Abstract
With the aim to utilize deoiled rice bran, an agro-industrial waste, as a feedstock for the co-production of multiple carbohydrases, a fungal strain was isolated which could utilize DORB to co-produce a consortium of cellulases, hemicellulases, pectinase and amylases and was named as Aspergillus niger P-19 after molecular identification. Further, optimization for the co-production of all the enzymes was carried out by one factor at a time approach. Time profile studies of the production of enzymes revealed that 5th day of incubation was best suited for the extraction of enzymes. An initial solid to moisture ratio of 1:1.5 and an inoculum size of 5 × 107 spores gds−1 were found to be optimum for maximum productivities. Enzyme yields were significantly improved with the exogenous supplementation of carbon source, nitrogen source, surfactants and lignocellulosic inducers. This is the first report of its kind where DORB has been utilized for the co-production and co-optimization of eight different enzymes which can have a potential application in biofuel industry as the enzyme preparation could effectively hydrolyze steam pre-treated DORB releasing a total reducing sugars of 356.17 ± 9.58 mg gds−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid rate of fossil fuel utilization and release of CO2 into the atmosphere has gathered global attention and prompted efforts to develop alternate energy using renewable sources [1]. Waste plant biomass appears to be one such promising source as it has a store of solar energy in the form of chemical bonds which can be exploited for various applications including fuel generation. Moreover, CO2 released by burning biomass in any form is equivalent to the amount of CO2 absorbed by the plant while growing [2]. This makes biomass fuel desirable as it can deal with two major problems: waste biomass disposal and depleting fossil fuels.
In pursuance of generating fuel out of waste, an economical method is required which governs the hydrolysis of all the major polysaccharides present in waste biomass [3]. Polysaccharides present in plant biomass include the part of food that is left in the waste during processing that mainly comprises of starch and the plant cell wall components which include cellulose, hemicelluloses and pectin [4]. As eco-friendly processes are always favoured, enzymatic hydrolysis of these polysaccharides is a core area of interest for fermentation biologists. Thus, a consortium of amylases, cellulases, hemicellulases and pectinase would be highly desirable which can hydrolyse nearly all the polysaccharides present in plant biomass. The ability of some microbes to produce a mixture of enzymes can be a source of such consortium. Among the various bacteria and fungi capable of producing extracellular depolymerising enzymes, the latter have been found better in terms of enzyme varieties and their yields [5]. Therefore, there is growing interest for identifying fungal species that are the producers of multiple carbohydrases.
Many of the residues like wheat straw [6], rice straw [7], groundnut waste [8], palm kernel cake [9], cassava waste [10], corn stover [11], coir waste and saw dust [12], soybean hulls [13], waste paper [14], kitchen waste residues [15], brewer’s spent grain [16] have been exploited for the production of various industrial enzymes, but deoiled rice bran (DORB) is one of the abundantly available residues which are underexploited and is generally used as an animal feed supplement. World annual rice production has increased from 472 million tonnes in 2012–2013 to 476 million tons in 2013–2014 with China and India being the top two producers [17]. One hundred kilograms of paddy on milling generally yields 56–58 kg white rice, 10–12 kg broken rice, 18–20 kg husk, and 10–12 kg rice bran [18]. It can thus be estimated that 47.6–57.12 million tonnes of rice bran could be obtained from 476 million tonnes of paddy. Rice bran, which is a by-product of rice milling industry, contains about 20 % oil, 15 % protein, 5 % lignin and approximately 50 % carbohydrates comprising cellulose, starch, hemicelluloses and pectin [18]. DORB is the left over residue after the extraction of rice bran oil from rice bran that is rich in carbohydrates which can thus be explored for the production of multiple carbohydrases. To the best of our knowledge, there is only one report where DORB has been used as a substrate for the production of enzymes in which only endoglucanase (CMCase) and α-amylase have been produced by co-culture of Aspergillus oryzae and Trichoderma reesei [19]. As it contains a mixture of polysaccharides, it can be explored for the production of a wide range of carbohydrases which has been attempted in the present study.
Above and beyond the type of substrate and microbe, the production of enzymes is also influenced by various cultural and nutritional factors like the moisture content, inoculum size, C and N sources in the medium and many more [20]. Thus, to obtain maximum yields, the recognition of suitable ingredients and cultural conditions is requisite. Although optimization for the production of enzymes has been carried out by many researchers [20–25], but there is hardly any report where the co-production of eight enzymes has been optimized for obtaining maximum yields of all the enzymes which has been tried in the present study.
In this study, we report the isolation of a fungal species capable of producing multiple carbohydrases including cellulases, hemicellulases, pectinase and amylases utilizing DORB as the feedstock. Optimization of various factors for obtaining maximum enzyme yields has also been attempted by using one variable at a time approach.
Materials and Methods
Microorganism
The fungal strain used in the study named as P-19 was isolated from decaying papers, vegetables and soil samples collected from various places of Chandigarh and Punjab, India. This strain was selected on the basis of levels of cellulolytic, hemicellulolytic, pectinolytic and amylolytic enzymes produced by solid state fermentation of DORB.
Identification of the Strain
The strain was identified on the basis of the molecular analysis done by 18S ribosomal sequencing taking the services of Xcelris, India. The phylogenetic tree was generated using the MEGA 4 software [26].
Time Course for the Production of Multiple Hydrolytic Enzymes by the Fungus Under Solid State Fermentation
The production of multiple hydrolytic enzymes was carried out under solid state cultivation conditions in 250 ml Erlenmeyer flasks containing 5 g rice bran moistened with 5 ml of distilled water. The flasks were sterilized by autoclaving at 15 psi for 20 min, inoculated with 2.5 ml of fungal spore suspension (2.5 × 107 spore/ml) and incubated at 30 °C in stationary state for 10 days. The profile of enzymes production was studied by withdrawing the flasks at regular intervals of 24 h and the enzymes were extracted by adding 200 ml of distilled water to each flask and churning the contents in a blender. After churning, the contents were filtered through metallic sieve and the solid residue was thoroughly pressed to extract the remaining liquid. Filtrate thus obtained was centrifuged at 10,000 rpm at 4 °C for 15 min and the clear supernatant thus obtained was used as enzyme preparation.
Enzyme Assays
The supernatants obtained from solid state cultures were assayed at 50 °C, pH 4.0 for cellulases (endo-β-1,4-glucanase, exo-β-1,4-glucanase, and β-1,4-glucosidase), hemicellulases (xylanase, mannanase), pectinase and amylases (α-amylase, glucoamylase) by standard protocols [27–32]. The activity of enzymes has been expressed in International Units (IU) where one unit of the CMCase, FPase, β-glucosidase, glucoamylase, xylanase, mannanase and pectinase is equivalent to the enzyme that releases one µmole of end product in one min under standard assay conditions while for α-amylase, it is equivalent to the amount of enzyme which reduces the color of starch-iodine complex by 10 % in 10 min. The productivities of the enzymes have been expressed as IU per gram dry substrate (IU gds−1).
Optimization of the Co-Production of Multiple Enzymes
Production of all the enzymes was optimized by following one variable at a time (OVAT) approach. The effect of various physic-chemical factors including substrate to moisture ratio (1:0.5–1:3), inoculum size (1 × 107 to 6 × 107 spores gds−1), supplementation of different defined carbon and nitrogen sources (2.5 % w/w on dry weight basis), surfactants (0.05 % w/w on dry weight basis) and various raw lignocellulosic residues and their delignified counterparts (10 % w/w on dry weight basis) was analyzed. The delignification of such residues was carried out by treating the powdered residues with 2 % NaOH for overnight, at room temperature and then autoclaving at 15 psi for 1 h. These were then neutralized, washed and dried before use.
Application of In-House Produced Enzyme Preparation in the Hydrolysis of DORB
Total carbohydrate of DORB was analyzed by phenol–sulphuric acid method [33]. Ten grams of DORB dispensed in 250 ml screw capped Erlenmeyer flasks containing 15 ml of 0.1 M acetate buffer, pH 4.0 was steam pre-treated at 15 psi for 15 min. This was followed by the addition of crude enzyme preparation from Aspergillus niger P-19 to make the enzyme to substrate ratio of 2, 0.3, 0.8, 20, 2, 1.6, 200 and 4 IU of CMCase, FPase, β-glucosidase, xylanase, mannanase, pectinase, α-amylase and glucoamylase respectively per gram of DORB and the final volume was made to 40 ml with buffer. Enzymatic hydrolysis was carried out by keeping the flasks in water bath shaker at 50 °C and 150 rpm for 72 h. The sample was withdrawn at a regular interval of 24 h, centrifuged at 10,000 rpm for 10 min and the supernatant was analysed for total reducing sugars by DNSA method [34] and glucose by glucose oxidase–peroxidase method [35].
Data Analysis
All the values have been expressed as mean ± standard deviation of three independent experiments with three replicates each. The results were statistically analysed using one way analysis of variance with the Holm–Sidak method using SigmaPlot (Systat Software, San Jose, CA).
Results
Screening and Isolation of Fungal Strain
After massive screening, a total of 12 fungal isolates were subjected to solid state fermentation of DORB to check for the production of cellulolytic enzymes. Among them, strain P-19 was selected as it was able to produce all the required carbohydrases like cellulases, hemicellulases, pectinase and amylases in appreciable amounts by solid state fermentation of DORB.
Molecular Identification of the Fungal Strain
18S rDNA sequencing of the fungal strain P-19 revealed 855 bp sequence and it was found to share maximum similarity with the species of genus Aspergillus. The aligned sequence has been submitted to Genbank and has an accession number KT336354. Phylogenetic analysis through the alignment and cladistic analysis of homologues nucleotide sequences revealed that it was closely related to the strain of Aspergillus niger PRK3 (NCBI accession no. KJ938685.1) and was thus named as A. niger P-19.
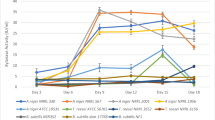
Time Course of Enzyme(s) Production by A. niger P-19
Aspergillus niger P-19 colonised well under SSF on DORB and started producing all the enzymes as early as after 1 day of incubation (Fig. 1). The peak of CMCase (113.28 ± 2.16 IU gds−1) production was obtained at the end of 5th day, whereas maximum FPase (31.34 ± 1.04 IU gds−1) and β-glucosidase (33.08 ± 0.51 IU gds−1) were produced after 4 and 8 days respectively. Xylanase production gave a sharp peak (996.81 ± 25.37 IU gds−1) at the completion of 4 days of incubation and mannanase was obtained maximally (86.72 ± 0.38 IU gds−1) after 5 days. Pectinase production peaked (93.19 ± 2.19 IU gds−1) at the end of 6th day. Among the amylases, production of α-amylase was maximum (32,200 ± 220 IU gds−1) after 5 days while glucoamylase gave highest yield (247.25 ± 7.12 IU gds−1) after 6 days. Though all the enzymes had different demands of incubation time to give their production maxima, 5 days of incubation was chosen for further studies as this time period was giving appreciable yields of all the enzymes with least compromises corresponding to 113.28 ± 2.16, 30.34 ± 1.15, 26.38 ± 0.41, 918.94 ± 26.06, 86.72 ± 0.38, 91.36 ± 2.25, 32,200 ± 220 and 238.54 ± 7.34 IU gds−1 of CMCase, FPase, β-glucosidase, xylanase, mannanase, pectinase, α-amylase and glucoamylase respectively.
Optimization of the Enzyme(s) Production by Altering One Variable at a Time (OVAT)
The optimization of enzymes production was attempted by studying the effect of a variety of physic-chemical parameters by altering one factor at a time and keeping all the others constant.
Effect of Moisture Content
The moisture in solid state fermentation was varied by altering the substrate to moisture ratio from 1.0:0.5 to 1.0:3.0. The data, as depicted in Table 1, shows that cellulases (CMCase, FPase, β-glucosidase) and hemicellulases (xylanase, mannanase) were maximally produced when the initial substrate to moisture ratio was kept at 1.0:1.5 thus maintaining a moisture content of 60 %, but the highest titres of pectinase and amylases were obtained when the moisture was kept to its minimum of 33.3 % at a substrate to moisture ratio of 1.0:0.5. There was 14.6, 56.6, 22.6, 43.7 and 6.13 % loss in the yields of CMCase, FPase, β-glucosidase, xylanase and mannanase respectively upon reducing the moisture content from 60 % to the lowest value of 33.3 %. On the other hand a decrease of 19.0, 37.0 and 10.8 % was observed in the yields of pectinase, α-amylase and glucoamylase upon increasing the moisture content from 33.33 to 60 %. As most of the enzymes revealed highest yields in presence of 60 % moisture, the same was maintained for further studies.
Effect of Inoculum Size
The effect of inoculum size was studied by varying the volume of the spore suspension having a count of 1 × 108 spores/ml used for inoculation by maintaining 1 × 107 to 6 × 107 spores gds−1 in the medium. All the components of cellulases and xylanase revealed the highest productivities with comparable yields with an inoculum size of 4 × 107 and 5 × 107 spores gds−1 while mannanase was produced maximally with an inoculums size of 1 × 107 spores gds−1. Pectinase and amylases were produced maximally with 5 × 107 spores gds−1 and a loss of 30.4, 4.3 and 28.2 % in the yields of pectinase, α-amylase and glucoamylase respectively was observed when inoculum size was reduced from 5 × 107 to 4 × 107 spores gds−1 (Table 2). Taking into consideration the productivities of all the enzyme components, an inoculum size of 5 × 107 spores gds−1 was used in further experiments.
Effect of Exogenous Supplementation of Defined Carbon Sources
The effect of supplementation of various carbon sources on the production of various enzyme components is depicted in Table 3. CMC and cellulose powder enhanced the productivity of complete cellulase complex with 17.8 and 16.1 % increase in the yield of CMCase, 33.9 and 16.5 % improvement in FPase and 15.6 and 29.5 % increase in the productivities of β-glucosidase respectively. Xylanase was produced to its maximum on the supplementation of CMC followed by xylan with 25.0 and 6.1 % improvement respectively compared to control. The production of mannanase was enhanced by 5.0 % with the addition of guar gum while cellulose and xylan improved pectinase production by 30.1 and 24.7 % respectively. Addition of starch yielded best amounts of amylases with 16.7 % improvement in the yield of α-amylase and 1.3 % increase in glucoamylase.
Effect of Supplementation of Nitrogen Sources
Of the various organic and inorganic nitrogen sources, sodium nitrate proved to be most favourable for improving the yields of all the enzymes except xylanase for which it proved to slightly inhibitory. It prompted an increase of 11.3, 11.1, 20.8, 14.2, 52.7, 15.12 and 32.3 % in the yields of CMCase, FPase, β-glucosidase, mannanase, pectinase, α-amylase and glucoamylase respectively and 14.1 % decrease in xylanase productivity (Table 3). This was followed by ammonium chloride which improved the productivities of CMCase, FPase, β-glucosidase, pectinase, α-amylase and glucoamylase by 11.8, 14.5, 53.2, 17.7, 13.0 and 22.1 %. It, however, decreased the yields of xylanase and mannanase by 54.2 and 24.1 % respectively.
Effect of the Supplementation of Surfactants
Of the various surfactants, Tween 20 was found to enhance the productivities of all the enzymes with 7.5, 32.0, 6.0, 12.4, 2.8, 13.4, 17.3 and 6.9 % improvement in the yields of CMCase, FPase, β-glucosidase, xylanase, mannanase, pectinase, α-amylase and glucoamylase respectively (Table 3).
Effect of Supplementation of Lignocellulosic Residues
Of the various raw and delignified cellulosic residues supplemented in deoiled rice bran, delignified corn stover induced the yields of CMCase, FPase, β-glucosidase, xylanase, mannanase, pectinase, α-amylase and glucoamylase by 3.5, 19.4, 14.0, 41.4, 6.0, 15.7, 7.4 and 20.4 % respectively. On the other hand delignified rice straw improved the production of CMCase, FPase, β-glucosidase and xylanase by 9.4, 27.5, 10.8 and 30.4 % respectively but decreased the yields of mannanase, pectinase, α-amylase and glucoamylase respectively by 29.3, 22.8, 5.6 and 18.9 % (Table 4).
Application of In-House Produced Enzyme Cocktail in the Hydrolysis of Steam Pre-treated DORB
The crude enzyme preparation worked well in the hydrolysis of steam pre-treated DORB as was evident from gradual increase in total reducing sugars and glucose after 24 h and 48 h of the reaction. Maximum bioconversion was observed during the first 24 h of enzymatic hydrolysis where the rates of total reducing sugar and glucose production were 2.25 and 2.12 mg ml−1h−1. There was slight increase in the sugars during the next 24 h revealing release of 0.28 and 0.05 mg ml−1h−1 total reducing sugars and total glucose respectively thus maintaining a total concentration of 89 and 72.2 g l−1 respectively for total reducing sugars and glucose. The overall recovery of total reducing sugars and glucose after 48 h of enzymatic hydrolysis were 356.17 ± 9.58 and 288.60 ± 8.13 mg gds−1 respectively with conversion efficiency of 72.65 % (Table 5).
Discussion
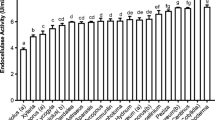
As fungi have been found to be better than bacteria in terms of yields and variability of extracellular hydrolytic enzymes, hunt was carried out for a fungal strain that is skilled to produce a range of carbohydrases required for the depolymerisation of cellulose, hemicelluloses, pectin and starch so that a consortium of enzymes could be obtained by fermenting deoiled rice bran, an agro-industrial waste. A natural variant of A. niger P-19 was thus isolated which could produce all these enzymes in appreciable amounts that can find its application in the complete hydrolysis of various agricultural and agro-industrial waste residues. A comparison of the yield of all these enzymes produced in the present study by A. niger P-19 under SSF with other similar studies has been made in Table 6 which suggests that the variety and amount of enzymes obtained in the present study is quite good and stands out well in the lot.
Time course for the co-production of cellulases, hemicellulases, pectinase and amylases revealed the peaks of the enzyme productivities on different days but appreciable yields of all the enzymes were noticed after 120 h of incubation. The levels of all the enzymes declined gradually after attaining the peaks. Grover et al. [19] have also reported best CMCase and α-amylase productivities on DORB at the end of 5th day of incubation. Another study reported different requirements of incubation time for various enzymes with day 5 being the best compromised day for obtaining best yields of all the enzymes on composite kitchen waste [15]. The decrease in yields with increasing incubation time after a certain period might be due to the release of proteases and/or some inhibitory metabolites during the stationary phase which may have degraded or inactivated the enzyme(s). This might also be due to the repression effect of the products of enzymatic reactions which had resulted in reduction in enzyme(s) production.
The growth and the enzymes production by the organism during solid state fermentation is affected by several factors including the moisture content, inoculum size and the presence of nutrients, inducers and surfactants. In solid state fermentation, microbes grow at the surface of the solid substrate particle having low moisture content. As the water availability in lower or higher concentrations adversely affects the microbial activity, it is critical to provide optimized water content to the fermenting substrate. Optimum level of moisture depends on the type of substrate, the requirements of the microorganism involved and the product required from the fermentation [44]. Different moisture requirements were observed for the best yields of all the enzymes with 60 % moisture most favourable for cellulases and hemicellulases and appreciable titres of the other enzymes. Sohail et al. [45] reported that different components of cellulase complex were produced to their maximum at different phases of growth by Aspergillus niger MS82. Similarly, the variation in moisture requirements for maximum production of different enzymes could also be attributed to the phase of growth at which the organism starts producing that particular enzyme. Contrary to our results, Grover and co-workers reported that 70 % moisture was best suited for the production of cellulase and amylase by solid state fermentation of DORB using co-culture of Aspergillus oryzae and Trichoderma reesei [19].
Inoculum size also plays an important role during fermentations. Lower inoculum requires longer time for sporulation while higher inoculum leads to rapid proliferation [46], thus, a balance between substrate utilization and biomass proliferation is required to obtain maximum productivities and an inoculums size of 5 × 107 spores gds−1 of DORB was selected in the present study as it was found to be sufficient for obtaining the appreciable yields of all the enzymes.
Exogenous supplementation of carbon sources to obtain maximum yields of all the enzyme components revealed that CMC enhanced the productivities of cellulases, xylanase as well as amylase. Many researchers have agreed upon the positive effect of CMC on the production of cellulases by various fungi [38, 47–49]. An increase in the production of xylanase by Trichoderma sp. in CMC supplemented pressmud extract moistened rice bran mix has already been reported [50]. The results obtained by Chantorn and group where guar gum was found to improve the productivity of mannanase by Penicillium oxalicum are also in agreement to our study [51]. Supplementation of xylan to banana peels improved the production of xylanase and pectinase by Aspergillus niger MS23 in a study which is in accordance to our results where xylan has improved the yields of xylanase and pectinase [49]. Starch was found to enhance the productivity of amylases in this study and other groups have also insisted upon the positive effect of the addition of starch on the production of amylases by Penicillium expansum MT-1 and Aspergillus oryzae respectively [52, 53].
Inorganic nitrogen sources like sodium nitrate and ammonium chloride were found to give improved yields of the enzymes. The positive effect of sodium nitrate on the production of mannanase by Fusarium oxysporum SS-25 via solid state fermentation of brewer spent grain has already been reported [16]. Another study has reported a positive effect of ammonium chloride on the production of cellulases by Aspergillus niger C-5 [25]. Also, ammonium nitrate has been reported to improve the production of cellulase by Trichoderma reesei NRRL 11460 [21]. Ammonium sulphate has been found to be the best nitrogen source for Mucor plumbeus and Aspergillus terreus [54]. In contrast, many workers have reported the positive effect of various organic sources on the yields of these enzymes [55–57]. Peptone has been found to improve the production of CMCase while tryptone improved the yields of FPase and β-glucosidase produced by Aspergillus niger NS-2 [58]. Also, contrary to our findings, Goyal and group has reported a positive effect of sodium nitrate on the production of xylanase by Trichoderma viride [59] and Rana et al. [60] have emphasized on the good effects of ammonium sulphate and peptone on the yields of xylanase by Fusarium oxysporum SS-25.
The surfactants probably increase enzyme yields in SSF by increasing penetration of water into the solid substrate matrix and increasing surface area for microbial growth [61]. Many workers have published the reports on simulative effect of various surfactants on the production of various enzymes like cellulases [62], protease [63, 64], amylases [65], laccases [66], phytase [67]. Tween 20 has been found to give a positive effect on the production of enzymes in the present work which was also found too increase the yield of cellulases by Bacillus pumilus [68]. In contrast, two fold increase in the yields of cellulase by Aspergillus terreus with the addition of tween 80 to the production medium has also been reported [69]. Tween 80 has also been found to enhance the yield of endoglucanase by Aspergillus fumigates [70].
The production of hydrolytic enzymes has been reported to be inducible and is affected by the form of the substrate used in fermentation. Thus, an appropriate combination of inducing substrates is vital to be recognized for better enzyme yields [7]. In the present study, delignified corn stover and delignified rice straw positively affected the yields of enzymes. Delignification of substrates makes the cellulose readily available for the growth of microorganism which might have led to the superior productivities of enzymes. Pretreated sugarcane baggase as a supporting carbon source with wheat bran has also been reported to improve the yields of cellulases and hemicellulases by Penicillium echinulatum [37]. Reddy and group reported a combination of rice bran and wheat bran to be beneficial for the production of cellulases by Aspergillus niger in submerged as well as solid state fermentation processes [71].
Our study has revealed a strain of A. niger P-19 which has the potential to produce multiple hydrolytic enzymes in appreciable titres. To the best of our knowledge, this is the first report of its kind where a consortium of eight hydrolytic enzymes has been co-produced by solid-state fermentation of DORB, which is an agro-industrial waste. Moreover, co-optimization of eight different enzymes also adds novelty to the work. If the production of enzymes by A. niger P-19 is further optimized using statistical tools like response surface methodology (RSM), even higher yields of all the enzyme components can be obtained. The enzyme preparation reported in this study has also proved its potential in the hydrolysis of DORB which indicates that it might also be applied to saccharify various other lignocellulosics for obtaining value-added products from them. However, the processes of pre-treatment and hydrolysis need further standardization to improve the overall carbohydrate conversion efficiency.
References
Nigam, P.S., Singh, A.: Production of liquid biofuels from renewable resources. Prog. Energ. Combust. 37, 52–68 (2011)
Biernat, K., Malinowski, A., Gnat, M.: The possibility of future biofuels production using waste carbon dioxide and solar energy. In: Fang, Z. (ed.) Biofuels-Economy, Environment and Sustainability (2013). doi:10.5772/53831
Maitan-Alfenas, G.P., Visser, E.M., Guimares, V.M.: Enzymatic hydrolysis of lignocellulosic biomass: converting food waste in valuable products. Curr. Opin. Food Sci. 1, 44–49 (2015)
Poli, A., Anzelmo, G., Fiorentino, G., Nicolaus, B. et al.: Polysaccharides from wastes of vegetable industrial processing: new opportunities for their eco-friendly re-use. In: Elnashar, M. (ed.) Biotechnology of Biopolymers (2011). doi:10.5772/16387
Soni, S.K., Batra, N., Bansal, N., Soni, R.: Bioconversion of sugarcane baggase into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulases from Aspergillus sp. S4B2F. BioRes. 5, 741–758 (2010)
Yang, S.Q., Yan, Q.J., Jiang, Z.Q., Li, L.T., et al.: High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation. Bioresour. Technol. 97, 1794–1800 (2006)
Kang, S.W., Park, Y.S., Lee, J.S., Hong, S.I., et al.: Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91, 153–156 (2004)
Vyas, A., Vyas, D.: Production of fungal cellulases by solid state bioprocessing of groundnut shell wastes. J. Sci. Ind. Res. 64, 767–770 (2005)
Kheng, P.P., Omar, I.C.: Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake (PKC) as substrate. Songklanakarin J. Sci. Technol. 27, 325–336 (2005)
Pothiraj, C., Balaji, P., Eyini, M.: Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. Afr. J. Biotechnol. 5, 1882–1885 (2006)
Gao, J., Weng, H., Zhu, D., Yuan, M.: Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour. Technol. 99, 7623–7629 (2008)
Samuel, S., Muthukkaruppan, S.M., Gayathri, S.N., Kumar, P.K.: Cellulase production by Bacillus spp and Aspergillus niger using coir waste and saw dust and partial purification. Int. J. Curr. Res. 2, 31–34 (2010)
Brijwani, K., Oberoi, H.S., Vadlani, P.V.: Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 45, 120–128 (2010)
Juwaied, A.A., Adnan, S., Al-Amiery, A.A.H.H.: Production of cellulase by different co- culture of Aspergillus niger and Tricoderma viride from waste paper. J. Yeast Fungal Res. 1, 108–111 (2010)
Janveja, C., Rana, S.S., Soni, S.K.: Environmentally acceptable management of kitchen waste residues by using them as substrates for the co-production of a cocktail of fungal carbohydrases. Int. J. Chem. Environ. Eng. Sys. 4, 20–29 (2013)
Rana, S.S., Janveja, C., Soni, S.K.: A β-mannanase from Fusarium oxysporum SS-25 via solid state fermentation on brewer’s spent grain: medium optimization by statistical tools, kinetic characterization and its applications. Int. J. Biol. Vet. Agr. Eng. 9, 115–125 (2015)
Department of Agriculture and Cooperation, India: Commodity Profile for Rice-March 2015. http://agricoop.nic.in/imagedefault/trade/Ricenew.pdf (2015). Accessed 24 July 2015
Kahlon, T.S.: Rice Bran: production, composition, functionality and food applications, physiological benefits. In: Cho, S.S., Samuel, P. (eds.) Food Applications and Health Benefits, pp. 305–321. CRC Press, Florida (2009)
Grover, A., Maninder, A., Sarao, L.K.: Production of fungal amylase and cellulase enzyme via solid state fermentation using Aspergillus oryzae and Trichoderma reesei. Int. J. Adv. Res. Technol. 2, 108–124 (2013)
Deswal, D., Khasa, Y.P., Kuhad, R.C.: Optimization of cellulase production by a brown rot fungus Famitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 102, 6065–6072 (2011)
Singhania, R.R., Sukumaran, R.K., Pillai, A., Prema, P., et al.: Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J. Biotechnol. 5, 332–336 (2006)
Lee, C.K., Darah, I., Ibrahim, C.O.: Production and optimization of cellulase enzyme using Aspergillus niger USM AI 1 and comparison with Trichoderma reesei via solid state fermentation system. Biotechnol. Res. Int. (2011). doi:10.4061/2011/658493
Puri, S., Arora, M., Sarao, L.: Production and optimization of amylase and glucoamylase using Aspergillus oryzae under solid state fermentation. Int. J. Res. Pure Appl. Microbiol. 3, 83–88 (2013)
Rana, S.S., Janveja, C., Soni, S.K.: Brewer’s spent grain as a valuable substrate for low cost production of fungal cellulases by statistical modelling in solid state fermentation and generation of cellulosic ethanol. Int. J. Food. Ferment. Technol. 3, 41–55 (2013)
Janveja, C., Rana, S.S., Soni, S.K.: Optimization of valorization of biodegradable kitchen waste biomass for production of fungal cellulase system by statistical modelling. Waste Biomass Valorization 5, 807–821 (2014)
Tamura, K., Dudley, J., Nei, M., Kumar, S.: MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007)
Mandels, M., Andreotti, R.E., Roche, C.: Measurements of saccharifying cellulases. Biotechnol. Biophys. Symp. 6, 21–23 (1976)
Bailey, M.J., Biley, P., Poutanen, K.: Inter laboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23, 257–270 (1992)
Stalbrand, H., Siika-aho, M., Viikari, L.: Purification and characterization of two β-mannanases from Trichoderma reesei. J. Biotechnol. 29, 229–242 (1993)
Minjares-Carranco, A., Trejo-Aguilar, B.A., Guillermo, A., Viniegra-Gonzalez, G.: Physiological comparision between pectinase producing mutants of Aspergillus niger adopted either to solid state fermentation or submerged fermentation. Enzyme Microb. Technol. 21, 25–31 (1997)
Fuwa, H.: A new method for micro determination of amylase activity by the use of amylose as substrate. J. Biochem. 41, 583–603 (1954)
Cori, G.T.: Amylo-1,6-glucosidase. Methods Enzymol. 1, 211–214 (1955)
Dubois, M., Gilles, K.A., Hamilton, K., Rebers, P.A., Smith, F.: Calorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Miller, G.L.: Use of DNS reagent for determination of reducing sugars. Anal. Chem. 31, 426–428 (1959)
Morin, L.G., Prox, J.: Single glucose oxidase-peroxidase reagent for two-minute determination of serum glucose. Clinical Chem. 19, 959–962 (1973)
Pirota, R.D.P.B., Delabona, P.S., Farinas, C.S.: Enzymatic hydrolysis of sugarcane bagasse using enzyme extract and whole solid-state fermentation medium of two newly isolated strains of Aspergillus Oryzae. Chem. Eng. Trans. 38, 259–264 (2014)
Camassola, M., Dillon, A.J.P.: Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J. Appl. Microbiol. 103, 2196–2204 (2007)
Rehman, S., Aslam, H., Ahmad, A., Khan, S.A., et al.: Production of plant cell wall degrading enzymes by monoculture and co-culture of Aspergillus niger and Aspergillus terreus under SSF of banana peels. Braz. J. Microbiol. 45, 1485–1492 (2014)
Ang, S.K., Shaza, E.M., Adibah, Y., Suraini, A.A., et al.: Production of cellulases and xylanase by Aspergillus fumigates SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48, 1293–1302 (2013)
Dhillon, G.S., Kaur, S., Brar, S.K., Verma, M.: Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulases bioproduction through solid-state fermentation. Ind. Crop Prod. 38, 6–13 (2012)
Kumar, S., Sharma, H.K., Sarkar, B.C.: Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci. Biotechnol. 20, 1289–1298 (2011)
Bansal, N., Tewari, R., Gupta, J.K., Soni, R., et al.: A novel strain of Aspergillus niger producing a cocktail of hydrolytic depolymerising enzymes for the production of second generation biofuels. BioRes. 6, 552–569 (2011)
Bansal, N., Tewari, R., Soni, R., Soni, S.K.: Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manage. 7, 1341–1346 (2012)
Maurya, D.P., Singh, D., Pratap, D., Maurya, J.P.: Optimization of solid state fermentation conditions for the production of cellulase by Trichoderma reesei. J. Environ. Biol. 33, 5–8 (2012)
Sohail, M., Siddiqi, R., Ahmad, A., Khan, S.A.: Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnol. 25, 437–441 (2009)
Baysol, Z., Uyar, F., Aytekin, C.: Solid state fermentation for production of alpha amylase by a thermotolerant Bacillus subtilis from hot spring water. Process Biochem. 38, 1665–1668 (2003)
Ahmed, S., Bashir, A., Saleem, H., Saadia, M., et al.: Production and purification of cellolose-degrading enzymesfrom a filamentous fungus Trichoderma herzanium. Pak. J. Bot. 41, 1411–1419 (2009)
Soni, S.K., Soni, R.: Regulation of cellulase synthesis in Chaetomium erraticum. BioRes. 5, 81–98 (2010)
Sohail, M., Ahmad, A., Khan, S.A.: Production of cellulases from Alternaria sp. MS28 and their partial characterization. Pak. J. Bot. 43, 3001–3006 (2011)
Balakrishnan, K., Kumar, R., Devi, R.A., Jayasri, S., et al.: Utilization of fortified rice husk for the fermentative production of xylanase by Trichoderma sp. Int J. Curr. Microbiol. Appl. Sci. 2, 174–187 (2013)
Chantorn, S.T., Buengsrisawat, K., Pokaseam, A., Sombat, T., et al.: Optimization of extracellular mannanase production from Penicillium oxalicum KUB-SN2-1 and application for hydrolysis property. Songklanakarin J. Sci. Technol. 35, 17–22 (2013)
Erdal, S., Taskin, M.: Production of α-amylase by Penicillium expansum MT-1in solid state fermentation using waste Loquat (Eriobotrya japonica Lindley) kernels as substrate. Rom. Biotechnol. Lett. 15, 5342–5350 (2010)
Sivaramakrishnan, S., Gangadharan, D., Nampoothiri, K.M., Soccol, C.R., et al.: Alpha amylase production by Aspergillus oryzae employing solid state fermentation. J. Sci. Ind. Res. 66, 621–626 (2007)
Padmavathi, T., Agarwal, P., Nandy, V.: Exploring marine fungal strains for cellulase production. Ann. Biol. Res. 3, 3602–3613 (2012)
Akinyele, J.B., Fabunmi, A.O., Olaniyi, O.O.: Effect of variations in growth parameters in cellulase activity of Trichoderma viride NSPR006 cultured on different wood-dusts. Malays. J. Microbiol. 9, 193–200 (2013)
Azzaz, H.H., Murad, H., Kholif, A.M., Hanfy, M.A., et al.: Optimization of culture conditions affecting fungal cellulase production. Res. J. Microbiol. 7, 23–31 (2012)
Kachlishvili, E., Penninckx, M.J., Tsiklauri, N., Elisashvili, V.: Effect of nitrogen source on lignocellulolytic enzyme production by white-rot basidiomycetes under solid-state cultivation. World J. Microbiol. Biotechnol. 22, 391–397 (2006)
Bansal, N., Janveja, C., Tewari, R., Soni, R., et al.: Highly thermostable and ph-stable cellulases from Aspergillus niger NS-2: properties and application for cellulose hydrolysis. Appl. Biochem. Biotechnol. 172, 141–156 (2014)
Goyal, M., Kalra, K.L., Sareen, V.K., Soni, G.: Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Braz. J. Microbiol. 39, 535–541 (2008)
Rana, S.S., Janveja, C., Soni, S.K.: Statistical modeling for enhanced xylanase production by Fusarium oxysporum SS-25 via solid state fermentation of Brewer’s spent grain. J. Technol. Innov. Renew. Energy 2, 173–185 (2013)
Bhardwaj, S., Vedamurthy, A.B., Bhattacharya, S., Das, A.: Effect of inorganic salts and surfactants on the production of α-amylase by a Mangrove isolate of Aspergillus flavus using solid-state fermentation. J. Chem. Biol. Phys. Sci. 2, 1390–1397 (2012)
Pardo, A.G.: Effect of surfactants on cellulase production by Nectria catalinensis. Curr. Microbiol. 33, 275–278 (1996)
Evans, E.C., Abdullahi, A.: Effect of surfactant inclusions on the yield and characteristics of protease from Bacillus subtilis. Proc. Rom. Acad., Series B 2, 108–112 (2012)
Uyar, F., Porsuk, I., Kizil, G., Yilmaz, E.I.: Optimal conditions for production of extracellular protease from newly isolated Bacillus cereus strain CA15. EurAsian J. BioSci. 5, 1–9 (2011)
Ikram-ul-haq, Shamim, N., Ashraf, H., Ali, S. et al.: Effect of surfactants on the biosynthesis of alpha amylase by Bacillus subtilis GCBM-25. Pak. J. Bot. 37, 373–379 (2005)
Usha, K.Y., Praveen, K., Reddy, B.R.: Enhanced production of ligninolytic enzymes by a mushroom Stereum ostrea. Biotechnol. Res. Int. (2014). doi:10.1155/2014/815495
Al-Asheh, S., Duvnjak, Z.: The effect of surfactants on the phytase production and the reduction of the phytic acid content in canola meal by Aspergillus carbonarius during a solid state fermentation process. Biotechnol. Lett. 16, 183–188 (1994)
Shankar, T., Isaiarasu, L.: Cellulase production by Bacillus pumilus EWBCM1 under varying cultural conditions. Middle-East J. Sci. Res. 8, 40–45 (2011)
Shahriarinour, M., Wahab, M.N.A., Mohamad, R., Mustafa, S., et al.: Effect of medium composition and cultural condition on cellulase production by Aspergillus terreus. Afr. J. Biotechnol. 10, 7459–7467 (2011)
Mehboob, N., Asad, M.J., Asgher, M., Gulfraz, M., et al.: Exploring thermophillic cellulolytic enzyme production potential of Aspergillus fumigates by the solid-state fermentation of wheat straw. Appl. Biochem. Biotechnol. 172, 3646–3655 (2014)
Reddy, G.P.K., Narasimha, G., Kumar, K.D., Ramanjaneyulu, G., et al.: Cellulase production by Aspergillus niger on different natural lignocellulosic substrates. Int. J. Curr. Microbiol. Appl. Sci. 4, 835–845 (2015)
Acknowledgments
Authors are highly thankful for the financial assistance provided by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India and Department of Science and Technology (DST). Junior Research Fellowship awarded under DST-INSPIRE scheme to Ms. Priya is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chugh, P., Soni, R. & Soni, S.K. Deoiled Rice Bran: A Substrate for Co-Production of a Consortium of Hydrolytic Enzymes by Aspergillus niger P-19. Waste Biomass Valor 7, 513–525 (2016). https://doi.org/10.1007/s12649-015-9477-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9477-x