Abstract
Pullulan has many useful characteristics but, its high cost limits its potential applications. In the present work, kitchen waste (KW), which otherwise has zero commercial value, was evaluated for the economical production of pullulan. Before fermentation, the KW was hydrolyzed into free sugars using an in-house produced cocktail of enzymes. During hydrolysis, 46 ± 3.5 g/l and 31 ± 2.2 g/l of total reducing sugars and glucose were released, respectively. Hydrolyzed kitchen waste was then used as substrate for fermentation by Aureobasidium pullulans MTCC 2013 yielding 20.46 ± 2.01 g/l pullulan. Further, effect of different nitrogen sources was evaluated and yeast extract (3%) was found to be the best, yielding (24.77 ± 1.06 g/l) exopolysaccharide (EPS). The pullulan produced from KW was characterized in terms of organoleptic properties, physical strength, Fourier-transform infrared spectroscopy (FTIR), and H nuclear magnetic resonance (H NMR) analysis. The results corroborated well with commercial pullulan. The biodegradable nature and water solubility of the film developed from pullulan was also confirmed. To the best of our knowledge, this is the first report on the validation of the biodegradability of in-house produced pullulan. Thus, kitchen waste appears to be a promising option for economical pullulan production. Additionally, the method may also prove to be helpful for managing the increasing load of municipal solid waste in an eco-friendly and scientific way.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World plastic production has grown from 1.9 tons to about 330 million tons between 1950 and 2013 (Seltenrich 2015). There is rising environmental concern on dumping of non-biodegradable plastics in landfills. This has diverted the interest of scientific community to develop “eco-friendly” bioplastic material (Shamsuddin et al. 2017).
In this context, pullulan is one of the extensively studied naturally produced biopolymer (exopolysaccharide) having many beneficial characteristics. It is an extracellularly produced polysaccharide, soluble in water but insoluble in many organic solvents and forms viscous adhesive solution which is transparent and colorless in appearance (Morris 1995; Shingel 2004). It comprises of maltotriose-units interconnected by α-1,6-glycosidic linkages. These linkages provide pullulan with high flexibility and great aqueous solubility. Pullulan is majorly synthesized by a yeast-like polymorphic fungus Aureobasidium pullulans, which is non-pathogenic to humans. Pullulan is very widely used in textile, pharmaceutical, food, and cosmetic industry (Singh et al. 2008a). Pullulan can be used to develop films of various strengths required for different purposes which are colorless, tasteless, odorless, tenacious, resistant to oil and grease, and are not affected by slight thermal variations. Besides, the films are also impermeable to oxygen, non-toxic, biodegradable, and edible (Leathers 2003). However, as quoted earlier (Hilares et al. 2019), pullulan is a costly product which is generally produced from hydrolyzed starch with prices ranging from US$30 to US$40/Kg for food grade pullulan, thereby limiting its well-acclaimed applications. To make its development cost-effective, one of the strategies is to exploit cheaper and nutritionally rich sources to sustain the growth of A. pullulan (An et al. 2017). Municipal solid waste (MSW) including sugarcane bagasse (Hilares et al. 2017), rice hull (Wang et al. 2014), and sweet potato (Wu et al. 2009) are being exploited as cheap sources of carbon for production of pullulan by A. pullulans.
A developing country like India which is subjected to rapid economic development and constant increase in population results in increased generation of MSW. This proportionately enhances the plastic production, as 25% of MSW is non-biodegradable, which is associated with the problem of its disposal. MSW collected from the cities is disposed of at the defined sites around the cities. However, due to revenue constraints, the collection centers often remain inappropriately equipped. Therefore, municipalities are not able to cater to high costs involved in the whole process of proper disposal of MSW. This gradually results in massive accumulation of MSW at the designated collection sites. Such practices culminate into serious concerns of health and environment. These landfills not only result in contamination of groundwater due to production of leachates but also serve as niches for breeding of harmful insects, rodents, and pests. This leads to generation of bad odors and accumulation of methane gas which may result in explosions causing serious health hazards particularly for the communities residing in the surrounding areas.
Keeping in view the necessity of the disposal of food waste residues in a scientific way and a need to develop a cost-effective process for the production of pullulan, the present study was executed using MSW, which comprises of 60% biodegradable (BMSW) component. This biodegradable fraction being rich in carbohydrates with proteins, minerals, and vitamins is considered as complete food for microorganism. Therefore, BMSW can be acted upon by microorganisms and transformed into several value-added products including exopolysaccharides such as gellan gum, xanthum gum, and bioplastics. This study, thus envisages use of carbohydrate rich, zero value food waste residues for bioconversion into pullulan and evaluation of its use for the production of packaging materials, as the substitute for plastic bags, which are mainly responsible for environmental pollution. The outcome of the study may prove to be beneficial for production of eco-friendly biodegradable plastic by managing the biodegradable municipal waste.
Materials and methods
Fungal strain
The yeast strain Aureobasidium pullulans MTCC 2013 was procured from Microbial Type Culture Collection of Institute of Microbial Technology, Chandigarh, India. Identity of the strain was confirmed on the basis of its morphological and microscopic characteristics. The strain was cultivated on Yeast extract peptone dextrose (YPD) (Yeast extract 1%, peptone 2%, dextrose 2%) medium and was incubated at 28 °C for 48 h. After 48 h, colony morphology was studied by staining with lactophenol blue dye and examining under light microscope. The culture was stored in the form of glycerol stocks and YPD agar slants.
Enzyme cocktail
Enzyme cocktail produced in-house using solid-state cultures of Aspergillus niger P-19 (Chugh et al. 2016) earlier in the laboratory was used in the present study also. The enzyme cocktail comprised multiple carbohydrases including CMCase (4.5 IU/ml), FPase (1.25 IU/ml), β-glucosidase (3.3 IU/ml), xylanase (180 IU/ml), mannanase (6.8 IU/ml), pectinase (13.0 IU/ml), α-amylase (1700 IU/ml), and glucoamylase (21.0 IU/ml).
Collection and pre-treatment of kitchen waste
The segregated kitchen waste (KW) was collected from a hostel of Panjab University, Chandigarh, India. The waste was dried, grounded properly, and was autoclaved for 30 min. The total carbohydrate content present in the grounded KW was estimated by Anthrone method (Dreywood 1946).
Bioconversion of carbohydrates present in the kitchen waste into free sugars
After cooling the autoclaved KW, 350 g KW was mixed with in-house produced enzyme cocktail. For the bioconversion, the enzyme dose used (per gram dry KW) was 9 U CMCase, 2.5 U FPase, 6.6 U β-glucosidase, 360 U xylanase, 13.6 U mannanase, 26 U pectinase, 3400 U α-amylase, and 42 U glucoamylase/gds. For this, 350 g autoclaved KW was mixed with 700 ml enzyme preparation and the final volume was adjusted to 3500 ml by the addition of sterile distilled water. This mixture was then added to a fermenter of 5 l capacity (pH 5.0; aeration 1 vvm; temperature 28 °C; rpm 150). The effectiveness of hydrolysis was checked by withdrawing the samples periodically at an interval of 24 h and analyzing for the release of total reducing sugar by DNSA and glucose by GOD-POD Kit. Free sugars so produced were used for pullulan production by the yeast A. pullulans.
Estimation of total reducing sugar
Total reducing sugars were estimated by Anthrone method (Dreywood 1946). Briefly, 100 mg of the sample was taken in 100 ml volumetric flask. To this, 5 ml of 2.5 N HCl was added and the contents were boiled for 3 h for the complete hydrolysis of the substrate. After boiling, the sample was neutralized with sodium bicarbonate till the effervescence was ceased. The volume was made upto 100 ml thereafter and the contents were centrifuged. To 1.0 ml of the supernatant taken in a fresh test tube, 4.0 ml of freshly prepared Anthrone reagent (prepared by dissolving 200 mg anthrone in 100 ml chilled sulfuric acid) was dispensed. The tubes were kept for boiling for 8 min and cooled rapidly. The absorbance was read at 630 nm and the concentration of total carbohydrates was calculated as per the standard graph plotted using different concentrations of glucose (10–100 μg/ml).
Glucose estimation
Glucose (GOD-PAP) diagnostic reagent kit from Reckon Diagnostics Pvt. Ltd. was used for glucose estimation in the sample. For this, 0.1 ml of appropriately diluted sample was taken in a test tube and was mixed with 1.0 ml of working reagent. The assay was performed as per the manufacturer’s instructions. Glucose (1 mg/ml) was used as standard.
Preparation of A. pullulans inoculum
The inoculum was prepared in YPD broth by inoculating 100 ml of the broth with pure culture of Aureobasidium pullulans strain from freshly prepared slant under sterilized conditions. The broth was incubated under shaking conditions at 150 rpm and 28 °C to obtain a cell count of 1 × 108 cells. Five percent of this inoculum was used for further inoculations.
Fermentation of hydrolysates obtained from the kitchen waste to pullulan by A. pullulans
The hydrolyzed KW was supplemented with peptone and yeast extract (0.25% each) (pH adjusted to 6.5) and was autoclaved at 15 psi for 15 min for proper sterilization. The hydrolyzed medium was inoculated with A. pullulans MTCC 2013 culture and was incubated at 28 °C at 150 rpm for 5 days. Samples were withdrawn periodically from the culture to determine the biomass, residual sugars, and pullulan production.
Biomass estimation
For biomass estimation, 2.0 ml samples were drawn periodically in pre-weighed micro centrifuge tubes. The samples were centrifuged at 10,000 rpm for 10 min. After centrifugation, supernatant was transferred to falcon tubes for sugar and pullulan yield estimation. The tube containing the pellet was placed in hot air oven at 60 °C overnight for drying the biomass. Weight of the micro centrifuge containing dry biomass was again taken to estimate weight of biomass.
Pullulan estimation
Pullulan estimation was carried out by the method of Sugumaran et al. 2013 with slight modifications. Briefly, 1.0 ml of the supernatant (obtained while performing biomass estimation) was transferred to a pre-weighed falcon tube. Supernatant was mixed with chilled absolute ethanol in 1:2 ratios at 4 °C for 1 h to precipitate the crude pullulan followed by centrifugation at 5000 RPM for 10 min. The precipitates were again washed with absolute ethanol and kept in hot air oven at 60 °C overnight to dry it completely. The falcon was again weighed to measure dry weight of pullulan.
Effect of supplementation of nitrogen sources on the growth of A. pullulans and EPS production
The effect of various nitrogen sources on the growth of A. pullulans and EPS production was studied by supplementing the kitchen waste with various organic nitrogen sources (Urea, peptone, yeast extract, soybean meal, tryptone) and inorganic nitrogen sources (Sodium nitrite, ammonium chloride, sodium nitrate, potassium nitrate, and ammonium sulfate) at a final concentration of 0.5% w/v. Control was run in parallel with peptone and yeast extract (0.25% each) as the nitrogen source.
Effect of varying concentrations of yeast extract on the growth of A. pullulans and EPS production
Since yeast extract was found to be the best nitrogen source for EPS production, different concentrations of yeast extract (0.5–3.5%) were tried to find out the optimal concentration of yeast extract for maximum possible pullulan production by A. pullulans.
Structural characterization of the EPS produced
Fourier-transform infrared spectroscopy
FTIR spectroscopy of the precipitated sample was performed for product analysis and pullulan from Sigma was used as standard. One hundred milligrams of completely dry sample was finely crushed and blended manually, till it formed a powder. The technique was performed in the Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, using the instrument Spectrum 400 by Perkin Elmer, USA. FTIR was carried out according to the potassium bromide pellet method and the samples were analyzed in the range of 4000–5000 cm−1.
H nuclear magnetic resonance spectroscopy
The NMR spectra were obtained using a Bruker Advance 400 MHz spectrometer with a 5-mm inverse probe available at SAIF facility Panjab University, Chandigarh. For analysis, 100 mg of sample was dried, crushed, and dissolved in D2O (99.96%). Pullulan from Sigma was used as standard.
Casting of pullulan film
Biopolymer film forming potential of the exopolysaccharide formed from kitchen waste was determined by the method described by Kandemir et al. 2005. Briefly, 100 ml of 1.5% (w/v in distilled water) solution of the laboratory-made pullulan was mixed with 2 ml of 4% acetic acid and 1 ml of glycerol. The mixture was concentrated by boiling till the total volume reduces to 1/4 of the original volume. The concentrate was pipetted and spread on to the plastic petriplates (15 cm diameter). These petriplates were kept for drying the content in an incubator overnight at 40 °C. Later, the transparent film was removed from the petriplates very carefully.
Quality assessment of developed pullulan
Evaluation of organoleptic properties
In regard to food and industrial applications, the organoleptic properties of pullulan, including color, odor, and taste of the EPS, were studied as per method of food safety organization and the results were obtained on the basis of experience made by the senses and visualization. Odor and taste was evaluated by smelling and tasting.
Strength of the developed pullulan film
Strength of the developed film was evaluated using standard procedure with the help of Texture Analyzer (TA.XT Plus, Stable Microsystems) (Sánchez-González et al. 2009). Specimens with dimension (1.0 cm wide × 6 cm long) were cut from the pullulan film and were evaluated for thickness, tensile strength, and elongation at break.
Water solubility
The water solubility of the developed pullulan film was ascertained by cutting a piece (10 mg) of the film and dissolving it in 1.0 ml of sterile distilled water. Different aliquots of the water were removed aseptically at different time intervals (48 h and 168 h) to check the total sugar content released in the water from film.
Validation of the biodegradability of the developed film
Biodegradation of the developed pullulan film was checked in the natural way by dumping it in the soil pots and observing its degradation over a period of 0–25 days using synthetic plastic as the control.
Statistical analysis
All the values have been expressed as mean ± standard deviation of three independent experiments with three replicates each. The results were statistically analyzed using Student’s t test.
Results
Identification of the fungal strain
The colonies of A. pullulans were observed to be irregular, leathery and non-sticky, opaque, and elevated from the center. The colonies were initially cream in color but later turned light pink. Microscopically, vegetative and hyaline hyphae with transversely septate were seen. Budding of dark brown conidia and chlamydospores was frequently seen.
Bioconversion of carbohydrates present in kitchen waste into free sugars
The in-house produced enzyme cocktail added to the kitchen waste for the bioconversion of the polysaccharides into the simple sugars revealed that the hydrolysis was initiated within initial 24 h (Fig. 1). Sugar release increased gradually upon further incubation and analysis was done until the sugar release reached a constant value, indicating the complete hydrolysis. After day 1, total reducing sugar and glucose released was 25 ± 1.9 g/l (250 mg/g) and 15 ± 1.2 g/l (150 mg/g), respectively, which corresponds to 0.94 g/l/h and 0.56 g/l/h, respectively. Further, on day 2, the levels of total reducing sugar and glucose released were 35 ± 2.3 g/l (350 mg/g) and 21 ± 2.1 g/l (210 mg/g), respectively, which corresponds to 0.416 g/l/h and 0.25 g/l/h, respectively. The total reducing sugar and glucose release was 40 ± 3.1 g/l (400 mg/g) and 28 ± 2.7 g/l (280 mg/g) on day 3, and 45 ± 3.3 g/l (450 mg/g) and 30 ± 2.3 (300 mg/g) on day 4, respectively. By the 5th day, total reducing sugar and glucose levels were 46 ± 3.5 g/l (460 mg/g) and 31 ± 2.2 g/l (310 mg/g), respectively.
Fermentation of hydrolysates obtained from kitchen waste to pullulan by A. pullulans
Figure 2 depicts the residual reducing sugars, biomass produced, and EPS formed when KW hydrolysate containing sugars was fermented by A. pullulans. Maximum EPS yield was obtained on the 5th day of fermentation and decrease in the concentration of residual reducing sugars was observed with the increase in the biomass.
Effect of supplementation of nitrogen sources on the growth of A. pullulans and EPS production
Nitrogen has been reported to be an essential component for cellular growth and EPS production (Göksungur et al. 2004; Yang et al. 2018). The effect of supplementation of different organic and inorganic nitrogen sources on EPS production has been shown in Table 1. Out of the various nitrogen sources used, yeast extract was found to support maximum biomass (20.54 g/l) and pullulan (20.46 g/l) yields. The values were found to be comparable with the control containing yeast extract and peptone (0.25% each). In case of other nitrogen sources, both the biomass and pullulan production was reduced significantly as compared with the control.
Effect of varying concentration of yeast extract on the growth of Aureobasidium pullulans and EPS production
The yield of EPSs can be influenced by the varied concentrations of different nitrogen sources (Klein and Kupper 2018). Since yeast extract was found to be optimal for EPS production in our study, supplementation of KW with different concentrations (0.5 to 3.5%) of yeast extract was also optimized. The results are shown in Table 2. Both the cell growth (30.70 ± 1.05) and pullulan yields (24.77 ± 1.06) were found to be increased with increasing concentrations of yeast extract (upto 3%). Further increase in yeast extract concentration (3.5%) leads to a decrease in both the biomass and pullulan yields. Figure 3 summarizes the schematic representation of the various steps involved in pullulan production from kitchen waste.
Structural characterization of the EPS
FTIR
Fourier-transform infrared (FTIR) spectroscopy of laboratory-made pullulan sample was performed and FTIR spectrum of commercial pullulan (Sigma) was used as reference. Basically, FTIR spectrum reveals the type of functional groups present in test EPS. IR spectra showed absorption bands and peaks characteristic of carbohydrate, carboxylate, and hydroxyl groups. Supplementary figure S1a and S1b shows the FTIR spectra of standard pullulan and spectra of laboratory-made pullulan from kitchen waste, respectively. The strong absorptions at 3298.3 cm−1 and 2936.0 cm−1 indicated that the EPS has some repeating units of –OH (found in sugars) and C–H bonds (found in alkane compounds), respectively. Similar features were observed in the spectra of both standard pullulan and the pullulan produced from KW in the specific area (1500–650 cm−1), which is characteristic for the pullulan molecule. Absorptions at 845.8 cm−1, 756.2 cm−1, and 1040.1 cm−1 are characteristic of the α-d-glucopiranoside units, α-(1-4)-d-glucosidic bonds, and α-(1-6)-d-glucosidic bonds, respectively. The results of the FTIR analysis confirmed that the EPS produced by growing Aureobasidium pullulans on kitchen waste is pullulan (Choudhury et al. 2012; Mitić et al. 2018). Functional groups along with particular absorption present in standard exopolysaccharide from kitchen waste have been mentioned in Table 3.
H NMR spectroscopy
One hundred milligrams of very well-dried and crushed sample along with pullulan standard was analyzed at NMR facility available at SAIF, Panjab University, Chandigarh. H nuclear spectra of laboratory-made pullulan was compared with respective spectra of standard pullulan obtained from sigma. Pullulan is a glucose polysaccharide consisting of maltotriose [α(1 → 4)] units attached by a [α(1 → 6)] linkages. H NMR spectra recorded in this study included all the protons of pullulan but the anomeric protons were focus of interest for comparison of pullulan with standard pullulan sample. Anomeric protons which are protons attached to number 1 or anomeric carbon of monosaccharide ring were well resolved from all other carbohydrate protons and showed resonance in the region from δ = 4.4 to 5.6. Resonance positions of anomeric protons of α (1 → 4) linkages were 5.3281, 5.3498 and 5.2917, 5.2830 and in laboratory-made pullulan by growing Aureobasidium pullulans on kitchen waste hydrolysate, while standard pullulan sample showed resonance at 5.3275, 5.3179 and 5.2908, 5.2813. The resonance positions of anomeric proton of α (1 → 6) and linkage in laboratory-made pullulan and standard pullulan were 4.8834, 4.8755 and 4.9928, 4.8740, respectively. The other protons overlapped in the region between δ = 3.0 and δ = 4.0. H NMR spectra of standard and laboratory-made pullulan from kitchen waste is shown in figure S2a and S2b, respectively.
Casting of the laboratory-made pullulan film and its characterization
The film casting potential of the pullulan produced from KW was evaluated at a concentration of 1.5% (w/v in distilled water) (as per the standardizations in our laboratory). A readily peelable film was formed at this concentration (Fig. 4a)
Quality assessment of pullulan
Evaluation of the organoleptic properties
Organoleptic properties play an important role for the appeal and acceptance of the product and also aids in the commercialization of a product. In the present study, the organoleptic characterization of the EPS was done and the results are shown in the Table 4. The pattern of the results obtained suggests that the developed EPS can be used in the bio-packaging as well as in the food industry for suitable applications. The organoleptic properties of the developed EPS were found to be very similar to that of standard pullulan.
Strength and water solubility of the developed pullulan film
The thickness and tensile strength of the developed pullulan film was found to be 0.113 ± 0.025 mm and 785.831 g force, respectively. The elongation at break was found to be 54.914 mm distance.
Further, the water solubility of the developed pullulan film was evaluated by placing a piece of the film (10 mg/ml) in sterile distilled water and assessing the release of sugar content thereafter. After 48 h, 3.7 mg/ml sugar content was recorded indicating the release of sugar content from the film into the water. The released sugar content was 9.53 mg/ml after 7 days.
Validation of the biodegradability of the developed bio-packaging film
The biodegradability of the developed bio-packaging film by soil microflora was checked by placing developed pullulan film over the soil sample in pots. Figure 4 b shows the biodegradability of the developed pullulan film. Panels 1 and 2 in the figure shows placing and labeling of the pots. Pots P1 and P2 show the placement of conventional plastic samples of different strengths. T1 is the test sample of laboratory-made pullulan film. The pot wherein no material was placed served as control (C). Panel 3 shows that these pots were covered with soil. Interestingly, the developed pullulan film was degraded by the 7th day. This experiment (repeated in triplicates) showed that conventional plastic could not be degraded by microflora of soil whereas the film formed by the laboratory-made pullulan got degraded as early as in 7 days. In order to assess the role of microflora in degradation, a sample from the same soil was autoclaved in a flask wherein the film was placed, incubated, and observed for several days. Film could not get degraded by the sterile soil devoid of microbiota.
Discussion
Though pullulan possesses many useful properties, high cost of its production limits its potential applications. Various agro-industrial wastes are being used for the economical production of pullulan. In the present study, we used biodegradable kitchen waste hydrolyzed by in-house produced multiple enzyme cocktail for economical production of pullulan. Enzymatic hydrolysis offers many advantages like high substrate specificity, hydrolysis under mild conditions, and low corrosion problem, thus making the process environmental friendly. Enzymatic saccharification of biomass has been performed for a variety of substrates including agricultural wastes for the production of various valuable products like lactic acid and ethanol (Duff and Murray 1996; Thomas et al. 2016; Adsul et al. 2007). Since kitchen waste contains a variety of carbohydrates in high amounts which can be converted to fermentable sugars, in the present study, we used a cocktail of enzymes for the efficient saccharification of the kitchen waste. The cooperative action of different enzymes like cellulases, amylases, and hemicellulases has been reported to enhance the efficiency of biomass hydrolysis, resulting in higher sugar release (Duff and Murray 1996). Prior reports are also available wherein kitchen waste has been reported to be enzymatically hydrolyzed for the release of sugars (Cekmecelioglu and Uncu 2013; Li et al. 2016; Kiran et al. 2015). It may be noted, though sucrose is the preferred substrate for pullulan production; however, several reports are available that indicate the use of glucose, starch, and xylose for pullulan production by A. pullulans (Barnett et al. 1999; Chen et al. 2014; Duan et al. 2008; Singh et al. 2008b). In the present study, we hydrolyzed kitchen waste which is heterogenous in composition containing starch, cellulose, hemicellulose, and pectin and the resultant hydrolysate contained mainly glucose and xylose which might have contributed to the production of pullulan. Although kitchen waste is not a uniform material and its composition varies depending upon the factors like consumption, custom of the family, and the season, three main polysaccharides invariably constitute the kitchen waste including cellulose, hemicelluloses, and starch. The sugars produced upon the hydrolysis of these polysaccharides produce a mixture of glucose and xylose with variation in the relative proportion depending upon the composition of kitchen waste. Both these sugars can be utilized by Aureobasidium pullulans for pullulan production.
Enzymatic hydrolysis of other substrates using multiple enzymes has also been carried out by different researchers, e.g., an enzyme preparation containing combination of enzymes; cellulase from Penicillium chrysogenum BCC4504 and β-glucosidase and xylanase from Aspergillus flavus BCC7179 was reported to markedly improve saccharification of sugarcane bagasse (84.0% and 70.4% for glucose and xylose, respectively) as compared with the individual enzyme preparations (Buaban et al. 2010) which may be attributed to the improved saccharification of the disaccharides. In a recent study wherein CRW (coffee residue waste) samples were hydrolyzed with cellulase and pectinase (in-house produced), a total sugar yield of 59.2 g/l was reported (Nguyen et al. 2017). Likewise, Thomas et al. 2016 used a cocktail of crude xylanase from Aspergillus sp. supplemented with cellulose (Zytex, India) and β-glucosidase (In-house) for the saccharification of rice straw (alkali-pretreated) and higher total reducing sugars (574.8 mg/g) were reported with the use of enzyme cocktail in comparison to the cocktail without xylanase (430.2 mg/g).
Though higher yield of pullulan has been reported using pure glucose (Xue et al. 2019), to make the process commercially viable, other substrates that are being used for pullulan production include grape skin pulp, potato starch hydrolysates, spent grain palm kernel, and cassava bagasse (An et al. 2017; Arvanitoyannis et al. 2006; Aliyu and Bala, 2011; Sugumaran and Ponusami 2010). In the present study, sugars produced from kitchen waste when utilized by A. pullulans indicated that the yield of pullulan if not much higher may at least be considered at par with the yields reported by other studies (Table 5).
Among various nitrogen sources used, yeast extract was found to give maximum pullulan yield. Yeast extract is a rich source of amino acids, peptides, proteins, vitamin B and, several mineral salts (Sharmila et al. 1989; Shen et al. 1993) and therefore has been found to have positive effect on the growth of microorganisms. Sugumaran et al. (2014) studied the effect of various nitrogen sources on pullulan production by A. pullulans MTCC 2670 under solid-state fermentation wherein maximum pullulan yield (33.4 mg/gds) was reported with NaNO2 followed by yeast extract (32.1 mg/gds). In another study, soybean pomace was found to support highest (7.5 g/l) pullulan yield by A. pullulans HP-2001 followed by yeast extract (5.5 g/l) (Seo et al. 2004). Thirumavalavan et al. (2009) also reported high pullulan yields with (58.6 g/l) yeast extract. Other nitrogen sources like ammonium nitrate, ammonium sulfate, and soytone have also been reported to favor pullulan production by A. pullulans (Auer and Seviour 1990; Reed-Hamer and West 1994).
The EPS produced from KW was characterized by FTIR and NMR spectroscopic studies. The spectra generated correlated well with that of the standard pullulan (Sigma). Finally, the film produced from pullulan was found to have reasonably good tensile strength and was water soluble and biodegradable. Biodegradability of pullulan in soil may be attributed to different microbiota present in the soil as the sterile soil could not do so.
The study indicates the process development for the management of kitchen waste (which otherwise is subjected to landfilling thus causing global warming), in addition to obtaining an appreciable yield of pullulan. In the previous studies, different substrates like coconut kernel, palm kernel, rice bran, sugarcane bagasse, and wheat bran have been evaluated for the production of pullulan by A. pullulans. Kitchen waste, a major component of BMSW, considered as zero-value food waste residue, serves as a complete food for the microorganisms. Therefore, kitchen waste may be exploited as yet another substrate to develop biodegradable packaging material with appreciable yields. It is worth noting that (i) though KW has impurities in addition to polysaccharides, pullulan is synthesized from simple sugars derived from the breakdown of polysaccharides, so other components/impurities are not likely to affect the properties of pullulan, (ii) even if pullulan is sticky (at higher concentrations), it can be purified simply by precipitation, and (iii) the whole process of conversion of KW to pullulan appears to be economically viable because in-house generation of enzyme cocktail produced from a natural variant of A. niger has been used in the present study as reported by us earlier (Chugh et al. 2016). This study gives the proof of concept of production of bioplastic and its biodegradability. Further studies are underway to prepare such bio-packaging material of different strengths required for different purposes along with the assessment of their shelf-lives at different temperatures. Thus, this double-edged approach of managing the BMSW in a scientific way indicates a great potential of usage of KW for the production of value-added products such as biodegradable pullulan resulting in low-cost and eco-friendly product.
References
Adsul MG, Varma AJ, Gokhale DV (2007) Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem 9(1):58–62. https://doi.org/10.1039/B605839F
Aliyu S, Bala M (2011) Brewer’s spent grain: a review of its potentials and applications. Afr J Biotechnol 10(3):324–331. https://doi.org/10.5897/AJBx10.006
An C, Ma SJ, Chang F, Xue WJ (2017) Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol 48(1):180–185. https://doi.org/10.1016/j.bjm.2016.11.001
Arvanitoyannis IS, Ladas D, Mavromatis A (2006) Potential uses and applications of treated wine waste: a review. Int J Food Sci Technol 41(5):475–487. https://doi.org/10.1111/j.1365-2621.2005.01111.x
Auer DP, Seviour RJ (1990) Influence of varying nitrogen sources on polysaccharide production by Aureobasidium pullulans in batch culture. Appl Microbiol Biotechnol 32(6):637–644. https://doi.org/10.1007/BF00164732
Barnett C, Smith A, Scanlon B, Israilides CJ (1999) Pullulan production by Aureobasidium pullulans growing on hydrolysed potato starch waste. Carbohydr Polym 38:203–209. https://doi.org/10.1016/S0144-8617(98)00092-7
Buaban B, Inoue H, Yano S, Tanapongpipat S, Ruanglek V, Champreda V, Pichyangkura R, Rengpipat S, Eurwilaichitr L (2010) Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J Biosci Bioeng 110(1):18–25. https://doi.org/10.1016/j.jbiosc.2009.12.003
Cekmecelioglu D, Uncu ON (2013) Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manag 33(3):735–739. https://doi.org/10.1016/j.wasman.2012.08.003
Chen Y, Guo J, Li F, Liu M, Zhang X, Guo X, Xiao D (2014) Production of pullulan from xylose and hemicellulose hydrolysate by Aureobasidium pullulans AY82 with pH control and DL-dithiothreitol addition. Biotechnol Bioprocess Eng 19(2):282–288. https://doi.org/10.1007/s12257-013-0715-4
Choudhury AR, Bhattacharyya MS, Prasad GS (2012) Application of response surface methodology to understand the interaction of media components during pullulan production by Aureobasidium pullulans RBF-4A3. Biocatal Agric Biotechnol 1(3):232–237. https://doi.org/10.1016/j.bcab.2012.02.003
Chugh P, Soni R, Soni SK (2016) Deoiled rice bran: a substrate for co-production of a consortium of hydrolytic enzymes by Aspergillus niger P-19. Waste Biomass Valori 7(3):513–525. https://doi.org/10.1007/s12649-015-9477-x
Dreywood R (1946) Qualitative test for carbohydrate material. Ind Eng Chem Anal Ed 18(8):499–499
Duan X, Chi Z, Wang L, Wang X (2008) Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohydr Polym 73:587–593. https://doi.org/10.1016/j.carbpol.2007.12.028
Duff SJ, Murray WD (1996) Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour Technol 55(1):1–33
Göksungur Y, Uçan A, Güvenç U (2004) Production of pullulan from beet molasses and synthetic medium by Aureobasidium pullulans. Turk J Biol 28(1):23–30
Göksungur Y, Uzunoğulları P, Dağbağlı S (2011) Optimization of pullulan production from hydrolysed potato starch waste by response surface methodology. Carbohydr Polym 83(3):1330–1337. https://doi.org/10.1016/j.carbpol.2010.09.047
Hilares RT, Orsi CA, Ahmed MA, Marcelino PF, Menegatti CR, da Silva SS, dos Santos JC (2017) Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour Technol 230:76–81. https://doi.org/10.1016/j.biortech.2017.01.052
Hilares RT, Resende J, Orsi CA, Ahmed MA, Lacerda TM, da Silva SS, Santos JC (2019) Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int J Biol Macromol 127:169–177. https://doi.org/10.1016/j.ijbiomac.2019.01.038
Kandemir N, Yemeniciogwlu A, Mecitogwlu Ç, Elmaci ZS, Arslanogwlu A, Göksungur Y, Baysal T (2005) Production of antimicrobial films by incorporation of partially purified lysozyme into biodegradable films of crude exopolysaccharides obtained from Aureobasidium pullulans fermentation. Food Technol Biotechnol 43(4):343–350
Kiran EU, Trzcinski AP, Liu Y (2015) Enhancing the hydrolysis and methane production potential of mixed food waste by an effective enzymatic pretreatment. Bioresour Technol 183:47–52. https://doi.org/10.1016/j.biortech.2015.02.033
Klein MN, Kupper KC (2018) Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol 69:1–10. https://doi.org/10.1016/j.fm.2017.07.008
Leathers TD (2003) Biotechnological production and applications of pullulan. Appl Microbiol Biotechnol 62(5-6):468–473. https://doi.org/10.1007/s00253-003-1386-4
Li Y, Jin Y, Li J, Li H, Yu Z (2016) Effects of thermal pretreatment on the biomethane yield and hydrolysis rate of kitchen waste. Appl Energy 172:47–58. https://doi.org/10.1016/j.apenergy.2016.03.080
Mitić Ž, Nikolić GM, Cakić M, Nikolić GS, Živanović S, Mitić S, Najman S (2018) Synthesis, spectroscopic and structural characterization of Co (II)-pullulan complexes by UV-Vis, ATR-FTIR, MALDI-TOF/TOF MS and XRD. Carbohydr Polym 200:25–34. https://doi.org/10.1016/j.carbpol.2018.07.032
Morris VJ (1995) Food polysaccharides and their applications, in: A. M. Stephen (Ed.), Marcel Dekker, New York, pp. 341–375.
Nguyen QA, Cho E, Trinh LT, Jeong JS, Bae HJ (2017, 244) Development of an integrated process to produce D-mannose and bioethanol from coffee residue waste. Bioresour Technol:1039–1048. 1039-1048. https://doi.org/10.1016/j.biortech.2017.07.169
Reed-Hamer B, West TP (1994) Effect of complex nitrogen sources on pullulan production relative to carbon source. Microbios 80(323):83–90
Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M (2009) Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll 23(8):2102–2109. https://doi.org/10.1016/j.foodhyd.2009.05.006
Seltenrich N (2015) New link in the food chain? Marine plastic pollution and seafood safety. Environ Health Perspect 123(2):A34–A41. https://doi.org/10.1289/ehp.123-A34
Seo HP, Son CW, Chung CH, Jung DI, Kim SK, Gross RA, Kaplan DL, Lee JW (2004) Production of high molecular weight pullulan by Aureobasidium pullulans HP-2001 with soybean pomace as a nitrogen source. Bioresour Technol 95(3):293–299. https://doi.org/10.1016/j.biortech.2003.02.001
Shamsuddin IM, Jafar JA, Shawai AS, Yusuf S, Lateefah M, Aminu I (2017) Bioplastics as better alternative to petroplastics and their role in national sustainability: a review. Adv Biosci Biotechnol 5(4):63. https://doi.org/10.11648/j.abb.20170504.13
Sharmila M, Ramanand K, Sethunathan N (1989) Effect of yeast extract on the degradation of organophosphorus insecticides by soil enrichment and bacterial cultures. Can J Microbiol 35(12):1105–1110. https://doi.org/10.1139/m89-185
Shen CF, Kosaric N, Blaszczyk R (1993) Properties of anaerobic granular sludge as affected by yeast extract, cobalt and iron supplements. Appl Microbiol Biotechnol 39(1):132–137. https://doi.org/10.1007/BF00166862
Shingel KI (2004) Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr Res 339(3):447–460. https://doi.org/10.1016/j.carres.2003.10.034
Singh P, Suman A, Tiwari P, Arya N, Gaur A, Shrivastava AK (2008a) Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J Microbiol Biotechnol 24(5):667–673. https://doi.org/10.1007/s11274-007-9522-4
Singh RS, Saini GK, Kennedy JF (2008b) Pullulan: microbial sources, production and applications. Carbohydr Polym 73:515–531. https://doi.org/10.1016/j.carbpol.2008.01.003
Sugumaran KR, Ponnusami V (2010) Conventional optimization of aqueous extraction of pullulan in solid-state fermentation of cassava bagasse and Asian palm kernel. Biocatal Agric Biotechnol 10:204–208. https://doi.org/10.1016/j.bcab.2017.03.010
Sugumaran KR, Gowthami E, Swathi B, Elakkiya S, Srivastava SN, Ravikumar R, Gowdhaman D, Ponnusami V (2013) Production of pullulan by Aureobasidium pullulans from Asian palm kernel: a novel substrate. Carbohydr Polym 92(1):697–703. https://doi.org/10.1016/j.carbpol.2012.09.062
Sugumaran KR, Jothi P, Ponnusami V (2014) Bioconversion of industrial solid waste—cassava bagasse for pullulan production in solid state fermentation. Carbohydr Polym 99:22–30. https://doi.org/10.1016/j.carbpol.2013.08.039
Thirumavalavan K, Manikkadan TR, Dhanasekar R (2009) Pullulan production from coconut by-products by Aureobasidium pullulans. Afr J Biotechnol 8:254–258
Thomas L, Parameswaran B, Pandey A (2016) Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew Energy 98:9–15. https://doi.org/10.1016/j.renene.2016.05.011
Wang D, Ju X, Zhou D, Wei G (2014) Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresour Technol 164:12–19. https://doi.org/10.1016/j.biortech.2014.04.036
Wu S, Jin Z, Tong Q, Chen G (2009) Sweet potato: a novel substrate for pullulan production by Aureobasidium pullulans. Carbohydr Polym 76(4):645–649. https://doi.org/10.1016/j.carbpol.2008.11.034
Xue SJ, Chen L, Jiang H, Liu GL, Chi ZM, Hu Z, Chi Z (2019) High pullulan biosynthesis from high concentration of glucose by a hyperosmotic resistant, yeast-like fungal strain isolated from a natural comb-honey. Food Chem 286:123–128. https://doi.org/10.1016/j.foodchem.2019.01.206
Yang J, Zhang Y, Zhao S, Zhou Q, Xin X, Chen L (2018) Statistical optimization of medium for pullulan production by Aureobasidium pullulans NCPS2016 using fructose and soybean meal hydrolysates. Molecules 23(6):1334. https://doi.org/10.3390/molecules23061334
Acknowledgments
The authors gratefully acknowledge Assistant Professor Gargi Ghoshal, University Institute of Chemical Engineering and Technology, Panjab University, Chandigarh, for the help provided by her in an experimental procedure in the study. We also acknowledge Ms. Shania Vij for providing secretarial assistance and Mr. Abhishek Thakur for helping in the collection of the kitchen waste. The authors also acknowledge Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, India, for providing assistance in performing FTIR and H NMR.
Author information
Authors and Affiliations
Contributions
SKS and SS conceived and designed the study. VR, AKS, and JS performed the experiments. SKS, AK, and SS analyzed the data. AK and VR drafted the manuscript. SKS, AK, and SS read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 395 kb)
Rights and permissions
About this article
Cite this article
Rishi, V., Sandhu, A.K., Kaur, A. et al. Utilization of kitchen waste for production of pullulan to develop biodegradable plastic. Appl Microbiol Biotechnol 104, 1307–1317 (2020). https://doi.org/10.1007/s00253-019-10167-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10167-9


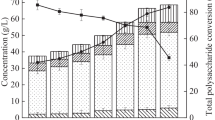


 , biomass produced during fermentation
, biomass produced during fermentation  , and EPS produced from the hydrolysate during fermentation
, and EPS produced from the hydrolysate during fermentation 

