Abstract
Detergent enzymes are currently added to all powder and liquid detergents that are manufactured. Cellulases, lipases, amylases, and proteases are used in the detergency to replace toxic phosphates and silicates and to reduce high energy consumption. This makes the use of enzymes in detergent formulation cost effective. Fungi are producers of important extracellular enzymes for industrial use. The fungal and bacterial cellulases maintain the shape and color of the washed garments. There is a high demand for cellulases at the market by detergent industries. With this high demand, genetic engineering has been a solution due to its high production of detergent-compatible cellulases. Fungi are the famous source for detergent-compatible cellulases production, but still, there is a lack of the cost-effective process of alkaline fungal cellulase production. Review papers on detergent-compatible bacterial cellulase and amylase and detergent-compatible fungal and bacterial proteases and lipases are available, but there is no review on detergent fungal cellulases. This review aims to highlight the production, properties, stability, and compatibility of fungal cellulases. It will help other academic and industrial researchers to study, produce, and commercialize the fungal cellulases with good aspects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulase is an hydrolase enzyme, cleaving β-1,4-glycosidic bonds of cellulose or its derivatives like cellooligosaccharide, to glucose monomers. To completely hydrolyze these polymers to glucose units, three enzymes act synergistically. These are endoglucanases (EC 3.2.1.4) that break down internal glycosidic bonds, exoglucanases (EC 3.2.1.91) cleaving chain termini liberating cellodextrins, and β-glucosidases (EC 3.2.1.21) that liberate glucose units following cellodextrins degradation (Uhlig 1998; Sajith et al. 2016). Fungi are the microorganisms of choice to produce industrial cellulases owing to desired properties like extracellular secretion of an enzyme in huge amounts with cost-effective substrates (Bhat 2000; Ahmed and Bibi 2018). Detergent-compatible cellulases are easily obtained abundantly from fungi compared to plants and animals (Acharya and Chaudhary 2012). In addition, the genetic material of fungal species is easily cloned into the bacterial strain for overproduction of cellulases because fungal cellulases are less complex in structure compared to bacterial ones (Uhlig 1998; Maki et al. 2009; Acharya and Chaudhary 2012).
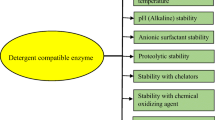
Cellulases are used in different industries such as detergent, biofuel, food, textile, cosmetics, feed, chemicals, pulp, and paper (Acharya and Chaudhary 2012; Juturu and Wu 2014; Sajith et al. 2016). The fungal cellulases are importantly used in the detergent preparation to aid in the defibrillation processes. They increase the color brightness/softness of the fabric and minimize the defibrillation cost (Miettinen-Oinonen and Suominen 2002; Koga et al. 2008). Oxidizing agents, anionic and non-ionic detergents, are the constituents of a detergent. The bacterial and fungal enzymes are stable and resistant to oxidizing agents (Niyonzima and More 2014c). In the detergency, the stability at alkaline pH is also a prerequisite for fungal cellulases (Miettinen-Oinonen and Suominen 2002; Koga et al. 2008; Niyonzima 2019). For instance, the cellulase of Humicola insolens that was active in the alkaline region of pH 8.5–9.0 and at temperature of 50 °C was used in washing powder to remove soil-related dirtiness. It was then commercialized due to these better properties (Uhlig 1998; Behera et al. 2017). Therefore, during washing, cellulases hydrolyze effectively sebum stain between inter-microfiber spaces without any fabric damage. Indeed, the inter-microfibers arise from mechanical damage during washing (Boisset et al. 1997; Uhlig 1998).
Enzymes used in the detergent industry may be immobilized. In the immobilization process, free cells or enzymes like cellulase are confined to an inorganic/organic or hybrid carrier to improve some properties. For instance, an improved washing capacity of commercial cellulase to remove indigo dye from denim fabrics was observed, compared to free cellulases, after enzyme immobilization on ZrOCl2-activated pumice support (Pazarlioglu et al. 2005). Zdarta et al. (2017) synthesized a lignin-TiO2 hybrid carrier. An increase of cellulase activity was observed for the cellulase purified from Aspergillus niger when immobilized to this support. Similarly, compared to free cellulase, the higher activity and stability of A. niger cellulase were observed when the enzyme was immobilized on a sponge through the glutaraldehyde as spacer group (Ahmed et al. 2013). Although cellulases immobilization can play a role in improving washing performance, the process should be checked for the cost-effectiveness.
Cellulases are utilized in detergent preparation along with other enzymatic detergents including fungal or bacterial proteases, amylases and lipases. The combination of these four enzymatic detergents facilitates the removal of all soil-related dirtiness and maintains the quality of all fabrics. For example, the SaniZyme® contains all these 4 detergent enzymes and removes various stains such as proteins, blood, carbohydrates, lipids, and mucous from the medical instrument because it is manufactured as a liquid detergent. Getinge Clean MIS Detergent® is another complex detergent enzyme; when combined with other components like surfactants at pH 8.0, it cleans all types of soils (Boisset et al. 1997; Uhlig 1998; Jayasekara and Ratnayake 2019).
Although branded cellulases such as Carezyme®, Celluzyme®, and SaniZyme® are available at the market for washing of fabrics and garments, they do not meet all the requirements. Therefore, the search for fungal cellulases with desirable aspects has to continue. The review papers on the detergent-compatible bacterial amylases and cellulases and detergent-compatible microbial proteases and lipases are available (Niyonzima and More 2014a; Niyonzima and More 2015a, b, c; Niyonzima 2018), but there is a gap for a dedicated report for detergent fungal cellulases. In this article, the production, properties, and compatibility of detergent fungal cellulases are addressed to the worldwide researchers.
Isolation and identification of detergent-compatible cellulases produced by fungi
Most of the detergent-compatible cellulases are screened from soil (Dave et al. 2015; Maharana and Ray 2015; Bagewadi et al. 2016; Bairagi 2016; Imran et al. 2018; El-Baroty et al. 2019). The other detergent-compatible enzymes such as bacterial and fungal proteases (Niyonzima and More 2015a), bacterial and fungal lipases (Niyonzima and More 2015a), bacterial amylases (Niyonzima and More 2014a), and bacterial cellulases (Niyonzima 2018) were also mostly isolated from soil microorganisms. Indeed, it is well known that soil harbors billions of microorganisms. The Aspergillus terreus that produces detergent-compatible cellulase was isolated from effluent. Iqbal et al. (2011), Pham et al. (2012), and Trinh et al. (2013) also isolated fungi that were able to produce detergent-compatible cellulases (Table 1).
Carboxymethylcellulose (CMC) agar is one of the screening media for fungi that produce detergent-compatible cellulases (Pham et al. 2012; Trinh et al. 2013; Bairagi 2016; Imran et al. 2018) (Table 1). Lignocellulosic substrate agar was the best screening medium for detergent-compatible cellulase secretion by T. viride (Iqbal et al. 2011). Sometimes, fungal growth media such as potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) are supplemented to CMC (Dave et al. 2015; Bagewadi et al. 2016) or to the filter paper strip (El-Baroty et al. 2019), respectively, for the secretion of detergent-compatible cellulases. A stain such as Congo red or chloramphenicol is added to the culture medium for better visualization of the cellulase hydrolysis zone. For instance, Congo red was used to observe the hydrolysis zone for the cellulase of Aspergillus terreus strain AKM-F3 (Maharana and Ray 2015). Sometimes, chloramphenicol is supplemented to CMC agar to prevent the bacterial growth. For instance, Bekele et al. (2015) inhibited bacterial growth by adding chloramphenicol to the cultivation medium when producing detergent-compatible cellulase from Aspergillus terreus.
The fungi producing detergent-compatible cellulases are identified based on morphological characteristics and microscopic features (Iqbal et al. 2011; Bekele et al. 2015; Bairagi 2016; Imran et al. 2018). However, most of them are currently identified with the help of ITS rDNA/RNA region sequencing (Trinh et al. 2013; Dave et al. 2015; Maharana and Ray 2015; Bagewadi et al. 2016; El-Baroty et al. 2019) (Table 1). Similarly, bacteria secreting detergent-compatible cellulases were generally identified based on morphological and microscopic characteristics followed by genetic material sequencing (Niyonzima 2018).
Production of detergent-compatible cellulases by fungi
Fungi secrete extracellular enzymes like cellulases that are important for industrial use (Nazir et al. 2010; Acharya and Chaudhary 2012). They are preferred because they secrete large quantities of enzymes when fermented on cheap materials like agro wastes or byproducts (Ahmed and Bibi 2018). These fungal cellulases are mainly obtained from 2 genera, viz., Aspergillus (Nguyen and Quyen 2010; Pham et al. 2012; El-Hadi et al. 2014; Bekele et al. 2015; Maharana and Ray 2015; Imran et al. 2018; El-Baroty et al. 2019) and Trichoderma (Iqbal et al. 2011; Ahmed et al. 2016; Bagewadi et al. 2016; Bairagi 2016). Similarly, Kirk et al. (2002) and Iqbal et al. (2011) reported that most of the cellulases that are commercialized are produced from Trichoderma and Aspergillus species. Peniophora sp. (Trinh et al. 2013) and Thermoascus sp. (Dave et al. 2015) were also reported as producers of the detergent fungal cellulases (Table 1).
Cost-effective and easily available growth supporting substrates are used to produce detergent compatible fungal cellulases. For instance, agricultural wastes, corn stalks, and sugarcane bagasse were utilized by Aspergillus species to produce detergent-compatible cellulases (Pham et al. 2012; Maharana and Ray 2015; Imran et al. 2018; El-Baroty et al. 2019). Sweet sorghum bagasse, vegetable waste, and wheat straw were cost-effective substances to produce detergent fungal cellulases in significant amounts (Iqbal et al. 2011; Ahmed et al. 2016; Bagewadi et al. 2016; Bairagi 2016). Dave et al. (2015) use a readily available cheap Jatropha deoiled seed cake to produce the cellulase from Thermoascus aurantiacus RBB-1. Therefore, the utilization of cellulosic material residues for fungal cellulases makes the production inexpensive and the pollution of the environment is monitored and decreased (Phitsuwan et al. 2013; Saxena and Singh 2014).
Cellulases production by fungal species is carried in solid-state fermentation (SSF) or submerged fermentation (SmF) and both strategies are generally cost-effective (Zhang and Zhang 2013). Indeed, some researchers prefer to use the SSF due to its simple process, functions under static conditions, minimal water output, high productivity, foam absence, cheap substrate utilization, less secretion of waste products, and low energy requirement; while others prefer SmF due to its simplicity of sterilization, simple enzyme recovery, heat and mass transfer, and controlled physicochemical and nutritional conditions (Bairagi 2016; Ahmed and Bibi 2018). Some detergent compatible fungal cellulases were produced under SSF. For instance, the detergent-compatible cellulases of Trichoderma species were produced with SSF (Iqbal et al. 2011; Ahmed et al. 2016; Bairagi 2016). T. aurantiacus RBB-1 produced a detergent-compatible cellulase under SSF with the response surface methodology carried out with Box-Behnken design (Dave et al. 2012). The detergent-compatible cellulases were also produced under SmF with Aspergillus species (Nguyen and Quyen 2010; El-Hadi et al. 2014; Bekele et al. 2015; Imran et al. 2018; El-Baroty et al. 2019). SSF or SmF is carried out sometimes at low tempearture to reduce energy utilization and to reduces the contamination exposures. For example, the detergent-compatible cellulase of A. terreus strain AKM-F3 was conducted at 15 °C and energy consumption was enormously reduced (Maharana and Ray 2015). More efforts are still needed to find the optimal parameters for cellulases production by fungi with improved quality to minimize the cost.
Fungal co-culturing was found to be beneficial because the co-produced enzymes are produced in important amounts in both solid and submerges fermentations. For instance, Trichoderma viride and Aspergillus niger produced cellulases in a significant quantity with waste paper (Juwaied et al. 2010) or wheat bran as substrate (Ikram-ul-Haq et al. 2005). Similarly, Jayant et al. (2011) screened A. niger and Penicillium chrysogenum that secreted cellulases in high amounts under SSF with waste paper. The co-culture of Penicillium and Cladosporium species was able to produce four hydrolases utilized in the detergent industry. These are cellulase, amylase, protease, and lipase (Abe et al. 2015). Two yeasts, viz., Tetracladium sp. and M. gelida, were co-cultured and produced high amounts of cellulases and amylases using a medium containing CMC or starch as a carbon source (Carrasco et al. 2016). T. viride and A. niger, after co-culturing, secreted cellulase and xylanase maximally (compared to monocultures) when wheat straw and sugarcane bagasse were used as substrates (Irfan et al. 2013). Emericella nidulans AUMC 5687 was able to concomitantly produce cellulase, xylanase, and pectinase in significant amounts (Moubasher et al. 2016). The co-culturing of fungi leads to a higher amount of cellulases and other enzymes production than corresponding monocultures. The process is environmentally friendly and cost-effective.
Optimization of nutritional and physicochemical factors for significant fungal cellulases production
The nutritional (like C and N sources) and physicochemical (inoculum size, pH, etc.) factors are the vital parameters affecting detergent-compatible cellulases. They have to be optimized for any fungus-producing the enzyme.
Initial pH of cultivation medium effect on detergent fungal cellulases production
Among physicochemical parameters, the initial pH of the cultivation medium is crucial because it induces morphological changes leading to detergent enzyme production (Cascalheira and Queiroz 1999; Niyonzima and More 2013a). The initial pH of Aspergillus (Nguyen and Quyen 2010; Pham et al. 2012; Bekele et al. 2015; Imran et al. 2018), Thermoascus (Dave et al. 2015), and Trichoderma (Iqbal et al. 2011; Ahmed et al. 2016; Bagewadi et al. 2016; Bairagi 2016) species producing detergent-compatible cellulases are in the 4.5 to 6.0 range (Table 2). However, the initial neutral pH was also observed for cellulase production by Aspergillus hortai (El-Hadi et al. 2014) and Peniophora sp. NDVN01 (Trinh et al. 2013). A low pH of 3.0 was also recorded for cellulase secretion by A. terreus (El-Baroty et al. 2019) (Table 2). After optimum pH, a decrease in bacterial or fungal detergent enzyme was noticed. This could be attributed to the alteration of enzyme structure (Niyonzima and More 2013b; Bairagi 2016). The lower pHs below optimum were also found to reduce detergent-compatible enzyme activity because the growth of fungi gets inhibited (Niyonzima et al. 2013; Maharana and Ray 2015).
Influence of incubation temperature on fungal cellulases production
The temperature was reported as a factor influencing the enzyme production including detergent-compatible cellulases (Karmakar and Ray 2011; Niyonzima 2018). Aspergillus species (Pham et al. 2012; El-Hadi et al. 2014; Bekele et al. 2015; Imran et al. 2018; El-Baroty et al. 2019) and Trichoderma species (Ahmed et al. 2016; Bagewadi et al. 2016; Bairagi 2016) that secrete detergent-compatible cellulases are in the range of 30–37 °C. However, a low incubation temperature of 15 and 28 °C was observed for detergent-compatible cellulase production by A. terreus strain AKM-F3 (Maharana and Ray 2015) and Peniophora sp. NDVN01 (Trinh et al. 2013), respectively. The optimal temperatures for cellulases production by Trichoderma viride (Iqbal et al. 2011) and T. aurantiacus RBB-1 (Dave et al. 2015) noticed were 45 and 50 °C, respectively (Table 2). The reduction in detergent enzyme secretion after optimum temperature may occur due to the inhibition of microbial growth and thermal inactivation (Niyonzima and More 2014d; Maharana and Ray 2015).
Influence of shaking on fungal cellulases secretion
The fermentation flasks are normally agitated at moderate rpm to favor maximum enzyme production (Kuhad et al. 2011). Most of the fungal cellulases are produced under static conditions (Iqbal et al. 2011; Dave et al. 2015; Bekele et al. 2015; Maharana and Ray 2015; Bagewadi et al. 2016; Imran et al. 2018; El-Baroty et al. 2019). However, when shaken, 150–200 rpm agitation range is considered. For instance, 150 rpm was optimum for cellulase secretion by Aspergillus hortai (El-Hadi et al. 2014). Ahmed et al. (2016) and Bairagi (2016) reported 180 rpm as the best shaking condition for cellulases secretion by Trichoderma species. Aspergillus species (Nguyen and Quyen 2010; Pham et al. 2012) and Peniophora sp. NDVN01 (Trinh et al. 2013) produce cellulases at the maximum level when agitated at 200 rpm (Table 2). Indeed, when cultivation flasks are shaken, the nutrients are well mixed and available to all bacterial or fungal cells, booting enzyme production. The shaking also maintains the aeration at optimum.
Influence of inoculum size on fungal cellulases secretion
The inoculum level is one of the important process parameters to be investigated when producing detergent enzymes during fermentation (Gaur and Tiwari 2015). Fungi producing cellulases are usually inoculated with 10% (v/v) (Iqbal et al. 2011; El-Hadi et al. 2014; Maharana and Ray 2015; Ahmed et al. 2016). However, 5 and 7% were seen as the best inoculum levels for detergent-compatible cellulases production by A. terreus (El-Baroty et al. 2019) and Trichoderma harzianum strain HZN11 (Bagewadi et al. 2016), respectively. Lower inoculation size leads to lower fungal growth owing to less conidial cells formation, whereas a higher inoculum concentration after optimum decreases detergent enzyme activity. This decrease can be attributed to the abundant fungal growth leading to fungal cell autolysis because of the nutritional imbalance. The decrease could also be attributed to the initial lag phase increase or depletion of important C and N sources or oxygen transfer (El-Hadi et al. 2014; Maharana and Ray 2015; Niyonzima 2019).
Influence of incubation time on fungal cellulases secretion
The incubation period has to be short to make fermentation inexpensive (Olama et al. 1993). Most fungi produce detergent-compatible cellulases from 5 to 7 days (Nguyen and Quyen 2010; Iqbal et al. 2011; Pham et al. 2012; Trinh et al. 2013; Dave et al. 2015; Maharana and Ray 2015; Ahmed et al. 2016; Bagewadi et al. 2016; Bairagi 2016; Imran et al. 2018; El-Baroty et al. 2019). However, a low incubation period of 4 days was recorded for cellulase secretion by Aspergillus species (El-Hadi et al. 2014; Bekele et al. 2015). A decrease in detergent enzyme production was observed after the optimal incubation period. This may be ascribed to the lack or decrease of important nutritional parameters in the production medium or poor oxygen transfer for fermentation carried out at 0 rpm (Maharana and Ray 2015; Bairagi 2016). The different incubation period reported may be attributed to the difference in the C sources bioavailability (Niyonzima and More 2014d; El-Baroty et al. 2019).
Influence of carbon sources on fungal cellulases secretion
A good choice of carbon sources is required since they have a vital role in the metabolism of a cell, and thus the detergent enzymes synthesis (Saha 2004; Bairagi 2016). Various C sources were reported to produce detergent in significant amounts (Table 2). For example, CMC and microcrystalline cellulose were C sources of choice for cellulase production by Aspergillus species (Bekele et al. 2015; Imran et al. 2018; El-Baroty et al. 2019). Low-cost substances such as sugarcane bagasse (Pham et al. 2012), pulp (Trinh et al. 2013), Jatropha deoiled seed cake (Dave et al. 2015), and lignocellulosic substrate (Iqbal et al. 2011; Ahmed et al. 2016) were also reported to produce fungal cellulases. Disaccharides such as lactose (El-Hadi et al. 2014; Bagewadi et al. 2016) and sucrose (Bairagi 2016) were also the best C sources. Indeed, when the lactose is used as a carbon source and an inducer, increase in enzyme activity is observed as the disaccharide penetration through the cell membrane is favored (El-Hadi et al. 2014). The glucose was found in many cases to decrease fungal growth and thus enzyme activity due to carbon catabolite repression (Ahmed and Bibi 2018; Niyonzima 2019).
Influence of nitrogen sources on detergent-compatible cellulases production by fungi
Preferred N sources were reported to be different for the cellulases secretion by fungi. Some fungal species used inorganic sources like ammonium nitrate (Bekele et al. 2015), sodium nitrate (Maharana and Ray 2015), ammonium monohydrogen phosphate (Trinh et al. 2013), and ammonium hydrogen sulfate (Ahmed et al. 2016) or organic sources such as yeast extract (El-Hadi et al. 2014; Bairagi 2016), soybean (Pham et al. 2012), and peptone (El-Baroty et al. 2019). The combination of nitrogen and organic nitrogen sources is sometimes considered to produce cellulase in an important amount. For instance, soybean and ammonium sulfate were mixed to produce cellulase in huge quantity from A. oryzae VTCC-F045 (Nguyen and Quyen 2010), whereas ammonium sulfate and protease peptone were combined to over-secrete cellulase from T. harzianum strain HZN11 in higher amounts (Bagewadi et al. 2016). A mixture of nitrogen organic sources was also taken into account for cellulase production by Aspergillus niger IMMIS1 (Imran et al. 2018). In some cases, a substance serves as both N and C source. Dave et al. (2015) utilized Jatropha deoiled seed cake as both C and N sources for detergent-compatible cellulase production by T. aurantiacus RBB-1. Sometimes, amino acids are supplemented to the production medium to increase cellulase secretion. For instance, aspartate acts as an inducer and boosts the detergent-compatible cellulase production from A. terreus strain AKM-F3 (Maharana and Ray 2015). A decrease in cellulase activity noticed after optimum could be ascribed to N source repression.
Purification of detergent-compatible cellulases produced by fungi
For partial purification of detergent-compatible enzymes, salts like ammonium sulfate and organic solvents like acetone and ethanol are used (Niyonzima and More 2014c). All the detergent-compatible fungal cellulases were precipitated by ammonium sulfate with saturation varying from 40 to 95%. The fungal cellulases need not to be in pure for detergent application. For example, the cellulase of A. terreus strain AKM-F3 was utilized in detergent formulation after only precipitation step (Maharana and Ray 2015). The fungal cellulases were mainly purified by gel filtration chromatography with Sephadex G-100 column packing materials (Nguyen and Quyen 2010; Iqbal et al. 2011; Pham et al. 2012; Ahmed et al. 2016; Imran et al. 2018). A combination of chromatographic methods was also reported. For example, DEAE cellulose DE-52 resin ion-exchange chromatography followed by Biogel P-100 size exclusion chromatography was the desired chromatographical method to purify the cellulase of T. aurantiacus RBB-1 (Dave et al. 2015). The cellulase obtained from T. harzianum strain HZN11 was purified with DEAE-Sepharose ion exchange and Sephadex G-100 gel filtration chromatography (Bagewadi et al. 2016). Trinh et al. (2013) use the Bio-Gel P-100 and Sephadex G-75 gel filtration chromatography procedure to totally purify the detergent-compatible cellulase of Peniophora sp. NDVN01. The specific activity of cellulases from fungi is in the range of 40–160 U/mg protein with fold increase varying from 2.0 to 33.0 and with a recovery yield of 1.5–24% range (Nguyen and Quyen 2010; Iqbal et al. 2011; Trinh et al. 2013; Dave et al. 2015; Maharana and Ray 2015; Ahmed et al. 2016; Bagewadi et al. 2016). The lower and higher specific activity of 14.122 and 388 U/mg protein were also observed for A. niger VTCC-F021 (Pham et al. 2012) and A. niger IMMIS1 (Imran et al. 2018), respectively (Table 3).
Properties of detergent compatible fungal cellulases
Molecular weight of fungal cellulases
Due to genomic composition and differences, molecular weights of detergent-compatible enzymes were reported to be different. The molecular weights of cellulases purified from Aspergillus (Nguyen and Quyen 2010; Pham et al. 2012; Maharana and Ray 2015; Imran et al. 2018), Peniophora (Trinh et al. 2013), Thermoascus (Dave et al. 2015), and Trichoderma (Iqbal et al. 2011; Ahmed et al. 2016; Bagewadi et al. 2016) species are in the 31–71 kDa range. All these detergent-compatible cellulases were found to be monomeric proteins. Similar ranges were report for enzymes used in the detergent preparations like 22–80 kDa for detergent bacterial cellulases (Niyonzima 2018), 30–94.5 kDa for detergent bacterial amylases (Niyonzima and More 2014a), and 16.1–60 kDa for detergent bacterial and fungal lipases (Niyonzima and More 2015b). Table 4 shows the characteristics of detergent fungal cellulases.
Influence of pH on fungal cellulases activity and stability
The enzyme to be used in the detergent industry has to be stable broadly in the alkaline region. The acidic pH in the range of 4.0–6.0 was observed for most of the fungal cellulases (Nguyen and Quyen 2010; Pham et al. 2012; Trinh et al. 2013; Dave et al. 2015; Maharana and Ray 2015; Ahmed et al. 2016; Bagewadi et al. 2016; Imran et al. 2018). However, 8.0 was the optimum pH of cellulase from T. viride (Iqbal et al. 2011). Although the optimum pH of fungal cellulases is in the acidic region, they are also stable in the alkaline region and thus can be used in detergent formulations. For instance, the cellulase of A. terreus strain AKM-F3 was 90% stable in the pH 5.0–8.0 range (Maharana and Ray 2015). Similarly, 71% residual activity at pH 8.0 for 1 h was noticed for the cellulase obtained from T. aurantiacus RBB-1 (Dave et al. 2015). Iqbal et al. (2011) and Ahmed et al. (2016) purified detergent-compatible cellulases from Trichoderma species that had stability in the pH range of 5.0 to 8.0.
Celluzyme® obtained from Humicola sp. functions actively in the pH and temperature ranges of 4–10 and 25–70 °C, respectively. Similarly, Carezyme® from the same species has a similar temperature range, but a different pH application range of 5–10.5. Both commercial enzymes were developed by Novo Nordisk (Bagsvaerd, Denmark) (Olsen and Falholt 1998). The different optimum pH observed for these detergent enzymes may be ascribed to the variability between their genetic materials (Li et al. 2008; Niyonzima and More 2014c; Iqbal et al. 2011). The decline in enzyme activity observed after the optimum pH could be due to enzyme 3D structure alteration owing to action of the H+ or OH− in the cellulase active site (Niyonzima and More 2014b; Maharana and Ray 2015).
Influence of temperature on fungal cellulases activity and stability
The optimum temperature observed for fungal cellulases varies from 35 to 60 °C (Nguyen and Quyen 2010; Iqbal et al. 2011; Pham et al. 2012; Trinh et al. 2013; Ahmed et al. 2016; Bagewadi et al. 2016; Imran et al. 2018). However, a low temperature of 15 °C was observed for a cellulase purified from A. terreus strain AKM-F3 (Maharana and Ray 2015). A higher optimum temperature was also recorded for cellulase of T. aurantiacus RBB-1 (Dave et al. 2015). All these fungal cellulases are 60 to 100% stable in the specified range. The optimum temperature of 50 °C was observed for commercial cellulases that were active in the washing powder at pH 8.5–9.0 range (Uhlig 1998; Behera et al. 2017). Similarly, the range found was the one recorded by Ito et al. (1989) where the real washing conditions took place at pH 10.0 and 40 °C for 20 min. Thermostability is a vital aspect of alkaline cellulase to be used in the detergent industries. Some detergent fungal cellulases are stable at high temperatures. For instance, the cellulase of T. aurantiacus RBB-1 was 100% stable at 70 °C and 90% stable for 1 h at 80 °C (Dave et al. 2015). This was a good aspect of industrial enzyme as there is no enzyme thermal inactivation. But currently, due to the high cost of electricity, detergent-compatible cellulases functioning at low temperatures are preferred. The denaturation of fungal cellulases noticed after optimum temperature could be due to non-covalent bonds rupture, disrupting thus the 3D enzyme structure (Okoshi et al. 1990; Maharana and Ray 2015).
Influence of various cations on the fungal cellulases
The detergent fungal cellulases behave differently when reacting with cations. Indeed, the cellulase of Aspergillus niger VTCC-F021 was stimulated by Cu2+, K+, and Fe2+; moderately inhibited by Ag+ and Ca2+; strongly inhibited by Zn2+, Ni2+, and Mn2; and no effect with Co2+ (Pham et al. 2012). Nguyen and Quyen (2010) purified the cellulase from Aspergillus oryzae VTCC-F045 that does not affect K+, Ni2+, Cu2+, and Mn2+; but it was slightly inhibited by Ca2+, Co2+, Fe2+, Zn2+, and Ag+. A. terreus strain AKM-F3 secretes a detergent-compatible cellulase that was stimulated by Ca2+ and Mn2+; and partially inhibited by Fe3+, Zn2+, Hg2+, Mg2+, Cu2+, Ba2+, Li+, Na+, and K+ (Maharana and Ray 2015). The cellulase from Peniophora sp. NDVN01 was activated by Ni2+; no inhibition with Ca2+ and Zn2+; but slight, moderate, and complete inhibitions were observed with K+ and Ba2+, Na+, Fe2+, Mn2+, and Mg2+, and Ag+ and Cu2+, respectively (Trinh et al. 2013). An activation by Ca2+, Co2+, Mg2+, Fe2+, and Mn2+ and an inhibition by Hg2+, Cd2+, Zn2+, and Pb2+ were observed for cellulase obtained from T. harzianum strain HZN11 (Bagewadi et al. 2016). Iqbal et al. (2011) and Ahmed et al. (2016) reported detergent-compatible cellulases from Trichoderma species that were activated by Co2+ and Mn2+, and inhibited by Hg2+. In most cases, Ni2+, K+, and Ca2+ stimulated and Hg2+ inhibited the detergent fungal species. Indeed, the detergent fungal enzymes may require Ni2+, K+, and Ca2+ for optimum activity and maximal stability; while Hg2+ may bind to some groups like carboxyl, thiol, and indole group present in the detergent enzyme active site and makes covalent bonds, thereby inhibiting enzyme (Trinh et al. 2013; Niyonzima and More 2014b; Maharana and Ray 2015; Niyonzima 2018).
Influence of specific reagents on fungal cellulases activity
The detergent-compatible cellulases purified from Aspergillus species are not inhibited by EDTA (Nguyen and Quyen 2010; Pham et al. 2012; Maharana and Ray 2015). They are therefore suitable for detergent use as stability in EDTA is needed. Indeed, the importance of EDTA in detergent preparation is that it improves soil removal by complexing the cations necessary for the hardness of water (Niyonzima and More 2015d). However, the inhibition was observed with cellulases of Trichoderma species (Iqbal et al. 2011; Ahmed et al. 2016) and thus not suitable for detergent industries. The decrease in detergent enzyme activity by EDTA may suggest the reaction between catalytic inorganic groups with EDTA, forming a complex which is inactive (Trinh et al. 2013; Niyonzima and More 2014c). 2-mercaptoethanol (2-ME) stimulated detergent-compatible cellulases of Peniophora sp. NDVN01 (Trinh et al. 2013) and Trichoderma harzianum strain HZN11 (Bagewadi et al. 2016). The stimulation of this cellulase shows that there is no thiol group in the active site (Niyonzima 2018). The cellulase of T. harzianum strain HZN11 was also activated by dithiothreitol (DTT) and urea. It was also inhibited by N-bromosuccinimide (NBS), p-chloromercuribenzoate (p-CMB), phenylmethylsulphonylfluoride (PMSF), iodoacetamide (IAA), dimethyl sulfoxide (DMSO), and 1,10-phenanthroline. The inhibition of this detergent-compatible cellulase by IAA and p-CMB may suggest the presence of thiol functional groups. These thiol groups in the active site were confirmed by stimulation of the enzyme by DTT and 2-ME. Tryptophan may also be present in the active site as the cellulase is inhibited by NBS (Bagewadi et al. 2016).
Broad substrate specificity and kinetic parameters of detergent fungal cellulases
CMC was the best substrate for detergent fungal cellulases in most cases compared to other substrates tested (Iqbal et al. 2011; Pham et al. 2012; Dave et al. 2015; Maharana and Ray 2015; Ahmed et al. 2016; Imran et al. 2018). For instance, Bagewadi et al. (2016) obtained a cellulase from T. harzianum strain HZN11 that was active against CMC, followed by filter paper and Avicel. However, the cellulase from Peniophora sp. NDVN01 has a higher specificity with barley β-glucan. The activity was four times compared to the one of CMC, but the cellulase was inactive against Avicel, locust bean gum, and birchwood xylan. It was therefore an endoglucanase (Trinh et al. 2013). The best substrate was also CMC for detergent bacterial cellulases (Niyonzima 2018). Kinetic parameters of fungal cellulases utilized in the detergent preparations are in Table 5. Vmax and Km are two main kinetic constants utilized to study detergent enzymes. Km found for most of fungal cellulases is in the 0.015–8 mg/ml range (Iqbal et al. 2011; Pham et al. 2012; Maharana and Ray 2015; Ahmed et al. 2016; Bagewadi et al. 2016; Imran et al. 2018). Low Km shows the highest cellulase activity for the CMC substrate. However, the cellulase of T. aurantiacus RBB-1 has relatively higher Km of 37 mg/ml compared to others (Dave et al. 2015). The difference in Km and Vmax observed between detergent enzymes from fungal species may be ascribed to their genetic variabilities (Iqbal et al. 2011; Niyonzima and More 2014b).
Influence of oxidizing and surfactant agents on fungal cellulases
Sodium dodecyl sulfate (SDS) is regarded as a component of detergent. The cellulases obtained from Aspergillus (Pham et al. 2012; Maharana and Ray 2015), Thermoascus (Dave et al. 2015), and Trichoderma species (Bagewadi et al. 2016) were stable in SDS and thus useful in detergent manufacturing. However, an inhibition was observed for some fungal cellulases (Nguyen and Quyen 2010; Iqbal et al. 2011; Trinh et al. 2013; Ahmed et al. 2016) (Table 6). SDS is importantly used in detergent formulation to react with positive ions necessary for water hardness (Niyonzima and More 2014c).
Nonionic surfactants are also important components of liquid and powder detergents. Most of the obtained cellulases were stable in Tween-20, Tween-80, and Triton X-100. Indeed, Pham et al. (2012) purified cellulase from A. niger VTCC-F021 that possessed significant activity in Tween-20, Tween-80, and Triton X-100. A. terreus strain AKM-F3 produced a detergent-compatible cellulase with good stability in Triton X-100, Tween-80, and Tween-20 (Maharana and Ray 2015). The cellulase from Peniophora sp. NDVN01 was stimulated by Tween-20, Tween-80, Triton X-100, and X-114 (Trinh et al. 2013). Tween 80, Triton X-100, Tween 20, and Tween 40 had no negative influence on the cellulase obtained from T. harzianum strain HZN11 (Bagewadi et al. 2016). Surfactants activate enzyme activities by favoring the permeability of surface-bound enzymes (Bairagi 2016). The cellulase of A. niger VTCC-F021 was partially inhibited with a residual activity of 78, 42, and 45% in Tween 20, Tween 80, and Triton X-100, respectively (Nguyen and Quyen 2010) (Table 6).
Fungal detergent-compatible cellulases have to be stable in oxidizing and bleaching agents. For example, T. strain HZN11 secreted a detergent-compatible cellulase with important activity in sodium tetraborate, sodium perborate, sodium hypochlorite, and hydrogen peroxide (Bagewadi et al. 2016). A small amount of oxidizing agents, surfactants, and bleaching agents have to be used as these can cause pollution to the environment. Bajpai and Tyagi (2007) proposed a detergent composition as follows: 0.004% (w/v) detergent enzymes, 0.14% (w/v) bleaching and oxidizing agents, and 0.02 and 0.03% (w/v) for nonionic and ionic surfactants, respectively.
Stability of fungal cellulases in local detergents
The fungal cellulases have to possess 100% stability in detergent components. The fungal cellulases were checked if they can resist in presence of commercial detergent components. An important stability was observed for cellulase from A. niger IMMIS1 in Ariel, Surf excel, Express power, Sunlight, and Bright (Imran et al. 2018). Dave et al. (2015) purified cellulase from T. aurantiacus RBB-1that had a significant activity in Arial, Rin, Wheel, and Surf Excel. Tide, Ariel, and Surf excel did not affect the cellulase activity of T. harzianum strain HZN11 (Bagewadi et al. 2016). The cellulase of T. harzianum was stimulated by Bonus and Surf Excel, and possessed maximum activity in in Wheel, and Ariel (Ahmed et al. 2016), whereas the cellulase of Trichoderma viride was activated by only Surf Excel and had 100% stability in Wheel, Ariel, and Bright Total (Iqbal et al. 2011) (Table 6).
Low velocities were necessary for BaCel5 and HiCel45 cellulases to remove the stains from fabrics at pH 10.0 and 40 °C for 20 min when used in liquid detergents. In these conditions, the softness and brightness of the fabrics are restored by using low dosages, and thus higher rates are not required for depilling as they can have destructive effects like loss of fabric strength (Caparrós et al. 2012). Celluzyme® and Carezyme® are also used in the powder and liquid detergents formulation to maintain the fabric softness, smoothness, and brightness. They are therefore known as color clarification cellulases (Olsen and Falholt 1998). Cellulase AP30K purified from Aspergillus niger supplied by Amano enzyme and Cellulase TRL obtained from Trichoderma longibrachiatum and Trichoderma reesei commercialized by Solvay Enzymes (Elkhart, IN) showed good properties to remove the soils from fabrics (Begum and Absar 2009). The detergent-compatible cellulase of Trichoderma harzianum strain HZN11 exhibited extraordinary storage stability of 60 days with 87% residual activity (Bagewadi et al. 2016).
In laundry detergent, 1–3% (w/w) of cellulase is incorporated to allow the soil removal and to give fabrics color brightening, softening, and whiteness maintenance. However, proteases, lipases, and amylases are added to both types of detergents in the range of 0.2–2% for laundry and 0.5–3% (w/w) for dish-wash detergents (Olsen and Falholt 1998). The fungal cellulase obtained from Trichoderma harzianum was able to remove ink-stain from a white cloth and the quality of the fabric was improved (Ahmed et al. 2016). In detergent industries, the fungal cellulase is used because it removes loose fibrils resulted from mechanical abrasion when used at a low dose. This selective degradation of only fibrils may be ascribed to the enzyme lower crystallinity enzyme and specificity in its action (Boisset et al. 1997). Ito et al. (1989) suggested that cellulases act by weakening cellulose amorphous areas where the soils prefer to bind.
Genetic engineering of fungal cellulases used in detergents
Genetic engineering and directed evolution can be used to enhance the activity and stability, to develop more efficient, and to reduce the cost of detergent-compatible cellulases production. For instance, the stability of Ce145 cellulase from Humicola insolens during detergent formulation was observed after the protein engineering step. Indeed, the surfactant C12-LAS inactivated the cellulase obtained from H. insolens Cel45 in detergent preparation. Site-directed mutagenesis was utilized to create mutations in the Cel45 crystal structure and cellulase with important stability in the detergent industry was obtained. The stability was shown by a significant resistance towards mechanical agitation while washing (Otzen et al. 1999). A high level of cellulase production was noticed after transferring the gene stce1 into the fungus H. insolens (Kuhad et al. 2011). Wang et al. (2014) expressed cellulase genes in T. reesei based on promoters and terminators. The bio-engineered alkaline cellulase was able to remove fibrils on the fabric surface, a process known as bio-stoning. Thus, research in genetic and protein engineering has to continue to get inexpensive detergent-compatible cellulases with desirable properties, as the production cost is still high.
Like other enzymes, cellulase secretion may be regulated by artificial or native transcription factors at the transcription level. Various filamentous fungi were reported to possess many transcription regulators. For example, artificial and natural transcription factors were manipulated in T. reesei to overproduce cellulases (Zhao et al. 2018). Krull et al. (2013) reported maximum cellulase production through pellet morphology engineering. The physicochemical parameters controlled may include shaking, temperature, inoculation concentration, pH, medium composition, and culture mode. Genome editing at the chromatin level was also utilized to develop improved fungal strains that over-produce cellulases (Zhao et al. 2018). Therefore, different and various genetic engineering methods can be applied to produce fungal cellulases for detergency by modifying the fungal genetic material. Indeed, genetic engineering can help fungi to cost-effectively produce significant amounts of detergent compatible-cellulases.
Conclusion
Detergent compatible fungal cellulases were reviewed in term of production, characteristics, and stability in the presence of other detergent components. Although important researches have been conducted on isolation, identification, production, purification, and characterization of detergent compatible fungal cellulases, future investigations should mostly focus on gene/protein engineering of these cellulases to overproduce stable and inexpensive fungal cellulases. The use of low-cost substrates including agricultural residues and spent byproducts has to continue to be exploited because the process is environmentally friendly and makes the enzyme production cost-effective. More efforts are still needed to fully understand the properties of detergent compatible fungal cellulases. The continual discovery of detergent enzymes with desirable aspects at low cost will allow the formulation of strong detergents that can remove all tough soil types. The eminent researchers from academia and industry have to work together to achieve all these goals.
References
Abe CAL, Faria CB, de Castro FF, de Souza S, Santos F, da Silva C, Tessmann D, Barbosa-Tessmann I (2015) Fungi isolated from maize (Zea mays L.) grains and production of associated enzyme activities. Int J Mol Sci 16:15328–15346. https://doi.org/10.3390/ijms160715328
Acharya S, Chaudhary A (2012) Bioprospecting thermophiles for cellulase production: a review. Braz J Microbiol 43:844–856. https://doi.org/10.1590/S1517-83822012000300001
Ahmed A, Bibi A (2018) Fungal cellulase, production and applications: minireview. Int J Health Life Sci 4:19–36
Ahmed I, Zia MA, Iqbal HMN (2016) Detergent-compatible purified endoglucanase from the agro-industrial residue by Trichoderma harzianum under solid state fermentation. BioResour 13:6393–6406. https://doi.org/10.15376/biores.11.3.6393-6406
Ahmed SA, El-Shayeb NMA, Hashem AM, Saleh SA, Abdel-Fattah AF (2013) Biochemical studies on immobilized fungal β-glucosidase. Braz J Chem Eng 30:747–758. https://doi.org/10.1590/S0104-66322013000400007
Bagewadi ZK, Mulla SI, Ninnekar HZ (2016) Purification and characterization of endo β-1,4-D-glucanase from Trichoderma harzianum strain HZN11 and its application in production of bioethanol from sweet sorghum bagasse. 3 Biotech 6:101. https://doi.org/10.1007/s13205-016-0421-y
Bairagi S (2016) Optimization of cellulase enzyme from vegetable waste by using Trichoderma atroviride in solid state fermentation. J Environ Sci Toxicol Food Technol 10:68–73. https://doi.org/10.9790/2402-1005026873
Bajpai D, Tyagi VK (2007) Laundry detergents: an overview. J Oleo Sci 56:327–340. https://doi.org/10.5650/jos.56.327
Begum MF, Absar N (2009) Purification and characterization of intracellular cellulase from Aspergillus oryzae ITCC-4857.01. Mycobiology 37:121–127. https://doi.org/10.4489/MYCO.2009.37.2.121
Behera BC, Sethi BK, Mishra RR, Dutta SK, Thatoi HN (2017) Microbial cellulases – diversity and biotechnology with reference to mangrove environment: a review. J Genet Eng Biotechnol 15:197–210. https://doi.org/10.1016/j.jgeb.2016.12.001
Bekele A, Abena T, Habteyohannes A et al (2015) Isolation and characterization of efficient cellulolytic fungi from degraded wood and industrialsamples. Afr J Biotechnol 14:3228–3234. https://doi.org/10.5897/AJB2015.14679
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383. https://doi.org/10.1016/S0734-9750(00)00041-0
Boisset C, Chanzy H, Schulein M, Henrissat B (1997) An ultrastructural study of the interaction of a fungal endoglucanase from Humicola insolens with cotton fibres. Cellulase 4:7–20. https://doi.org/10.1023/A:1018454900127
Caparrós C, Lant N, Smets J, Cavaco-Paulo A (2012) Effects of adsorption properties and mechanical agitation of two detergent cellulases towards cotton cellulose. Biocatal Biotransfor 30:260–271. https://doi.org/10.3109/10242422.2012.666840
Carrasco M, Villarreal P, Barahona S, Alcaíno J, Cifuentes V, Baeza M (2016) Screening and characterization of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiol 16:21. https://doi.org/10.1186/s12866-016-0640-8
Cascalheira JF, Queiroz JA (1999) Kinetic study of the cellobiase activity of Trichoderma reesei cellulose complex at high substrate concentrations. Biotechnol Lett 21:651–655. https://doi.org/10.1023/A:1005525015777
Dave BR, Sudhir AP, Pansuriya M, Raykundaliya DP, Subramanian RB (2012) Utilization of Jatropha deoiled seed cake for production of cellulases undersolid-state fermentation. Bioprocess Biosyst Eng 35:1343–1353. https://doi.org/10.1007/s00449-012-0723-3
Dave BR, Sudhir AP, Subramanian RB (2015) Purification and properties of an endoglucanase from Thermoascus aurantiacus. Biotechnol Rep 6:85–90. https://doi.org/10.1016/j.btre.2014.11.004
El-Baroty G, Abou-Elella F, Moawad H, El-Sebai TN, Abdulaziz F, Khattab AA (2019) Optimization and characterization of extracellular cellulaseproduced by native Egyptian fungal strain. Not Bot Horti Agrobot Cluj Napoca 47:743–750. https://doi.org/10.15835/nbha47311500
El-Hadi AA, El-Nour SA, Hammad A, Kamel Z, Anwar M (2014) Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. J Radiat Res Appl Sci 7:23–28. https://doi.org/10.1016/j.jrras.2013.11.003
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15:1–12. https://doi.org/10.1186/s12896-015-0129-9
Ikram-ul-Haq JMM, Khan TS, Siddiq Z (2005) Cotton saccharifying activity of cellulases produced by co-culture of Aspergillus niger and Trichoderma viride. Res J Agric Biol Sci 1:241–245
Imran M, Anwar Z, Zafar M, Ali A, Arif M (2018) Production and characterization of commercial cellulase produced through Aspergillus niger IMMIS1 after screening fungal species. Pak J Bot 50:1563–1570
Iqbal MH, Ahmed NI, Zia MA, Irfan M (2011) Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Adv Biosci Biotechnol 2:149–156. https://doi.org/10.4236/abb.2011.23024
Irfan M, Nadeem M, Syed Q (2013) Production of cellulases and hemicellulases from cellulytic fungal cultures in submerged fermentation using agricultural wastes. E J Biol 9:62–66
Ito S, Shikata S, Ozaki K, Kawai S, Okamoto K, Inoue S, Takei A, Ohta YI, Satoh T (1989) Alkaline cellulase for laundry detergent: production by Bacillus sp. KSM-635 and enzymatic properties. Agric Biol Chem 53:1275–1281. https://doi.org/10.1080/00021369.1989.10869489
Jayant M, Rashmi J, Shailendra M, Deepesh Y (2011) Production of cellulase by different co-culture of Aspergillus niger and Penicillium chrysogenum from waste paper, cotton waste and baggase. J Yeast Fungal Res 2:24–27
Jayasekara S, Ratnayake R (2019) Microbial cellulases: an overview and applications. IntechOpen, London. https://doi.org/10.5772/intechopen.84531
Juturu V, Wu JC (2014) Microbial cellulases: engineering, production and applications. Renew Sust Energ Rev 33:188–203. https://doi.org/10.1016/j.rser.2014.01.077
Juwaied AA, Adnan S, Al-Amiery AAHH (2010) Production cellulase by different co-culture of Aspergillus niger and Trichoderma viride from waste paper. J Yeast Fungal Res 1:108–111. https://doi.org/10.5897/JYFR.9000043
Karmakar M, Ray RR (2011) Current trends in research and application of microbial cellulases. Res J Microbiol 6:41–53. https://doi.org/10.3923/jm.2011.41.53
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351. https://doi.org/10.1016/s0958-1669(02)00328-2
Koga J, Baba Y, Shimonaka A, Nishimura T, Hanamura S, Kono T (2008) Purification and characterization of a new family 45 endoglucanase, STCE1, from Staphylotrichum coccosporum and its overproduction in Humicola insolens. Appl Environ Microbiol 74:4210–4217. https://doi.org/10.1128/AEM.02747-07
Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, Kampen I, Kwade A, Wittmann C (2013) Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol 163:112–123. https://doi.org/10.1016/j.jbiotec.2012.06.024
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res 2011:1–10. https://doi.org/10.4061/2011/280696
Li W, Zhang WW, Yang MM, Chen YL (2008) Cloning of the thermostable cellulase gene from newly isolated Bacillus subtilis and its expression in Escherichia coli. Mol Biotechnol 2:195–201. https://doi.org/10.1007/s12033-008-9079-y
Maharana AK, Ray P (2015) Optimization and characterization of cold-active endoglucanase produced by Aspergillus terreus strain AKM-F3 grown on sugarcane bagasse. Turk J Biol 39:175–185. https://doi.org/10.3906/biy-1408-22
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5:500–516. https://doi.org/10.7150/ijbs.5.500
Miettinen-Oinonen A, Suominen P (2002) Enhanced production of Trichoderma reesei endoglucanases and use of the new cellulase preparations in producing the stonewashed effect on denim fabric. Appl Environ Microbiol 68:3956–3964. https://doi.org/10.1128/aem.68.8.3956-3964.2002
Moubasher AAH, Ismail MA, Hussein NA, Gouda HA (2016) Enzyme producing capabilities of some extremophilic fungal strains isolated from different habitats of Wadi El-Natrun, Egypt. Part 2: Cellulase, xylanase and pectinase. Eur J Biol Res 6:103–111
Nazir A, Soni R, Saini HS, Kaur A, Chadha BS (2010) Profiling differential expression of cellulases and metabolite footprints in Aspergillus terreus. Appl Biochem Biotechnol 162:538–547. https://doi.org/10.1007/s12010-009-8775-9
Nguyen HQ, Quyen DT (2010) Purification and properties of an endoglucanase from Aspergillus oryzae VTCC-F045. Aust J Basic Appl Sci 4:6217–6222
Niyonzima FN (2018) Detergent compatible bacterial cellulases. J Basic Microbiol 59:134–147. https://doi.org/10.1002/jobm.201800436
Niyonzima FN (2019) Production of microbial industrial enzymes. Acta Sci Microbiol 2:75–89. https://doi.org/10.31080/ASMI.2019.02.0434
Niyonzima FN, More SS (2013a) Screening and optimization of cultural parameters for an alkaline protease production by Aspergillus terreus gr. under submerged fermentation. Int J Pharm Bio Sci 4:1016–1028
Niyonzima FN, More SS (2013b) Optimization of fermentation culture conditions for alkaline protease production by Scopulariopsis spp. Appl Biol Res 15:1–4
Niyonzima FN, More SS (2014a) Detergent compatible bacterial amylases. Appl Biochem Biotechnol 174:1215–1232. https://doi.org/10.1007/s12010-014-1144-3
Niyonzima FN, More SS (2014b) Biochemical properties of the alkaline lipase of Bacillus flexus XJU-1 and its detergent compatibility. Biologia 69:1108–1117. https://doi.org/10.2478/s11756-014-0429-x
Niyonzima FN, More SS (2014c) Purification and properties of detergent compatible extracellular alkaline protease from Scopulariopsis spp. Prep Biochem Biotechnol 44:738–759. https://doi.org/10.1080/10826068.2013.854254
Niyonzima FN, More SS (2014d) Concomitant production of detergent compatible enzymes by Bacillus flexus XJU-1. Braz J Microbiol 45:903–910. https://doi.org/10.1590/s1517-83822014000300020
Niyonzima FN, More SS (2015a) Detergent compatible proteases: microbial production, properties and stain removal analysis. Prep Biochem Biotechnol 45:233–258. https://doi.org/10.1080/10826068.2014.907183
Niyonzima FN, More SS (2015b) Microbial detergent compatible lipases. J Sci Ind Res 74:105–113
Niyonzima FN, More SS (2015c) Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J Basic Microbiol 55:1–10. https://doi.org/10.1002/jobm.201500112
Niyonzima FN, More SS (2015d) Purification and characterization of detergent compatible alkaline protease from Aspergillus terreus gr. 3. Biotech 5:61–70. https://doi.org/10.1007/s13205-014-0200-6
Niyonzima FN, More SS, Muddapur U (2013) Optimization of fermentation culture conditions for alkaline lipase production by Bacillus flexus XJU-1. Curr Trends Biotechnol Pharm 7:793–803
Okoshi H, Ozaki K, Shikata S, Oshino K, Kawai S, Ito S (1990) Purification and characterization of multiple carboxymethyl cellulases from Bacillus sp. KSM-522. Agric Biol Chem 54:83–89. https://doi.org/10.1080/00021369.1990.10869921
Olama ZA, Hamza MA, El-Sayed MM, Ab-del-Fattah M (1993) Purification properties and factors affecting the activity of Trichoderma viride cellulose. Food Chem 47:221–226. https://doi.org/10.1016/0308-8146(93)90153-7
Olsen HS, Falholt P (1998) Tehe role of enzymes in modern detergency. J Surfactant Deterg 1:555–567. https://doi.org/10.1007/s11743-998-0058-7
Otzen DE, Christiansen L, Schulein M (1999) A comparative study of the unfolding of the endoglucanase Ce145 from Humicola insolens in denaturant and surfactant. Protein Sci 8:1878–1887. https://doi.org/10.1110/ps.8.9.1878
Pazarlioglu NK, Sariisik M, Telefoncu A (2005) Treating denim fabrics with immobilized commercial cellulases. Process Biochem 40:767–771. https://doi.org/10.1016/j.procbio.2004.02.003
Pham TH, Quyen DT, Nghiem NM (2012) Purification and properties of an endoglucanase from Aspergillus niger VTCC-F021. Turk J Biol 36:694–701. https://doi.org/10.3906/biy-1202-30
Phitsuwan P, Laohakunjit N, Kerdchoechuen O, Kyu KL, Ratanakhanokchai K (2013) Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol 58:163–176. https://doi.org/10.1007/s12223-012-0184-8
Saha BC (2004) Production purification and properties of endoglucanase from a newly isolated strain of Mucor circinelloides. Process Biochem 39:1871–1876. https://doi.org/10.1016/j.procbio.2003.09.013
Sajith S, Priji P, Sreedevi S, Benjamin S (2016) An overview on fungal cellulases with an industrial perspective. J Nutr Food Sci 6:461. https://doi.org/10.4172/2155-9600.1000461
Saxena R, Singh R (2014) Contemporaneous production of amylase and protease through CCD response surface methodology by newly isolated Bacillus megaterium strain B 69. Enzyme Res 2014:1–12. https://doi.org/10.1155/2014/601046
Trinh DK, Quyen DT, Do TT, Nghiem NM (2013) Purification and characterization of a novel detergent- and organic solvent-resistant endo-beta-1,4-glucanase from a newly isolated basidiomycete Peniophora sp. NDVN01. Turk J Biol 37:377–384. https://doi.org/10.3906/biy-1207-37
Uhlig H (1998) Industrial enzymes and their applications. John Wiley and Sons Inc. USA, New York
Wang W, Meng F, Liu P, Yang S, Wei D (2014) Construction of a promoter collection for genes co-expression in filamentous fungus Trichoderma reesei. J Ind Microbiol Biotechnol 41:1709–1718. https://doi.org/10.1007/s10295-014-1508-2
Zdarta J, Jedrzak A, Klapiszewski L, Jesionowski T (2017) Immobilization of cellulase on a functional inorganic–organic hybrid support: stability and kinetic study. Catalysts 7:374. https://doi.org/10.3390/catal7120374
Zhang XZ, Zhang YHP (2013) Cellulases: characteristics, sources, production, and applications. https://doi.org/10.1002/9781118642047.ch8
Zhao XQ, Zhang XY, Zhang F, Zhang R, Jiang BJ, Bai FW (2018) Metabolic engineering of fungal strains for efficient production of cellulolytic enzymes. In: Fang X, Qu Y (eds) Fungal cellulolytic enzymes. Springer Nature, Singapore. https://doi.org/10.1007/978-981-13-0749-2_2
Acknowledgments
The author thank INES Ruhengeri for encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niyonzima, F.N. Detergent-compatible fungal cellulases. Folia Microbiol 66, 25–40 (2021). https://doi.org/10.1007/s12223-020-00838-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-020-00838-w