bone marrow contusion/edema [97, 112]. The avulsed fragment may or may not be visualized [97, 106, 112]. Other associated findings include abnormal signal intensities in the joint capsule (capsular edema) and paracapsular connective tissue (paracapsular edema) [112], including fluid surrounding the IT tract [106]. The anterolateral ligament can be visualized in almost all cases, even at 1.5-T MRI, with meniscal, femoral, and tibial portions, best visualized as a thin, linear, hypointense structure in the coronal plane attaching to the Segond fracture fragment [110, 114]. A joint effusion may also be present. In all cases of Segond fracture, MR imaging should be obtained to evaluate for concomitant ligamentous or meniscal injury [97].
Treatment
Segond fractures alone do not necessarily require operative fixation. Patients have the option to be treated with physiotherapy. However, operative treatment of any associated injury, such as an ACL tear, contributing to knee instability is usually undertaken [113, 115].
Conclusion
As an anatomical structure, the IT tract has been known in the literature for quite some time, yet its precise anatomy remains somewhat elusive. However, its contribution to knee stability is relatively well established, and the pathologies related to the IT tract are numerous and common. In addition, these pathologies often carry significant morbidity for patients. Plain radiography, CT, MR, and US imaging potentially all have a role in the diagnosis of the wide array of IT tract pathology. As such, the radiologist should have a firm appreciation of the complex anatomy of the IT tract, as well as the various imaging findings associated with its pathology. A clear understanding of the clinical presentation and pathophysiology of conditions affecting the IT tract are often helpful in making a correct diagnosis as well.
References
Gray H. Anatomy of the human body. 20th edition ed. Philadelphia: Lea & Febiger; 1918.
Vieira EL, Vieira EA, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269–74.
Hirschmann MT, Müller W. Complex function of the knee joint: the current understanding of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2780–8.
Vinson EN, Major NM, Helms CA. The posterolateral corner of the knee. AJR Am J Roentgenol. 2008;190(2):449–58.
Gage BE, McIlvain NM, Collins CL, Fields SK, Comstock RD. Epidemiology of 6.6 million knee injuries presenting to United States emergency departments from 1999 through 2008. Acad Emerg Med. 2012;19(4):378–85.
Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369–75.
Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12(3):200–8.
Vesalius A. The Epitome of Andrea Vesalius. New York: The Macmillan Company, 1949: 38.
Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14(1):39–45.
Gerdy PN. Troisième monographie maladies des organes du mouvement os muscles. Paris: chez Victor Masson. 1855.
Segond PF. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Prog méd. 1879:1–85.
KAPLAN EB. The iliotibial tract; clinical and morphological significance. J Bone Joint Surg Am. 1958;40-A(4):817–32.
Sher I, Umans H, Downie SA, Tobin K, Arora R, Olson TR. Proximal iliotibial band syndrome: what is it and where is it? Skeletal Radiol. 2011;40(12):1553–6.
Birnbaum K, Siebert CH, Pandorf T, Schopphoff E, Prescher A, Niethard FU. Anatomical and biomechanical investigations of the iliotibial tract. Surg Radiol Anat. 2004;26(6):433–46.
Netter FH. Atlas of Human Anatomy. 6th Edition ed. Philadelphia: Saunders; 2014.
Huang BK, Campos JC, Michael Peschka PG, et al. Injury of the gluteal aponeurotic fascia and proximal iliotibial band: anatomy, pathologic conditions, and MR imaging. Radiographics. 2013;33(5):1437–52.
Daggett M, Claes S, Helito CP, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee: letter to the editor. Am J Sports Med. 2016;44(4):NP14–5.
Fairclough J, Hayashi K, Toumi H, et al. The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat. 2006;208(3):309–16.
Evans P. The postural function of the iliotibial tract. Ann R Coll Surg Engl. 1979;61(4):271–80.
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–8.
Lieb FJ, Perry J. Quadriceps function. An anatomical and mechanical study using amputated limbs. J Bone Joint Surg Am. 1968;50(8):1535–48.
Kennedy JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior cruciate ligament. As determined by clinical and morphological studies. J Bone Joint Surg Am. 1974;56(2):223–35.
Kennedy JC, Stewart R, Walker DM. Anterolateral rotatory instability of the knee joint. An early analysis of the Ellison procedure. J Bone Joint Surg Am. 1978;60(8):1031–9.
Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–9.
Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21(1):55–60.
Kosy JD, Soni A, Venkatesh R, Mandalia VI. The anterolateral ligament of the knee: unwrapping the enigma. Anatomical study and comparison to previous reports. J Orthop Traumatol. 2016.
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3186–95.
Vincent JP, Magnussen RA, Gezmez F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):147–52.
Stijak L, Bumbaširević M, Radonjić V, et al. Anatomic description of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2083–8.
Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
LaPrade RF. Editorial commentary: defining the anatomy of the anterolateral aspect of the knee among experts is clearly needed. Arthroscopy. 2016;32(5):842–3.
LaPrade RF. Editorial commentary: it is all about how one defines the anatomy. Arthroscopy. 2016;32(5):849–50.
Haims AH, Medvecky MJ, Pavlovich R, Katz LD. MR imaging of the anatomy of and injuries to the lateral and posterolateral aspects of the knee. AJR Am J Roentgenol. 2003;180(3):647–53.
Murphy BJ, Hechtman KS, Uribe JW, Selesnick H, Smith RL, Zlatkin MB. Iliotibial band friction syndrome: MR imaging findings. Radiology. 1992;185(2):569–71.
Vasilevska V, Szeimies U, Stäbler A. Magnetic resonance imaging signs of iliotibial band friction in patients with isolated medial compartment osteoarthritis of the knee. Skeletal Radiol. 2009;38(9):871–5.
Muhle C, Ahn JM, Yeh L, et al. Iliotibial band friction syndrome: MR imaging findings in 16 patients and MR arthrographic study of six cadaveric knees. Radiology. 1999;212(1):103–10.
Ekman EF, Pope T, Martin DF, Curl WW. Magnetic resonance imaging of iliotibial band syndrome. Am J Sports Med. 1994;22(6):851–4.
Wang HK, Ting-Fang Shih T, Lin KH, Wang TG. Real-time morphologic changes of the iliotibial band during therapeutic stretching; an ultrasonographic study. Man Ther. 2008;13(4):334–40.
Goh LA, Chhem RK, Wang SC, Chee T. Iliotibial band thickness: sonographic measurements in asymptomatic volunteers. J Clin Ultrasound. 2003;31(5):239–44.
Gyaran IA, Spiezia F, Hudson Z, Maffulli N. Sonographic measurement of iliotibial band thickness: an observational study in healthy adult volunteers. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):458–61.
De Maeseneer M, Marcelis S, Boulet C, et al. Ultrasound of the knee with emphasis on the detailed anatomy of anterior, medial, and lateral structures. Skeletal Radiol. 2014;43(8):1025–39.
Bonaldi VM, Chhem RK, Drolet R, Garcia P, Gallix B, Sarazin L. Iliotibial band friction syndrome: sonographic findings. J Ultrasound Med. 1998;17(4):257–60.
Lewis CL. Extra-articular snapping hip: a literature review. Sports Health. 2010;2(3):186–90.
Chang KS, Cheng YH, Wu CH, Özçakar L. Dynamic ultrasound imaging for the iliotibial band/snapping hip syndrome. Am J Phys Med Rehabil. 2015;94(6):e55–6.
Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142–50.
Pelsser V, Cardinal E, Hobden R, Aubin B, Lafortune M. Extraarticular snapping hip: sonographic findings. AJR Am J Roentgenol. 2001;176(1):67–73.
Bonilla-Yoon I, Masih S, Patel DB, et al. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2014;21(1):35–43.
Scolaro JA, Chao T, Zamorano DP. The Morel-Lavallée lesion: diagnosis and management. J Am Acad Orthop Surg. 2016;24(10):667-72.
Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775–82.
Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289–94.
Reddix RN, Carroll E, Webb LX. Early diagnosis of a Morel-Lavallee lesion using three-dimensional computed tomography reconstructions: a case report. J Trauma. 2009;67(2):E57–9.
Neal C, Jacobson JA, Brandon C, Kalume-Brigido M, Morag Y, Girish G. Sonography of Morel-Lavallee lesions. J Ultrasound Med. 2008;27(7):1077–81.
Choudhary AK, Methratta S. Morel-Lavallée lesion of the thigh: characteristic findings on US. Pediatr Radiol. 2010;40 Suppl 1:S49.
Choi YS, Lee SM, Song BY, Paik SH, Yoon YK. Dynamic sonography of external snapping hip syndrome. J Ultrasound Med. 2002;21(7):753–8.
Allen WC, Cope R. Coxa Saltans: The snapping hip revisited. J Am Acad Orthop Surg. 1995;3(5):303–8.
Schaberg JE, Harper MC, Allen WC. The snapping hip syndrome. Am J Sports Med. 1984;12(5):361–5.
Zbojniewicz AM. US for diagnosis of musculoskeletal conditions in the young athlete: emphasis on dynamic assessment. Radiographics. 2014;34(5):1145–62.
Deslandes M, Guillin R, Cardinal E, Hobden R, Bureau NJ. The snapping iliopsoas tendon: new mechanisms using dynamic sonography. AJR Am J Roentgenol. 2008;190(3):576–81.
Krishnamurthy G, Connolly BL, Narayanan U, Babyn PS. Imaging findings in external snapping hip syndrome. Pediatr Radiol. 2007;37(12):1272–4.
Chang CY, Kreher J, Torriani M. Dynamic sonography of snapping hip due to gluteus maximus subluxation over greater trochanter. Skeletal Radiol. 2016;45(3):409–12.
Larsen E, Johansen J. Snapping hip. Acta Orthop Scand. 1986;57(2):168–70.
Inoue S, Noguchi Y, Mae T, Rikimaru S, Hotokezaka S. An external snapping hip caused by osteochondroma of the proximal femur. Mod Rheumatol. 2005;15(6):432–4.
Satku K, Chia J, Kumar VP. Snapping hip—an unusual cause. J Bone Joint Surg (Br). 1990;72(1):150–1.
Battaglia M, Guaraldi F, Monti C, Vanel D, Vannini F. An unusual cause of external snapping hip. J Radiol Case Rep. 2011;5(10):1–6.
Larsen E, Gebuhr P. Snapping hip after total hip replacement. A report of four cases. J Bone Joint Surg Am. 1988;70(6):919–20.
Hodnett PA, Shelly MJ, MacMahon PJ, Kavanagh EC, Eustace SJ. MR imaging of overuse injuries of the hip. Magn Reson Imaging Clin N Am. 2009;17(4):667–79. vi.
Lavine R. Iliotibial band friction syndrome. Curr Rev Musculoskelet Med. 2010;3(1–4):18–22.
Linenger JM, West LA. Epidemiology of soft-tissue/musculoskeletal injury among US Marine recruits undergoing basic training. Mil Med. 1992;157(9):491–3.
Renne JW. The iliotibial band friction syndrome. J Bone Joint Surg Am. 1975;57(8):1110–1.
Nemeth WC, Sanders BL. The lateral synovial recess of the knee: anatomy and role in chronic Iliotibial band friction syndrome. Arthroscopy. 1996;12(5):574–80.
Costa ML, Marshall T, Donell ST, Phillips H. Knee synovial cyst presenting as iliotibial band friction syndrome. Knee. 2004;11(3):247–8.
Hariri S, Savidge ET, Reinold MM, Zachazewski J, Gill TJ. Treatment of recalcitrant iliotibial band friction syndrome with open iliotibial band bursectomy: indications, technique, and clinical outcomes. Am J Sports Med. 2009;37(7):1417–24.
Fairclough J, Hayashi K, Toumi H, et al. Is iliotibial band syndrome really a friction syndrome? J Sci Med Sport. 2007;10(2):74–6. discussion 7–8.
Jelsing EJ, Finnoff JT, Cheville AL, Levy BA, Smith J. Sonographic evaluation of the iliotibial band at the lateral femoral epicondyle: does the iliotibial band move? J Ultrasound Med. 2013;32(7):1199–206.
Cowden CH, Barber FA. Arthroscopic treatment of iliotibial band syndrome. Arthrosc Tech. 2014;3(1):e57–60.
Lui TH. Endoscopic resection of lateral synovial cyst of the knee. Arthrosc Tech. 2015;4(6):e815–8.
Holmes JC, Pruitt AL, Whalen NJ. Iliotibial band syndrome in cyclists. Am J Sports Med. 1993;21(3):419–24.
Michels F, Jambou S, Allard M, Bousquet V, Colombet P, de Lavigne C. An arthroscopic technique to treat the iliotibial band syndrome. Knee Surg Sports Traumatol Arthrosc. 2009;17(3):233–6.
Martens M, Libbrecht P, Burssens A. Surgical treatment of the iliotibial band friction syndrome. Am J Sports Med. 1989;17(5):651–4.
Isusi M, Oleaga L, Campo M, Grande D. MRI findings in iliotibial band friction syndrome: a report of two cases. Radiologia. 2007;49(6):433–5.
Grando H, Chang EY, Chen KC, Chung CB. MR imaging of extrasynovial inflammation and impingement about the knee. Magn Reson Imaging Clin N Am. 2014;22(4):725–41.
van der Worp MP, van der Horst N, de Wijer A, Backx FJ. Nijhuis-van der Sanden MW. Iliotibial band syndrome in runners: a systematic review. Sports Med. 2012;42(11):969–92.
Aderem J, Louw QA. Biomechanical risk factors associated with iliotibial band syndrome in runners: a systematic review. BMC Musculoskelet Disord. 2015;16:356.
Louw M, Deary C. The biomechanical variables involved in the aetiology of iliotibial band syndrome in distance runners—A systematic review of the literature. Phys Ther Sport. 2014;15(1):64–75.
Baker RL, Fredericson M. Iliotibial band syndrome in runners: biomechanical implications and exercise interventions. Phys Med Rehabil Clin N Am. 2016;27(1):53–77.
Strauss EJ, Kim S, Calcei JG, Park D. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19(12):728–36.
Noehren B, Schmitz A, Hempel R, Westlake C, Black W. Assessment of strength, flexibility, and running mechanics in men with iliotibial band syndrome. J Orthop Sports Phys Ther. 2014;44(3):217–22.
Sangkaew C. Surgical treatment of iliotibial band friction syndrome with the mesh technique. Arch Orthop Trauma Surg. 2007;127(4):303–6.
Nishimura G, Yamato M, Tamai K, Takahashi J, Uetani M. MR findings in iliotibial band syndrome. Skeletal Radiol. 1997;26(9):533–7.
O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725–39. vii.
Draghi F, Danesino GM, Coscia D, Precerutti M, Pagani C. Overload syndromes of the knee in adolescents: sonographic findings. J Ultrasound. 2008;11(4):151–7.
Marra MD, Crema MD, Chung M, et al. MRI features of cystic lesions around the knee. Knee. 2008;15(6):423–38.
Beaman FD, Peterson JJ. MR imaging of cysts, ganglia, and bursae about the knee. Radiol Clin North Am. 2007;45(6):969–82. vi.
Gunter P, Schwellnus MP. Local corticosteroid injection in iliotibial band friction syndrome in runners: a randomised controlled trial. Br J Sports Med. 2004;38(3):269–72. discussion 72.
Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26(4):387–91.
LaPrade RF, Gilbert TJ, Bollom TS, Wentorf F, Chaljub G. The magnetic resonance imaging appearance of individual structures of the posterolateral knee. A prospective study of normal knees and knees with surgically verified grade III injuries. Am J Sports Med. 2000;28(2):191–9.
Gottsegen CJ, Eyer BA, White EA, Learch TJ, Forrester D. Avulsion fractures of the knee: imaging findings and clinical significance. Radiographics. 2008;28(6):1755–70.
Fay K, Mannem R, Baynes K, Sarin D, DuBois M. Iliotibial band avulsion fracture: a case report with differential diagnosis. Emerg Radiol. 2016;23(1):93–6.
Mackenzie R, Dixon AK, Keene GS, Hollingworth W, Lomas DJ, Villar RN. Magnetic resonance imaging of the knee: assessment of effectiveness. Clin Radiol. 1996;51(4):245–50.
Association AM. Standard Nomenclature of Athletic Injuries. Chicago: American Medical Association; 1968.
Arend CF. Sonography of the iliotibial band: spectrum of findings. Radiol Bras. 2014;47:33–7.
Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187–92.
Yoo JH, Hahn SH, Yang BK, et al. An en bloc avulsion fracture of tibial tuberosity and Gerdy’s tubercle in an adolescent basketball player: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(6):781–5.
Campos JC, Chung CB, Lektrakul N, et al. Pathogenesis of the Segond fracture: anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology. 2001;219(2):381–6.
Claes S, Luyckx T, Vereecke E, Bellemans J. The Segond fracture: a bony injury of the anterolateral ligament of the knee. Arthroscopy. 2014;30(11):1475–82.
De Maeseneer M, Boulet C, Willekens I, et al. Segond fracture: involvement of the iliotibial band, anterolateral ligament, and anterior arm of the biceps femoris in knee trauma. Skeletal Radiol. 2015;44(3):413–21.
Helito CP, Helito PV, Bonadio MB, et al. Correlation of magnetic resonance imaging with knee anterolateral ligament anatomy: a cadaveric study. Orthop J Sports Med. 2015;3(12):2325967115621024.
Helito CP, Helito PV, Costa HP, Demange MK, Bordalo-Rodrigues M. Assessment of the anterolateral ligament of the knee by magnetic resonance imaging in acute injuries of the anterior cruciate ligament. Arthroscopy. 2017;33(1):140–6.
Kosy JD, Mandalia VI, Anaspure R. Characterization of the anatomy of the anterolateral ligament of the knee using magnetic resonance imaging. Skeletal Radiol. 2015;44(11):1647–53.
Porrino Jr J, Maloney E, Richardson M, Mulcahy H, Ha A, Chew FS. The anterolateral ligament of the knee: MRI appearance, association with the Segond fracture, and historical perspective. AJR Am J Roentgenol. 2015;204(2):367–73.
Van Dyck P, Clockaerts S, Vanhoenacker FM, et al. Anterolateral ligament abnormalities in patients with acute anterior cruciate ligament rupture are associated with lateral meniscal and osseous injuries. Eur Radiol. 2016;26(10):3383–91.
Weber WN, Neumann CH, Barakos JA, Petersen SA, Steinbach LS, Genant HK. Lateral tibial rim (Segond) fractures: MR imaging characteristics. Radiology. 1991;180(3):731–4.
Boutry N, Dupont S, Glaude E, Demondion X, Laffargue P, Cotten A. Segond fracture revealed by ultrasonography. J Ultrasound Med. 2005;24(10):1431–5.
Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421–7.
Davis DS, Post WR. Segond fracture: lateral capsular ligament avulsion. J Orthop Sports Phys Ther. 1997;25(2):103–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Grants received
None
Disclosures
None
Rights and permissions
About this article
Cite this article
Flato, R., Passanante, G.J., Skalski, M.R. et al. The iliotibial tract: imaging, anatomy, injuries, and other pathology. Skeletal Radiol 46, 605–622 (2017). https://doi.org/10.1007/s00256-017-2604-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2604-y