Abstract
Seaweeds are considered as a health food partly due to the polysaccharide composition of the cell wall. Because conventional extraction methods have low yields and lead to environmental pollution, enzymatic methods have been proposed. In this study, a new strain of Bacillus sp. was isolated from cattle feces that produced a mannanase, a polysaccharide-degrading enzyme active against the green seaweed Codium fragile. The purified 39-kDa mannanase exhibited maximum activity at 55 °C and pH 6.0, and maintained its catalytic activity stably at temperatures up to 60 °C and at a broad pH range (5.0–11.0). Enzymatic activity was slightly enhanced by Cu2+ and Na+ but strongly inhibited by Fe2+, Ag+, and EDTA. The mannanase showed the highest specificity to the inexpensive substrates such as konjac powder and locust bean gum, and efficiently released various manno-oligosaccharides. This novel mannanase can thus be applicable in the food, feed, and pulp industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds are abundant marine resources in the coasts of Korea and Japan and have been used extensively in the edible, medicinal, feed, and cosmetic industries. Recently, seaweeds have been recognized as a health food, and are utilized as functional food ingredients [1]. Seaweed contains a high content of polysaccharides, which are a constituent of the cell walls. The structure of the polysaccharides composing the cell wall varies depending on the seaweed type. Brown seaweed contains alginate and fucans; red seaweed contains carrageenans, agar, cellulose, and xylan; and green seaweed contains cellulose, xylan, mannan, and glucuronoxylomannan [2]. These seaweed polysaccharides are used as raw materials for functional foods such as fucoidans and porphyran [3]. However, the use of solvents or hot water to extract functional components from seaweed shows a low extraction yield and leads to environmental pollution [4, 5]. In contrast, enzymatic hydrolysis is an eco-friendly process with higher extraction efficiency relative to conventional methods [6,7,8].
Endo-1,4-β-d-mannanase (EC. 3.2.1.78, mannanase), which catalyzes the random hydrolysis of the β-1,4-mannosidic linkage in the main chain of mannan, glucomannan, and galactomannan, is a useful tool to produce bioactive manno-oligosaccharides from seaweeds [9, 10]. Furthermore, this enzyme is useful in several processes of the food industry, including viscosity reduction in coffee extracts, clarification of fruit juices and wines, and oil extraction from coconut meat [11]. Mannanase is produced by a wide range of organisms and is classified in glycoside hydrolase family (GH) 5 or 26 via amino acid sequence similarities [11,12,13]. Bacterial mannanases have been characterized from several genera, including Bacillus, Bacteroides, Caldibacillus, Caldocellum, Cellulomonas, Clostridium, Dictyoglomus, Flavobacterium, Paenibacillus, Piromyces, Rhodothermus, Sclerotium, Streptomyces, and Thermotoga [11]. Mannanase is active against various mannan substrates, especially locust bean gum (LBG) and konjac. Mannanase from Bacillus sp. displays the highest specific activity against LBG, which is a water-soluble polysaccharide of the mannan family [14,15,16,17].
Codium fragile is a species of green seaweed of the family Codiacea. The cell wall of C. fragile is mainly composed of linear β-D-mannans (31%) that have a degree of polymerization (DP) between 20 and 10,000 [18, 19]. Sulfated polysaccharides, which show anticoagulant, antivirus, and immunostimulating activities, comprise a small proportion of the cell wall in C. fragile [20, 21], and β-D-mannans of medium DP form aggregate with sulfated arabinogalactans and protein in hot-water extractions [22]. Although the characteristics of several bacterial mannanases have been reported, few studies have tested mannanase on C. fragile cell walls. In this study, we used C. fragile as a target seaweed to screen mannanase-producing bacteria from various environmental samples, and isolated a mannanase-producing Bacillus strain. In addition, the mannanase from the isolated strain was purified and its catalytic properties were characterized in detail.
Materials and methods
Chemical and enzymes
Bacto peptone and yeast extract were obtained from BD Bioscience (Franklin Lakes, NJ, USA). DNA-modifying enzymes, including restriction endonucleases T4 DNA ligase and PrimeSTAR HS DNA polymerase, were purchased from New England Biolabs (Beverly, MA, USA) or Takara (Kyoto, Japan). All other chemicals used in this study were of analytical reagent grade from Sigma-Aldrich Chemical Co (St. Louis, MO, USA).
Screening of mannanase-producing bacteria
In total, 400 bacterial species with potential mannan-degrading activity were isolated from various environmental samples (e.g., soil, seawater, feces). The microorganisms were inoculated on locust bean gum (LBG) agar containing 0.1% LBG and 1.5% agar in artificial seawater (23.6 g/L NaCl, 0.64 g/L KCl, 4.53 g/L MgCl2·H2O, 1.3 g/L CaCl2·2H2O, 1 g/L yeast extract, and 0.5 g/L Bacto peptone in 50 mM Tris–HCl (pH 7.4)) at 25 °C for 7 days. LBG agar plates were stained with 0.2% Congo Red for 10 min and washed with 0.1 M NaCl for 10 min. Colonies with a clear zone were isolated and evaluated for LBG-degrading activity. Selected bacterial isolates were subjected to a secondary screening using artificial seawater agar plates containing 0.05% azurine-crosslinked (AZCL) galactomannan, AZCL cellulose, and AZCL xylan (Megazyme, Bray, Ireland). After incubation at 25 °C for 48 h, the colony showing the widest blue zone was selected for further study.
Identification of mannanase-producing bacteria
The 16S rRNA gene sequence of the isolated Bacillus strain, denominated as R2AL2A, was determined after genomic DNA extraction and PCR amplification as described by Seo et al. [23]. The 16S rRNA sequence was compared with the sequences of homologous strains in GenBank using EzTaxon [24].
Expression of mannanase from bacterial strain R2AL2A against C. fragile
Bacterial strain R2AL2A was inoculated on dried C. fragile with artificial seawater to express mannanase against the C. fragile cell wall. After incubation at 25 °C for 6 days, the C. fragile was filtered through Whatman No.1 filter paper (Whatman, Kent, England). The filtered supernatant was analyzed by thin-layer chromatography (TLC) as described below and dialyzed to remove reducing sugar. The polysaccharide-degrading activity of the dialyzed supernatant was measured by incubation with different polysaccharide substrates (0.5% (w/v) alginate, fucoidan, laminarin, low-melting agarose (LMA), carrageenan, and LBG) in 10 mM sodium phosphate buffer (pH 7.0) at 25 °C for 15 h. The amount of reducing sugars was estimated by the dinitrosalicylic acid (DNS) method [25].
Purification of mannanase from bacterial strain R2AL2A
The bacterial strain R2AL2A was inoculated in 0.5% LBG, 0.2% Tryptone, 0.1% yeast extract, and 0.2% NaCl and incubated at 25 °C for 2 days. The broth was then centrifuged at 10,620 × g for 30 min and subjected to ammonium sulfate precipitation (50–80%). The precipitated crude enzyme was dialyzed against 20 mM Tris–HCl (pH 7.6, buffer A) for 16 h to remove salt. The resulting sample was applied onto a DEAE Sepharose Fast Flow (16/10) column (GE healthcare, Uppsala, Sweden) pre-equilibrated with buffer A on an ÄKTA Purifier 100 system (GE healthcare). Fractions (5.0 mL) were collected at a flow rate of 2 mL/min by washing the column with buffer A, followed by a linear gradient of 0.1 M NaCl in buffer A. The fractions were monitored using an ultraviolet detector at 280 nm. Fractions with mannanase activity were collected and concentrated by Amicon Ultra Centrifugal filters (Millipore, Billerica, MA, USA). Next, samples were chromatographed on a Superdex 75 (10 × 300 mm, GE healthcare) column pre-equilibrated with buffer A. Fractions containing mannanase activity were stored at 4 °C until use. All purification steps were performed at 4 °C unless stated otherwise.
SDS-PAGE and zymogram analyses
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed in a 12% (w/v) polyacrylamide gel. Proteins were visualized by Coomassie brilliant blue R-250 staining. Zymography of mannanase was performed by gel activity assays using 12% (w/v) polyacrylamide gel with 0.2% LBG as substrate. After electrophoresis, the gel was washed once with Triton X-100 and twice with 50 mM Tris–HCl (pH 8.0) with gentle shaking to remove SDS. Active proteins were detected by incubation at 25 °C, staining with 0.2% (w/v) Congo red, and destaining with 1 M NaCl. The bands containing active proteins were detected according to the presence of a clear zone [26].
Mannanase activity assays
Mannanase activity assays were performed with a slight modification of the methods reported by Cann et al. [27]. Standard assay mixtures (total volume 100 μL) contained 0.5% LBG in 50 mM sodium citrate buffer (pH 6.0) with purified mannanase. After incubation at 55 °C for 10 min, the reaction was terminated by addition of 300 μL of ice-cold 0.5 N HCl containing 1% (w/v) p-hydroxy benzoic acid hydrazide and 1 N NaOH. The mixture was heated at 100 °C for 5 min, and then the absorbance of the cooled solution was measured at 410 nm. One unit (U) of mannanase activity was defined as the amount of enzyme that could hydrolyze LBG and release 1 mM mannose within 1 min of reaction at 55 °C.
Effects of temperature, pH, and various reagents on enzyme activity
The effect of temperature on mannanase activity was determined in 25 mM sodium citrate buffer (pH 6.0) in a temperature range of 10–80 °C using LBG as a substrate. For determination of thermal stability, the residual activity of mannanase was measured after pre-incubation at different temperatures at pH 6.0 for 30 min. The optimal pH of mannanase activity was determined at 55 °C in sodium citrate (pH 3.0–6.0), sodium phosphate (pH 6.0–7.5), Tris–HCl (pH 7.5–9.0), and glycine–NaOH (pH 9.0–11.0) buffers. The stability at different pH was determined using the same buffer system in the pH range of 3.0–11.0. After incubation of the enzyme at various pH values at room temperature for 2 h without substrate, the remaining enzymatic activity was measured. The effects of metal ions on mannanase activity were determined by assaying for residual activity after incubating the enzyme with various metal ions at concentrations of 5 and 10 mM, dissolved in 25 mM sodium citrate buffer (pH 6.0) at 25 °C for 2 h.
Substrate specificity of mannanase from bacterial strain R2AL2A
The substrate specificity of purified mannanase was determined by incubating the enzyme with each of the following substrates at 0.5%: LBG, konjac powder, xylan, guar gum, mannan, carboxymethyl cellulose, 4-nitrophenyl α-d-mannopyranoside, or 4-nitrophenyl β-d-mannopyranoside in 25 mM sodium citrate buffer (pH 6.0) at 55 °C for 10 min. The reducing sugars were determined by the DNS method.
Hydrolysis pattern analysis of mannanase from bacterial strain R2AL2A
The hydrolysis pattern of the purified mannanase was determined by TLC. The mannanase was incubated with 0.5% (w/v) LBG in 25 mM sodium citrate buffer (pH 6.0). After different time intervals, samples were spotted on Silica gel 60 F plates (Merck, Darmstadt, Germany) using 1-butanol/acetic acid/H2O (2:1:1, v/v/v) as the solvent. After development, the TLC plate was dried and visualized by dipping into 10% H2SO4 in 80% methanol. The plate was then dried and placed in an oven at 110 °C for 10 min.
Results and discussion
Screening and isolation of mannan-degrading bacteria from various environmental samples
To screen for mannanase enzymatic activity, 400 bacterial strains obtained from various environmental samples such as soil, seawater, and domesticated animal feces were grown in LBG agar plates. Mannanase-producing bacteria are able to hydrolyze LBG during growth, and a clear zone appears around the colony after Congo red staining [26]. Twenty-three bacterial strains isolated by preliminary screening were transferred to agar plates containing AZCL-galactomannan for secondary screening. Bacterial mannanase acting on AZCL-galactomannan releases the blue dye azurine. One colony of highly efficient mannan-degrading bacteria surrounded by a blue halo was isolated from cattle feces (Supplemental Fig. 1). Analysis of the 16S rRNA gene sequence of the isolated bacterial strain revealed that it is closely related to Bacillus subtilis subsp. subtilis DSM10T with 99.53% sequence similarity (Supplemental Fig. 2). Mannanase has been identified and purified from various B. subtilis strains [11, 16]. B. amyloliquefaciens CS47, a mannan-degrading bacteria, was isolated from horse feces by Cho [28]. The strain of Bacillus sp. producing mannanase was isolated, identified, designated as Bacillus sp. R2AL2A, and deposited in the Korean Culture Center of Microorganisms (KCCM 11722P).
Identification of the C. fragile cell wall-degrading enzyme from Bacillus sp. R2AL2A
The Bacillus sp. R2AL2A was grown on dried C. fragile as a carbon source to express the C. fragile cell wall-degrading enzyme. After incubation, the dry mass of C. fragile was reduced by 47%, and it could be assumed that the cell wall of C. fragile was hydrolyzed by some enzyme from Bacillus sp. R2AL2A. Supernatant of the growth media was separated and analyzed by TLC, and degraded manno-oligosaccharides from C. fragile were detected (data not shown). These results inferred that the cell wall from C. fragile can be degraded by Bacillus sp. R2AL2A. The supernatant reacted with various marine polysaccharides such as alginate, fucoidan, laminarin, LMA, carrageenan, and LBG, but only LMA and LBG were hydrolyzed to release reducing sugars (Supplemental Fig. 3). LBG hydrolysis by the enzyme present in the supernatant was especially efficient. Consistent with these results, microbial mannanase shows a more efficient breakdown of LBG when compared to other marine polysaccharides [11]. In addition, LBG and galactomannan are similar, both consisting of mannose and galactose units. Generally, the production of mannanase is inducible in microbial cells and appears to be regulated by the presence of mannan-like polysaccharides as a carbon source [29]. The C. fragile cell wall is mainly constituted of β-1,4 linear mannan, and mannanase from Aplysia kurodai preferably degrades β-1,4 mannan from C. fragile [9]. It can be proposed that Bacillus sp. R2AL2A expressed mannanase to degrade the C. fragile cell wall as a carbon source.
Purification of mannanase from Bacillus sp. R2AL2A
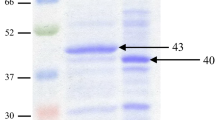
Mannanase from Bacillus sp. N16-5 was effectively expressed to use LBG as a carbon source in a previous study [30]. We thus investigated whether expression of a mannanase similarly occurred in Bacillus sp. R2AL2A to utilize LBG as a carbon source. Mannanase from Bacillus sp. R2AL2A was purified by ammonium sulfate and anion exchange chromatography. The results of each purification step are presented in Table 1. The final enzyme preparation was purified 17.89-fold with a yield of 3.59%. The specific activity of the purified enzyme, determined using LBG as substrate, was 4678.38 U/mg protein and was very similar to the value (4341 U/mg) for the Bacillus licheniformis enzyme used industrially [31]. The molecular mass of the purified enzyme was determined to be 39 kDa by SDS-PAGE and zymogram analyses (Fig. 1). The apparent molecular mass of mannanase from Bacillus sp. R2AL2A was similar to that of mannanase from B. subtilis WY32 (39.6 kDa) and B. subtilis WL-3 (38 kDa), and lower than that from Bacillus sp. N16-5 (55 kDa) and Bacillus nealsonii PN-11 (50 kDa) [11, 14, 16, 17, 32].
Physicochemical characterization of the purified mannanase from Bacillus sp. R2AL2A
The effects of temperature, pH, metal ions, and some chemical reagents on the activity of the purified mannanase from Bacillus sp. R2AL2A were determined. The optimal pH for the enzyme was 6.0, and it was stable from pH 5.0 to 11.0 (Figs. 2A, B). Consistent with our results, most mannanases from Bacillus strains have an acidic to neutral optimal pH [11]. However, some mannanases from Bacillus sp. N16-5 and Bacillus sp. JAMB-750 have an optimal pH in the alkaline range (pH 9.5–10.0) [11, 14]. In our results, purified mannanase had 80–100% residual activity in a broad pH range of 5.0–11.0. Similarly, the highly alkaline mannanases from Bacillus sp. N16-5 and Bacillus sp. JAMB-750 are very stable at pH 6.0–11.0 [33, 34]. Although the optimal pH of the purified mannanase from Bacillus sp. R2AL2A was in the acidic range, the pH stability of the enzyme extended from acidic to alkaline conditions.
Biochemical properties of purified mannanase from Bacillus sp. R2AL2A. (A) Optimal pH was determined in different buffers at 55 °C. (B) pH stability was determined by measuring the residual activities after pre-incubation for 2 h at different pHs at 25 °C: filled circle represents sodium citrate at pH 3.0–6.0, empty square sodium phosphate at pH 6.0–7.0, filled triangle Tris–HCl at pH 7.0–9.0, and open inverted triangle glycine–NaOH at pH 9.0–11.0. (C) Optimal temperature was determined at various temperatures for 10 min in 25 mM sodium citrate buffer (pH 6.0). (D) Thermostability was assayed by measuring the residual activity after pre-incubation for 30 min at different temperatures
Our results show that the purified enzyme had an optimal temperature of 50 °C (Fig. 2C), which is very similar to that of enzymes from B. subtilis B36, B. subtilis BM9602, Bacillus sp. M50, and Bacillus sp. 1633 [11]. In addition, the purified enzyme was stable at temperatures up to 60 °C (Fig. 2D). The thermostability of the purified enzyme is similar to that from B. subtilis WY34, Bacillus sp. N16-5, and B. nealsonii PN-11 [17, 32, 33]. The mannanase from Bacillus sp. R2AL2A, with a wide range of thermal and pH stability, has the potential to be used in many industrial applications.
The effects of various metal ions and chemical reagents on the activity of purified mannanase are shown in Table 2. The activity of the purified enzyme was slightly enhanced by Cu2+ and Na+, was unaffected by Ca2+ and Zn2+, and was strongly inhibited by Fe2+, Ag+, and EDTA. Although several studies have reported that microbial mannanases are affected by metal ions [10, 11, 17, 31, 32], the underlying mechanisms are not completely understood. It has been described that metal ions affect mannanase enzymatic activity because of oxidation of specific residues containing SH groups on the active site, or the substrate binding site is occupied by metal ions or chemical reagents [16, 17, 32].
Substrate specificity of the purified mannanase from Bacillus sp. R2AL2A
The substrate specificity of the purified mannanase from Bacillus sp. R2AL2A was also investigated, and its relative activity on various substrates is displayed in Table 3. The purified mannanase exhibited the highest activity against konjac powder, followed by LBG (74.56%). However, it showed relatively low activity on guar gum (11.76%), xylan (9.10%), and mannan (0.44%). Moreover, reducing sugars were not released by the purified mannanase with carboxymethyl cellulose, 4-nitrophenyl α-D-mannopyranoside, and 4-nitrophenyl β-D-mannopyranoside. Although most mannanases from Bacillus strains display high activity toward LBG [15,16,17, 32, 35], the highest activity of mannanase from Bacillus sp. R2AL2A was toward konjac powder. Konjac powder is a glucomannan consisting of mannose and glucose at a 3:1 ratio, whereas LBG (mannose and galactose at a 4:1 ratio) is a galactomannan similar to guar gum (mannose and galactose at a 2:1 ratio), consisting of a β-d-mannose backbone with side chains of α-d-galactose [36]. High konjac powder-hydrolyzing activity suggests that the mannanase from Bacillus sp. R2AL2A more readily hydrolyzes glucomannan than it does galactomannan. The weak hydrolysis of guar gum indicates that the mannanase from Bacillus sp. R2AL2A is limited by branched α-galactose residues, which was also reported for mannanases from B. subtilis WL-7, Bacillus sp. N16-5, and B. subtilis WY34 [14, 32, 35].
The hydrolysis products released from LBG and konjac powder were analyzed by TLC (Fig. 3). LBG degradation resulted in a mixture of mannobiose, mannotriose, mannopentaose, and other unidentified manno-oligosaccharides. Konjac powder hydrolysis generated mannobiose and glucomanno-oligosaccharide, and the main product was estimated to have a DP of 2–3. The hydrolysis pattern of the purified mannanase was similar to that of mannanase from Penicillium oxalicum GZ-2 and Reinekea sp. KIT-YO10 [37, 38]. Most microbial mannanases degrade various mannan polymers to produce mainly mannobiose and mannotriose with no mannose [37,38,39]. However, some mannanases show not only endo-β-1,4-mannnase activity but also 1,4-β-mannosidase activity, releasing mannose form mannan polysaccharides [11]. In conclusion, mannanase from Bacillus sp. R2AL2A is a typical endo-β-1,4-mannanase, and it produces manno-oligosaccharides primarily with DP of 2–6 from the inexpensive and widely available LBG and konjac powder.
TLC assays of hydrolysis products from locust bean gum (A) and konjac powder (B). Lane S, manno-oligosaccharide standards (mannose to mannohexaose): M1 mannose; M2 mannobiose; M3 mannotriose; M4 mannotetraose; M5 mannopentaose; M6 mannohexaose. Lanes 0–4; reaction product of substrate at different reaction times (0, 15, 30 min, 12, and 24 h)
In the present study, the isolated Bacillus sp. R2AL2A strain, a C. fragile cell wall-degrading bacteria, was shown to produce an endo-β-1,4-mannanase. The mannanase from Bacillus sp. R2AL2A showed thermostability and high stability across a broad pH range, and with a substrate specificity different from that of other Bacillus mannanases. Our results indicate that the mannanase from Bacillus sp. R2AL2A can be used in many applications in the food, feed, and pulp industries. Future work will focus on the production of functional manno-oligosaccharides as putative prebiotics from C. fragile using the mannanase from Bacillus sp. R2AL2A.
References
Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23: 543–597 (2011)
Mabeau S, Fleurence J. Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci. Tech. 4: 103–107 (1993)
Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar. Drugs 9: 1056 (2011)
Rioux LE, Turgeon SL, Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 69: 530–537 (2007)
Ye H, Wang K, C Zhou, J Liu, X Zeng. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 111: 428–432 (2008)
Wijesinghe WAJP, Jeon YJ. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 83: 6–12 (2012)
Siriwardhana N, Jeon YJ, Kim SH, Ha JH, Heo SJ, Lee KW. Enzymatic hydrolysis for effective extraction of antioxidative compounds from Hizikia fusiformis. Algae 19: 59–68 (2004)
Ale MT, Meyer AS. Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 3: 8131–8141 (2013)
Zahura UA, Rahman MM, Inoue A, Tanaka H, Ojima T. An endo-β-1,4-mannanase, AkMan, from the common sea hare Aplysia kurodai. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 157: 137–143 (2010)
Tamaru Y, Araki T, Amagoi H, Mori H, Morishita T. Purification and characterization of an extracellular β-1,4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl. Environ. Microbiol. 61: 4454–4458 (1995)
Dhawan S, Kaur J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 27: 197–216 (2007)
Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280: 309–316 (1991)
Hogg D, Pell G, Dupree P, Goubet F, Martín-Orúe SM, Armand S, Gilbert HJ. The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem. J. 371: 1027–1043 (2003)
Ma Y, Xue Y, Dou Y, Xu Z, Tao W, Zhou P. Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8: 447–454 (2004)
Piwpankaew Y, Sakulsirirat S, Nitisinprasert S, Nguyen TH, Haltrich D, Keawsompong S. Cloning, secretory expression and characterization of recombinant β-mannanase from Bacillus circulans NT 6.7. Springerplus 3: 430 (2014)
Yoon KH, Chung S, Lim BL. Characterization of the Bacillus subtilis WL-3 mannanase from a recombinant Escherichia coli. J. Microbiol. 46: 344 (2008)
Chauhan PS, Sharma P, Puri N, Gupta N. Purification and characterization of an alkali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur. Food Res. Technol. 238: 927–936 (2014)
Mackie W, Sellen DB. The degree of polymerization and polydispersity of mannan from the cell wall of the green seaweed Codium fragile. Polymer 10: 621–632 (1969)
Estevez JM, Fernández PV, Kasulin L, Dupree P, Ciancia M. Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 19: 212–228 (2009)
Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 84: 14–21 (2011)
Lee JB, Ohta Y, Hayashi K, Hayashi T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr. Res. 345: 1452–1454 (2010)
Ciancia M, Quintana I, Vizcargüénaga MI, Kasulin L, Dios A, Estevez JM, Cerezo AS. Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Int. J. Biol. Macromole. 41: 641–649 (2007)
Seo DH, Jung JH, Kim HY, Kim YR, Ha SJ, Kim YC, Park CS. Identification of lactic acid bacteria involved in traditional Korean rice wine fermentation. Food Sci. Biotechnol. 16: 994–998 (2007)
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 62: 716–721 (2012)
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428 (1959)
Agrawal P, Verma D, Daniell H. Expression of Trichoderma reesei β-mannanase in tobacco chloroplasts and its utilization in lignocellulosic woody biomass hydrolysis. Plos One 6: e29302 (2011)
Cann IKO, Kocherginskaya S, King MR, White BA, Mackie RI. Molecular cloning, sequencing, and expression of a novel multidomain mannanase gene from Thermoanaerobacterium polysaccharolyticum. J. Bacteriol. 181: 1643–1651 (1999)
Jeong CS. Isolation and characterization of mannanase producing Bacillus amyloliquefaciens CS47 from horse feces. J. Life Sci. 19: 1724–1730 (2009)
El-Sharouny EE, El-Toukhy NMK, El-Sersy NA, El-Gayar AAE-A. Optimization and purification of mannanase produced by an alkaliphilic-thermotolerant Bacillus cereus N1 isolated from Bani Salama Lake in Wadi El-Natron. Biotechnol. Biotechnol. Equip. 29: 315–323 (2015)
Lin SS, Dou WF, Xu HY, Li HZ, Xu ZH, Ma YH. Optimization of medium composition for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 using response surface methodology. Appl. Microbiol. Biotechnol. 75: 1015–1022 (2007)
Zhang J, He Z, Hu K. Purification and characterization of β-mannanase from Bacillus licheniformis for industrial use. Biotechnol. Lett. 22: 1375–1378 (2000)
Jiang Z, Wei Y, Li D, Li L, Chai P, Kusakabe I. High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr. Polym. 66: 88–96 (2006)
He X, Liu N, Li W, Zhang Z, Zhang B, Ma Y. Inducible and constitutive expression of a novel thermostable alkaline β-mannanase from alkaliphilic Bacillus sp. N16-5 in Pichia pastoris and characterization of the recombinant enzyme. Enzyme Microb. Tech. 43: 13–18 (2008)
Hatada Y, Takeda N, Hirasawa K, Ohta Y, Usami R, Yoshida Y, Grant WD, Ito S, Horikoshi K. Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme. Extremophiles 9: 497–500 (2005)
Kweun MA, Lee MS, Choi JH, Cho KH, Yoon KH. Cloning of a Bacillus subtilis WL-7 mannanase gene and characterization of the gene product. J. Microbiol. Biotechnol. 14: 1295–1302 (2004)
Dionísio M, Grenha A. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioall. Sci. 4: 175–185 (2012)
Liao H, Li S, Zheng H, Wei Z, Liu D, Raza W, Shen Q, Xu Y. A new acidophilic thermostable endo-1,4-β-mannanase from Penicillium oxalicum GZ-2: cloning, characterization and functional expression in Pichia pastoris. BMC Biotechnol. 14: 90 (2014)
Hakamada Y, Ohkubo Y, Ohashi S. Purification and characterization of β-mannanase from Reinekea sp. KIT-YO10 with transglycosylation activity. Biosci. Biotechnol. Biochem. 78: 722–728 (2014)
Kulcinskaja E, Rosengren A, Ibrahim R, Kolenová K, Stålbrand H. Expression and characterization of a Bifidobacterium adolescentis β-mannanase carrying mannan-binding and cell association motifs. Appl. Environ. Microbiol. 79: 133–140 (2013)
Acknowledgements
This research was supported by the Main Research Program (E0170602-01) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, S., Lee, MH., Lee, ES. et al. Characterization of mannanase from Bacillus sp., a novel Codium fragile cell wall-degrading bacterium. Food Sci Biotechnol 27, 115–122 (2018). https://doi.org/10.1007/s10068-017-0210-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0210-3