Abstract
Laccase production and pellet formation of transformants of Coprinopsis cinerea strain FA2222 of C. cinerea laccase gene lcc1 subcloned behind the gpdII-promoter from Agaricus bisporus were compared with a control transformant carrying no extra laccase gene. At the optimum growth temperature of 37 °C, maximal laccase yields of 2.9 U/ml were obtained by the best lcc1 transformant pYSK7-26 in liquid shake flask cultures. Reduction in temperature to 25 °C increased laccase yields up to 9.2 U/ml. The control transformant had no laccase activities at 37 °C but native activity at 25 °C (3.5 U/ml). Changing the temperature had severe effects on the morphology of the mycelial pellets formed during cultivation, but links of distinct pellet morphologies to native or recombinant laccase production could not be established. Automated image analysis was used to characterise pellet formation and morphological parameters (pellet area, diameter, convexity and mycelial structure). Cross sections of selected pellets showed that they differentiated in an outer rind and an inner medulla of loosened hyphae. Pellets at 25 °C had a small and dense outer zone and adopted with time a smooth surface. Pellets at 37 °C had a broader outer zone and a fringy surface due to generation of more and larger protuberances in the rind that when released can serve for production of further pellets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases are multi-copper oxidases that oxidise phenolic compounds and a number of other organic substances. The enzymes are therefore of interest for various applications in the food, textile, pulp and paper, and wood industries, in biofuel production, synthesis of biochemicals and drugs, soil bioremediation and others (Arora and Sharma 2010; Cannatelli and Ragauskas 2016; Kudanga et al. 2017; Kües 2015; Mate and Alcalde 2017; Osma et al. 2010; Yang et al. 2017). For native laccase production, usually basidiomycetes are selected that are most efficient in secretion of enzymes. Laccases are typically won from liquid cultures which are treated with suitable inducers for improving enzyme production (e.g. copper, ethanol, 2,5-xylidine and other phenolic compounds) (Martani et al. 2017; Tavares et al. 2005). Also other cultivation parameters can positively influence natural laccase production, for example temperature (Nyanhongo et al. 2002; Rühl et al. 2013; Šnajdr and Baldrian 2007), aeration (Fernández-Alejandre et al. 2016; Babič and Pavko 2012; Dekker and Barbosa 2001) and agitation (Fernández-Alejandre et al. 2016; Hublik and Schinner 2000; Michel et al. 1990). Production with wildtype strains is naturally restricted to laccases that are natively secreted in reasonable amounts. Any new strain will require defining new specific fermentation conditions for optimal growth and production (Domingos et al. 2017; Veiter et al. 2018).
Recombinant expression of laccases in other fungi with well-established fermentation procedures is an alternative way for high-level enzyme production (Bertrand et al. 2017; Piscitelli et al. 2010). Unfortunately, expression of laccase genes from basidiomycetes in ascomycete yeasts and filamentous ascomycetes results often in low yields or altered enzymatic properties, among due to faulty glycosylation or mis-folding of the secreted enzymes (Jolivalt et al. 2005; Larrondo et al. 2003; Madzak et al. 2005; Sigoillot et al. 2004; Bertrand et al. 2017; Yang et al. 2017). Basidiomycetes are thus also considered as hosts for recombinant laccase production (Alves et al. 2004; Arimoto et al. 2015; Coconi-Linares et al. 2015; Kajita et al. 2004; Kilaru et al. 2006; Kum et al. 2011; Ryu et al. 2013) although the fermentation technology with basidiomycetes is little studied compared to ascomycetous yeasts or selected filamentous ascomycetes such as Aspergillus and Penicillium species (Bentil et al. 2018; Domingos et al. 2017; Krull et al. 2013).
One of the basidiomycetous species easy to transform and easy to cultivate in the laboratory is the mushroom Coprinopsis cinerea (Dörnte and Kües 2012, 2016; Kües 2000). We exploit this agaricomycete as a producer of enzymes. A range of laccase genes have successfully been expressed in the fungus under control of the Agaricus bisporus gpdII promoter (Kilaru 2006; Kilaru et al. 2006; Dörnte and Kües 2016). In a former study, up to 3 U/ml laccase activity was obtained in shaken cultures of C. cinerea lcc1 transformants grown in a complete medium at the optimal growth temperature of 37 °C (Kilaru et al. 2006). Changing medium and reducing growth temperature considerably improved natural laccase production of various C. cinerea wildtype strains (Rühl et al. 2013). Here, we show that this is also the case for recombinant laccase production. However, the temperature change caused in addition a major morphological chance in fungal pellet structure. Since morphology is known in other systems to influence fungal productivity (Grimm et al. 2005; Papagianni 2004; Wucherpfennig et al. 2013) and it is in submerged fermentations of Agaricomycetes so far mostly a neglected parameter (Antecka et al. 2016; Babič and Pavko 2012; Cui et al. 2016; Fazenda et al. 2010; Hwang et al. 2004; Tang et al. 2007; Tao et al. 2018; Tepwong et al. 2012), pellet formation of C. cinerea transformants in shaken cultures was analysed in laccase-producing and non-producing cultures.
Material and methods
Fungal cultures
Prototrophic transformants (named pYSK7-26, -3, -23, -32, and -35) of the trp1-auxotrophic C. cinerea monokaryon FA2222 (DSM 28333) were used which express the C. cinerea laccase gene lcc1 under control of the gpdII-promoter of A. bisporus after ectopic genomic integration of the lcc1 plasmid pYSK7 and C. cinerea trp1+ vector pCc1001. A transformant of only trp1+-vector pCc1001 served as control (Kilaru et al. 2006). Clone pYSK7-26 had the highest laccase activity of 3 U/ml in liquid culture at 37 °C in YMG/T (YMG plus 100 mg/l tryptophan) complete medium plus 0.1 mM CuSO4 whereas other lcc1 transformants showed best activities between 0.4 and 1.7 U/ml (Kilaru et al. 2006).
For spore (oidia) collection, fungi were grown on YMG-agar plates (per litre, 4 g yeast extract; 10 g malt extract; 4 g glucose; 10 g agar; Rao and Niederpruem 1969) at 37 °C until the mycelium reached the border of the Petri dishes. Sterile water (ddH2O) was poured onto the plates and the mycelium with the oidia was scraped with a sterile spatula from the agar. The spore solution was filtered using a sterile funnel filled with glass wool and the spores counted (Kertesz-Chaloupková et al. 1998). Spores in amounts of 5 × 107 were transferred into 50 ml sterile mKjalke medium (per litre, 10 g yeast extract, 20 g glucose, 0.5 g CaCl2 × 2 H2O, 2 g KH2PO4, 50 mg MgSO4 × 7 H2O; Rühl et al. 2013) in 500 ml flasks. The inoculated flasks were incubated for 4 days at 37 °C as stationary (standing) pre-cultures. Then, they were homogenised by an Ultra-Turrax® (IKA Werke GmbH & Co. KG, Staufen, Germany) for 30 s at 8000 rpm and for 30 s at 9500 rpm (rotations per minute). Each 5 ml of a homogenised pre-culture was inoculated into 100 ml of fresh mKjalke medium supplemented with 0.1 mM CuSO4 in 500 ml flasks. Cultivation of the main cultures took place at 25 and 37 °C on a rotary shaker at 120 rpm. Per experimental set, at least 15 main cultures were inoculated, and per day of analysis, always 3 parallel cultures were analysed.
Laccase assay
Laccase activities in culture supernatants were determined in 100 mM sodium acetate buffer (pH 5.0) at room temperature (RT) with 5 mM ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate), AppliChem GmbH, Darmstadt, Germany] as substrate as described before (Kilaru et al. 2006; Rühl et al. 2013). Oxidation of the substrate was observed for 5 min with the help of a spectrophotometer (DU 800, Beckman Coulter, Fullerton, CA, USA) at 420 nm (ε = 36,000 M−1 cm−1) (Matsumura et al. 1986). One unit of enzyme activity was defined as the amount of enzyme needed to transform 1 μmol ABTS per min. Enzymatic activities are given in unit (U) per volume.
Glucose determination
Glucose concentrations in culture supernatants were determined indirectly with a glucose (HK) assay kit following the instructions of the supplier (Sigma-Aldrich Chemie GmbH, Munich, Germany).
C/N determination of culture supernatant
Tin capsules (HEKAtech GmbH, Wegberg, Germany) were weighted and filled with an adequate amount of culture supernatants (40–120 μl to reach ~ 800 ng dry weight/capsule). The tin capsules with the supernatant were lyophilised, weighted again and analysed with a CHNS-O elemental analyser (Carlo Erba Instruments, EA 1108-Elemental Analyzer, Milan, Italy).
Morphological observation
Pellet growth of cultures was observed and analysed using an automated image analysis system described by Rühl and Kües (2009). Complete cultures were evenly dispersed onto a 28.5 cm × 38.5 cm glass tray sealed by a silicon border; representative images from three different 6.4 cm × 8.5 cm-sized fields were taken with a CCD camera (Color View II, Soft Imaging System GmbH, Münster, Germany), and parameters of all photographed pellets were analysed via the software analySIS® (Soft Imaging System GmbH, Münster, Germany) as described (Rühl and Kües 2009). Pellets were clustered in distinct groups according to their individual pellet area (0–0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8, 0.8–1.0, 1–3, 3–5, 5–7, 7–9, 9–11, 11–13, 13–15, 15–17, 17–19, 19–21, 21–23, 23–25 mm2), their convexity (0.50–0.525, 0.525–0.55, 0.55–0.575, …, 0.975–1.00) and their diameter (0–0.5, 0.5–1.0, 1.0–1.5, …, 9.5–10.0 mm). Pellets per image were counted for calculation of pellet concentration (pellets per culture).
Embedding of mycelium pellets
For embedding into Roti®-Plast (Roth, Karlsruhe, Germany), around 10 mycelial pellets were collected per fungal culture per day of incubation and fixed in FAE solution (100 ml contains 90 ml of 70% ethanol (EtOH), 5 ml of glacial acetic acid and 5 ml of 37% formaldehyde) followed by several embedding steps: The pellets rested at least for 4 h each at room temperature (RT) in 70% EtOH, 80% EtOH, 90% EtOH, 96% EtOH, 96% EtOH/isopropanol 1:1, isopropanol, isopropanol/Roti®-Histol 3:1, isopropanol/Roti®-Histol 1:1, isopropanol/Roti®-Histol 1:3, and 3 times pure Roti®-Histol, and then in saturated Roti®-Plast in Roti®-Histol for at least 8 h, in saturated Roti®-Plast in Roti®-Histol at 40 °C for at least 12 h, and 3 times in pure melted Roti®-Plast at 60 °C for at least 12 h. The embedded pellets were fixed onto a wooden block and trimmed to fit in the microtome, and 10–12 μm cuttings were sliced with a rotary microtome (R. Jung, Heidelberg, Germany). The cuttings were transferred onto gelatinised microscope slides and washed three times with pure Roti®-Histol, once with isopropanol/Roti®-Histol 1:1 and twice with pure isopropanol for at least 5 min each. At the end, the slides were stored at RT for evaporation of the isopropanol. Radial sections of the pellets were photographed using a Color View II CCD camera linked to a binocular (Stemi 2000-C, Carl Zeiss MicroImaging GmbH, Göttingen, Germany), and the programme analySIS® was used to analyse the cross sections.
Determination of the mycelial dry weights
Following photographing for image analysis, complete fungal cultures were poured into a Büchner funnel containing a cellulose filter (Whatman, Dassel, Germany) of known dry weight (DW) and pellets were separated from the liquid by suction. The filters together with the wet biomass were dried at 80 °C, cooled down in a desiccator and weighted. DWs of the fungal biomass were determined in g/l culture.
Results
Growth and laccase production at 25 and 37 °C
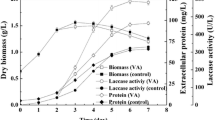
The laccase-producing lcc1 transformant pYSK7-26 and the pCc1001 control transformant were inoculated into Cu-supplemented mKjalke medium and cultivated in parallel at 25 and 37 °C, respectively. Biomass production, glucose consumption and C/N ratio followed a same general pattern for the pYSK7-26 transformant and the pCc1001 control transformant at 25 and at 37 °C, respectively (Fig. 1a–c). For both strains, growth at 25 °C was delayed compared to 37 °C, consistent with the optimal growth temperature of the fungus (Kües 2000). DW values of ~ 1 g/l were reached at day 2 of cultivation for both transformants in 25 °C and of ~ 3 g/l in 37 °C cultures (Fig. 1a). All cultures reached their maximum DW of around 8–10 g/l at day 6 of cultivation. Afterwards, the biomass declined in the cultures at 37 °C with DW values of 7 and 5 g/l at days 8 and 10 of cultivation, respectively. In the 25 °C cultures, the biomass levels kept high up to day 8 of cultivation and then afterwards slightly declined (Fig. 1a). Although the total biomass production of the two strains under same culture conditions was essentially comparable, a striking strain-specific difference was seen in pellet concentrations (Fig. 2a). pYSK7-26 reached a pellet concentration of 3000 and 2200 pellets per culture from day 6 onwards at both cultivation temperatures whereas the pCc1001 control transformant produced in parallel more than 4000 pellets per culture (Fig. 1d).
Shaken cultures of the lcc1 transformant pYSK7-26 (square) and the pCc1001 control transformant (triangle) at 25 °C (open symbol) and 37 °C (closed symbol) in Cu-supplemented mKjalke medium: dry weight of the fungal biomass (a), glucose concentration (b), C/N ratio (c), number of pellets per culture with a projected area ≥ 0.2 mm2 (d), pH (e), as well as laccase activity of the supernatant (f). Values are averages ± standard deviation calculated from three parallel cultures
Along with biomass production, glucose was fully consumed in the first 6 days of cultivation in all cultures, except for the cultures of pYSK7-26 at 25 °C where the glucose was fully depleted at day 8 of cultivation (Fig. 1b). The C/N ratios in the cultures followed exactly the glucose concentration curves. At day 6, respectively day 8 of cultivation, the C/N ratios levelled down to ~ 4 and kept for the rest of the cultivation period at this level. Generally, the 37 °C cultures showed a faster decrease in the glucose concentration and the C/N ratios compared to the 25 °C cultures (Fig. 1c).
During cultivation, noticeable changes in pH values were observed. The initial pH of the Cu-supplemented mKjalke medium is pH 6.0. The pH decreased in all cultures until day 4 to pH 5.0 to 5.5. Subsequently, a dramatic increase of the pH was observed at day 6 (pH 7.8) in the 37 °C cultures and final values of almost pH 9.0 occurred at day 10. The 25 °C cultures showed from day 6 onwards a slightly delayed increase in the pH of the supernatant and reached a final pH of ~ 8.0 at day 10 of cultivation (Fig. 1e).
Cultures of pYSK7-26 and the pCc1001 control transformant distinguished in laccase activity (Fig. 1f). As the untransformed strain FA2222 (Kilaru et al. 2006; Rühl et al. 2013), the pCc1001 control transformant showed no measurable activity at 37 °C. It reached however activities of about 3.5 U/ml at day 6 of cultivation in 25 °C (Fig. 1f), comparable to the untransformed strain FA2222 under the same cultivation conditions (Rühl et al. 2013). Laccase activity in the supernatant of pYSK7-26 cultures increased until day 6 at 25 and 37 °C, to a comparable enzymatic level of about 3 U/ml. In the next 4 days, the enzymatic activity at 37 °C decreased continuously to final levels below 0.3 U/ml (Fig. 1f). In contrast, the 25 °C cultures reached their maximum laccase activities of around 9.2 U/ml at day 8 of cultivation (Fig. 1f), along with the highest biomass values and full glucose depletion (Fig. 1a, b). Afterwards with reduction in biomass, laccase activities dropped to about 5 U/ml at day 10 of cultivation (Fig. 1f).
Pellet development in shake flask cultures
Fungal growth and pellet formation was followed up over the time by automated image analysis as described earlier (Rühl and Kües 2009). Selected photographs of the pellets in the culture supernatants are shown in Fig. 2. Pellet formation differed obviously between temperatures but not as much between the strains.
At 25 °C at the initial phase of growth at day 2 of cultivation, pellets for both transformants were small of irregular fringy shape and consisted of a loosely aggregated, overall thin mycelium. With prolonged cultivation time at day 4 of cultivation, pellets were compact and reached round and sometimes oval shapes (Fig. 2a, b, rows 25 °C). At day 6 of cultivation, pellets showed the highest compactness throughout the cultivation, as documented by their non-transparent appearance in the photographs (Fig. 2a, rows 25 °C). Until the end of the cultivation, the pellets retained their round and oval shapes but generally reduced in their mycelial density as is visualised by the lighter grey shading of the pellets in the photographs (Fig. 2a, rows 25 °C).
At 37 °C, both transformants produced large and dense pellets already at day 2 of the cultivation (Fig. 2a, rows 37 °C). However, from day 4 onwards high amounts of free mycelium (hyphal fragments; disperse mycelium, Grimm et al. 2005) were present in the supernatants of the cultures (Fig. 2a, rows 37 °C). In cultures of the pCc1001 control transformant, the amount of hyphal fragments appeared to be higher than in cultures of pYSK7-26. Overall, along with producing masses of mycelial fragments, the mycelial compactness of the pellets in both transformants reduced much more drastically at the end of the cultivation at 37 °C than in the 25 °C cultures (Fig. 2a, b). This is indicated by the lighter pellet colours in the photographs taken for the transformants on days 8 and 10 of cultivation at 37 °C, respectively (Fig. 2b, rows 37 °C).
Photographs of pellets taken by a CCD camera attached to a binocular revealed that pellet surfaces varied tremendously between 25 and 37 °C cultures (Fig. 2b). At 25 °C, initially the surfaces of pellets were fleecy-pocked but with time, the pellets of both transformants showed a very smooth surface area. In contrast, pellets derived from cultures at 37 °C had a much more hairy look throughout cultivation. Thereby, pellets of the pCc1001 transformants were more frayed than pellets of the pYSK7-26 transformant and had more small mycelial flocks peripherally attached to the main pellet bodies (Fig. 2b).
Pellet size distributions
In order to characterise pellet morphologies in more detail, in total about 800–1200 pellets per transformant per culture condition and per day of cultivation were characterised by automated image analysis. The study revealed that the distribution of the projected area of the individual pellets showed differences in size between strains, between cultivation temperatures and between times of cultivation.
At 25 °C, after 2 days of cultivation most of the pellets of pYSK7-26 covered a projected area of 1–3 mm2, whereas pellets of the pCc1001 control transformant were smaller with most of them having a projected area below 0.4 mm2 (Fig. 3a). At 37 °C after 2 days of cultivation, the pellets reached already an average size of around 7 and 3 mm2 in the cultures of pYSK7-26 and of the pCc1001 control transformant, respectively (Fig. 3a). Over the whole cultivation time, small pellets and loosely aggregated hyphal fragments covering a projected area lower than 0.2 mm2 were present in all cultures (represented in Fig. 3a by the height of the outer left peaks). At 37 °C, the fraction of pellets with a projected area lower than 0.2 mm2 was for both transformants almost 25% of total pellets at day 4. Their amounts increased in the case of the pCc1001 control transformant to 50% at day 6 and remained high (36–38%) until the end of cultivation. In cultures of transformant pYSK7-26 in contrast, amounts of small pellets and loosely aggregated hyphal fragments continually decreased, down to 10% at day 10 of cultivation. In the 25 °C cultures, amounts were generally lower. With prolonged cultivation time, the amounts of small pellets and loosely aggregated hyphal fragments increased from >5% at day 4 of cultivation up to 10 and 25% at day 10 for pYSK7-26 and the pCc1001 control transformant, respectively (Fig. 3a). However, at both temperatures and for both strains, most pellets were larger than 0.2 mm2, with the projected area mostly in the range of 1–5 mm2. Generally, the cultures of pYSK7-26 had slightly larger pellets than the cultures of the pCc1001 control transformant. From day 4 of cultivation onwards, the majority of pellets of pYSK7-26 had a projected area between 3 and 7 mm2, with average values between 4.8 ± 3.2 and 6.0 ± 4.7 mm2. Pellets from cultures of the pCc1001 control transformant had smaller average areas with values of 3.1 ± 2.1 to 4.7 ± 4.1 mm2 (Fig. 3a).
Distributions of pellets from C. cinerea shake flask cultures over the fermentation time (days of cultivation) according to their projected pellet area [mm2] (a), pellet diameter [mm] (b) and pellet convexity (c). The lcc1 transformant pYSK7-26 (black line) and the pCc1001 control transformant (grey line) were grown at 25 °C (solid line) and 37 °C (dashed line) in Cu-supplemented mKjalke medium, respectively. The distributions are given in % as a mean value of 3 replicates with each 200–800 analysed pellets per replicate. Standard deviations in the analyses were always below 21% for the projected pellet area (a), 18% for the pellet diameter (b) and 12% for the pellet convexity (c) (not further shown). Curves were drawn by extrapolation of calculated values in % for size ranges (see Material and methods), while calculated values were fixed within curves in position of the median value of a range. To indicate the change of scale, curves in (a) were interrupted at the value 1 mm2 for the pellet areas
The results on the projected pellet areas are reflected in the data on pellet diameters (Fig. 3a, b). Pellets at 25 °C of both transformants at day 2 of cultivation showed a sharp peak in the distribution curve of diameters. Most pellets had a diameter between 1.0 mm to 1.5 mm. From day 4 onwards, the pellet diameters increased mostly to values of 2.0–2.5 mm and the peaks of pellet diameters of these sizes remained stable over several days of cultivation. However, at day 10 of cultivation smaller fragments with diameters of 0.5–1.0 mm occurred once again, forming a second peak less in height (Fig. 3b).
Cultures grown at 37 °C generally showed a broader distribution of pellet diameters than the cultures at 25 °C (Fig. 3b). At day 2 of cultivation, larger pellet diameters were observed in the 37 °C cultures compared to cultures grown at 25 °C and pellet diameters showed a larger variability. Pellet diameters of the pCc1001 control transformant peaked at two distinct sizes, at 0.5–1.0 mm and at 2.0–2.5 mm, and pellet diameters of pYSK7-26 at one size at 3.0–3.5 mm. After day 2 of cultivation at 37 °C, the distribution of pellets in cultures of both transformants split clearly into two groups with diameters of < 1.0 mm and diameters of 2.5–3.5 mm, respectively. Although the heights of the two peaks fluctuated, they remained present throughout the cultivation (Fig. 3b).
As stated already above and seen in Fig. 2, pellet forms in all cultures were initially rather irregular with a broad distribution of convexity values between the pellets. With longer times of cultivation, large portions of pellets rounded up. The process was fastest in the cultures of transformant pSYK7-26 at 25 °C, resulting in a steep peak of convexity values of 0.95–0.975 already on day 4 of cultivation. Cultures of transformant pSYK7-26 at 37 °C followed delayed, with sharp peaks of convexity values of around 0.95 at days 8 and 10 of cultivation. In contrast, all cultures of the pCc1001 control transformant showed very broad peaks of convexity until the end of cultivation (Fig. 3c).
Summing up all the observations, pellet area and diameter varied strongly between cultivation temperatures at the beginning of cultivation (days 2 and 4; Fig. 3a, b) whereas morphological differences in overall shape of the two transformants overlaid the temperature effect at the end of cultivation (day 10; Fig. 3c).
Micromorphological structure of C. cinerea pellets
The morphological differences between pellets grown at 25 and 37 °C are very obvious when comparing microtome cuttings. The pellets of 37 °C cultures already reached their final size after 2 days, whereas at 25 °C the final size was acquired within 4 days (compare Figs. 3 and 4). At the first day of analysis (day 2 of cultivation), pellets were more or less uniformly structured without a different outer zone, whereas at day 4 of cultivation morphological differentiation within pellets became visible.
Microtome cuttings of paraffin embedded pellets from the lcc1 transformant pYSK7-26 and the pCc1001 control transformant cultures grown at 25 °C (upper rows of photos) and 37 °C (lower rows of photos) in Cu-supplemented mKjalke medium, respectively. The bar in the lower right part indicates 1 mm. Per day of cultivation and transformant, at least 3 pellets were analysed
At 25 °C, the smooth pellets of both transformants had a very dense and small mycelial outer zone (rind) and a less dense inner region (medulla). However, the outer mycelial rinds in pellets from the pCc1001 control transformant appeared to be less even than those of pYSK7-26 pellets (Fig. 4 and also Fig. 2b). In pellets of 37 °C cultures, the outer mycelial zones were irregular furry (pellets of the pCc1001 control transformants more than pellets of pYSK7-26) and less compact than the outer rind of pellets formed at 25 °C. Moreover, the outer zones of pellets formed at 37 °C were generally much broader than those formed at 25 °C (Fig. 4 and also Fig. 2b). Despite a more shaggy appearance of pellets of the pCc1001 control transformant (Fig. 2b), no obvious differences were seen under the binocular between pellets of the two transformants when grown at 37 °C. We noticed however that particularly the pellets from 10-day-old cultures were more instable, which explains why microtome cuttings were harder to obtain and why outer fringes could easily be lost during preparation. Higher enlargements revealed that hyphae in the outer rinds of pellets were generally stronger and highly intermingled whereas hyphae in the inner medulla loosened and degenerated with time. Partially, hyphae within the medulla converted into large chlamydospores (Fig. 5). Chlamydospores were seen in pellets of pYSK7-26 and of the pCc1001 control transformant at both temperatures from day 8 onwards, with more large spores at day 10.
To better compare the general structure of pellets from different cultures and at different temperatures with each other, ratios of the complete diameter to the width of the dense outer zone were calculated (Fig. 6). Because clear differentiation into an outer and an inner zone was only observed from day 4 onwards, pellets derived from day 2 of cultivation were excluded from the analysis.
Comparison of the ratio pellet diameter/width outer zone measured from cross sections of pellets of the lcc1 transformant pYSK7-26 and the pCc1001 control transformant cultivated at 25 and 37 °C, respectively, at day 4 (white), day 6 (shaded grey), day 8 (grey) and day 10 (black) of cultivation. The error bars indicate the standard deviation of three different pellet cross sections. The image shows an example for a microtome cutting of a pellet with arrows indicating the diameter of the pellet (large arrow) and the width of the outer zone (small bar) as used for calculation of the ratio: pellet diameter/width outer zone. Letters a and b refer to significantly different groups calculated by an ANOVA analysis for the four groups 25 °C–pYSK7-26, 25 °C–pCc1001, 37 °C–pYSK7-26 and 37 °C–pCc1001
The ratios diameter to outer zone width did not differ for a strain within a culture over the time or between strains at the same cultivation condition. The ratios for both strains for 37 °C were around 5 over the whole period of cultivation, with minor differences being within the range of the standard deviation (4.9 ± 1.1). At 25 °C, the ratios were more than twofold higher than at 37 °C with values ranging from 12 to 17 (14.1 ± 3.6).
To underline that the morphological phenotypes of the outer zone were not specific to the lcc1 transformant pYSK7-26, we tested four additional lcc1 transformants (clones -3, -23, -32 and -35, Fig. 7) from cultures grown either at 25 or at 37 °C. Day 6 and day 8 pellets were embedded and cross sections with the microtome were produced. The overall microscopic structure and hyphal morphologies did not differ between the five lcc1 transformants (not further shown). Ratios of pellet diameters to width of outer zones for all five lcc1 transformants were considerably larger at 25 °C than at 37 °C. At 25 °C, the calculated ratios for the different transformants ranged between 11.1 and 28.7 (in average 21.5 ± 5.2). At 37 °C, the ratios for the different transformants varied less with values between 2.8 and 4.6 giving an average over all clones of 3.6 ± 0.7 (Fig. 7).
Comparison of the ratios (pellet diameter/width outer zone) determined from cross sections of different lcc1 transformants (pYSK7-clone number) cultivated in Cu-supplemented mKjalke medium at 25 °C (a) and 37 °C (b). lcc1 transformant pYSK7-26 is included to indicate levels of variability between series of experiments. Samples were compared after 6 days (shaded grey) and 8 days (grey) of cultivation. The error bars indicate the standard deviation of parameters of at least three different pellet cross sections measured
Discussion
In this study, the changing morphology of C. cinerea pellets in Cu-supplemented mKjalke medium in liquid shaken cultures was observed together with enzyme production by a recombinant laccase producing strain (FA2222 lcc1 transformant pYSK7-26, trp1+ by co-transformation with trp1+-vector pCc1001) and a control transformant without extra laccase gene (carrying only trp1+-vector pCc1001). Results show that laccase activities in culture supernatants and morphology of fungal pellets in our experiments were primarily influenced by the cultivation temperature. The results however do not reveal an effect by morphology on recombinant laccase production or, vice versa, of recombinant laccase production on morphology.
Native and recombinant laccase production
At the optimal growth temperature at 37 °C, lcc1 transformant pYSK7-26 showed laccase activities of maximum 2.9 U/ml in Cu-supplemented mKjalke medium (Fig. 1f) which was comparable to the activities of 3.0 U/ml yielded before for the same transformant in Cu-supplemented YMG/T medium (Kilaru et al. 2006). Cultivation of the pCc1001 control transformant at 37 °C in contrast resulted in no laccase activities in both culture media (Fig. 1f; Kilaru et al. 2006). This suggests that the laccase activity of transformant pYSK7-26 at 37 °C is only due to recombinant expression (Kilaru et al. 2006).
Laccase yields of the lcc1 transformant pYSK7-26 were much higher in Cu-supplemented mKjalke medium at 25 °C (maximum 9.2 U/ml; Fig. 1f) which is comparable to reported best recombinant laccase production of 107 nkat/ml (equates in our rating system to 6.4 U/ml) in the agaricomycete Pycnoporus cinnabarinus (Alves et al. 2004) and presents the highest laccase activity reported in C. cinerea so far (Kilaru et al. 2006; Pan et al. 2014; Rühl et al. 2013). Based on the specific laccase activity of purified Lcc1 of 67 U/mg (Kilaru 2006), our highest activity of 9.2 U/ml equals 137 mg of laccase per litre culture supernatant.
However, part of these enzymatic activities of the C. cinerea lcc1 transformant pYSK7-26 likely derived from natural production of laccases since lower enzymatic activities (maximum 3.5 U/ml) were also measured in the supernatant of parallel cultures of the pCc1001 control transformant. The pCc1001 control transformant behaved comparable to the untransformed parental strain FA2222 which produced maximum laccase activities of 2.8 U/ml in Cu-supplemented mKjalke medium at 25 °C by secretion of the two laccases Lcc1 and Lcc5 and which had no laccase activity at 37 °C (Kilaru et al. 2006; Rühl et al. 2013).
As documented in Fig. 1, best enzymatic yields of native and recombinant laccase production at both growth temperatures correlated with high biomass amounts, depletion of glucose in the medium and a reduced C/N ratio. Moreover, there is a link in biomass production, glucose depletion and C/N ratio to a shift in pH from slightly acidic to alkaline. Although media parameters were previously not measured, we observed also in our previous studies that native and recombinant laccase activities were highest when C. cinerea cultures were fully grown (Kilaru et al. 2006; Rühl et al. 2013).
In this study, native laccase was already produced by the pCc1001 control transformant at 25 °C at slightly acidic pH values when glucose concentrations were fallen low and C/N ratios reduced, although full activity was seen with full glucose depletion at pH 8 (Fig. 1e, f). Previously, Hublik and Schinner (2000) reported for Pleurotus ostreatus a correlation of laccase production with an increase in the pH in the culture supernatant with maximum laccase activities at pH 8.5. In cultures of Trametes versicolor, onset of laccase production paralleled decrease in glucose content and increase in biomass. With the end of the exponential phase, the pH steadily raised up to pH 6.5, but optimum laccase activity was observed at pH 4.8 to 5.1 (Sun et al. 2013). In another study, the pH in batch cultures of the agaricomycete Trametes pubescens increased sharply from pH 4 to 7 after glucose depletion and with it the laccase activity. Under fed-batch conditions at low glucose influx, maximum laccase activities were obtained under slightly acidic conditions at pH 5 (Galhaup et al. 2002b). Accordingly, laccase activities in T. pubescens depend on the low glucose concentration rather than on the pH of the cultivation medium (Galhaup et al. 2002a, b). Laccase production is low-carbon-controlled also in many other Agaricomycetes and C/N ratios are of influence (Yang et al. 2017; Janusz et al. 2013). Native laccase production in C. cinerea likely also underlies such nutritional regulatory mechanisms whereas the observed change in pH seen in the cultures might be a secondary action commenced by the end of the mycelial growth phase (Rühl 2010).
How about regulation of recombinant laccase production in C. cinerea under the control of the gpdII-promoter from A. bisporus which is constitutive in the native host (Harmsen et al. 1992; Kilaru and Kües 2005)? Consistent with a constitutive behaviour of the gpdII-promoter also in C. cinerea, laccase activity by the pYSK7-26 transformant at 37 °C was detected in Cu-supplemented mKjalke medium when glucose levels were still significant high and activity levels raised with biomass production to decrease in the stationary phase with biomass ageing (Fig. 1a–f). Similar curves of laccase activities levelled with biomass production and ageing were observed before by Kilaru et al. (2006) in cultures at 37 °C for the pYSK7-26 strain and other transformants in Cu-supplemented YMG/T medium. At 25 °C in Cu-supplemented mKjalke medium however, there was a sharp peak of high laccase activity at day 8 of cultivation (Fig. 1f). Currently, it is unclear whether this is due to the ectopic genomic place of the vector integration in C. cinerea or due to a failure of appropriate regulation of the A. bisporus promoter in the foreign host at 25 °C or due to a type of interference with laccase activities such as enzyme degradation by peptidases.
Pellet morphology and laccase activities
Enzymes in filamentous fungi are believed to mainly be secreted by physiologically active hyphal tips, and fungal colonies differ spatially in gene expression and secretion activities in different growth zones (Conesa et al. 2001; Krijgsheld et al. 2012; Levin et al. 2007; Moukha et al. 1993; Nevalainen and Peterson 2014; Wösten et al. 1991). Therefore, it is very reasonable to study fungal growth and morphology during cultivation and fermentation. Here, the pellet production and morphology were compared between two C. cinerea transformants, one of which recombinantly produced laccase.
Importantly, the two transformants had comparable growth behaviours with a faster biomass production at 37 °C as compared to 25 °C and laccase production had little influence on biomass production (Fig. 1a, f). Clear differences in pellet morphology in surface structure and width and density of outer pellet zones were visible between different cultivation temperatures and to some degree also between the two transformants when grown at 25 °C (in pellet convexity, Fig. 3c) and at the higher temperature of 37 °C (in fringy pellet surface, compactness and pellet convexity, Figs. 2b and 3c). However, any clear link of specific pellet morphologies to native or recombinant laccase production is not apparent from our current observations.
Native laccase production occurred in liquid cultures at 25 °C, recombinant laccase production at 37 °C and even more at 25 °C (Fig. 1f; Rühl et al. 2013), indicating that reducing the temperature has a prominent role on increasing laccase yields. An analogous temperature effect on laccase activities is seen in growing agar cultures of both C. cinerea wildtype strains and transformants (our unpublished observations). Better laccase production at sub-optimum lower growth temperatures has also been reported for cultures of Trametes hirsuta (Dhakar and Pandey 2013). It is currently open whether it is just the temperature which influences degrees of native laccase activities in C. cinerea or whether there is possibly also an indirect effect by the temperature on hyphal morphology by creation of a smooth rather than a fringy pellet surface. However, microparticle-mediated morphological engineering of pellets of Cerrena unicolor and Pleurotus sapidus lead in both species to a rise in laccase yields with an increase in pellet numbers while producing C. unicolor pellets became more fringy and producing P. sapidus pellets in contrast became more smooth (Antecka et al. 2016). Moreover, solid particle waste or CaCO3 microparticles in liquid cultures of T. versicolor stimulated mycelial dispersal and laccase productivity (Tišma et al. 2012). Incidences of distinct pellet surface morphologies and shapes can thus lead to same practical results.
Timings of recombinant laccase production were different: In 37 °C cultures, it started in the logarithmic growth phase, in 25 °C cultures at entry in the stationary phase (Fig. 1f) and pellet morphology was clearly different in the different cultures (Figs. 2b and 4). More crucial than pellet morphology in the current work is probably the activity of the heterologous promoter (see above). At this stage of consideration, we can discuss the pellet morphology independently of laccase production. However, future studies need to further evaluate effects such as changing inocula and agitation and different rheological conditions on pellet size and morphology in connection to yields of laccase activities for further optimisation of cultivation conditions (Veiter et al. 2018). Tests with different inoculum amounts of recombinant laccase producers resulted in same enzymatic activities and alike pellet formations (Z. Fang, personal communication).
Pellet zonation and ageing
The most striking observation in this study is the different morphology of C. cinerea pellets at the different growth temperatures (Figs. 2b and 4). Pellets at 37 °C with broader outer rinds resembled aggregates of L. edodes formed at medium agitation (100 rpm) at 25 °C whereas low agitation (50 rpm) lead in L. edodes to larger loose mycelial clumps and high agitation (300 rpm) to breakage into smaller mycelial flocks (Tepwong et al. 2012). In a series of grey-scale images, Tepwong et al. (2012) showed pellet formation over the time. Albeit occurring over longer time (10–25 days), the sequence of events at medium agitation followed in L. edodes in the main what we observed at 37 °C for the faster growing C. cinerea. Initially, there were loose unstructured hyphal aggregates and later on hyphal differentiation in inner and outer pellet zones happened. Between outer and inner zones of a pellet, hyphal strengths and intertwinings differed. Molecular work in the ascomycete Aspergillus niger revealed clearly that fungal colonies within liquid cultures are heterogeneous in gene expression, between hyphal aggregates, between zones and even between hyphae within a zone (de Bekker et al. 2011; van Veluw et al. 2013; Wösten et al. 2013). The morphological differentiation in distinct zones and within the outer zone and also the localised formation of protuberances (Figs. 2b and 4) argue for similar spatial expression effects to possibly occur also in C. cinerea pellets.
Comparable protruding structures were observed for Ganoderma lucidum pellets and shown to be glucose-regulated and to develop into second-generation pellets after liberation from the parental structure (Wagner et al. 2004; Wan-Mohtar et al. 2016). As reported earlier for other fungi (Fang and Zhong 2002; Fazenda et al. 2010; Lejeune and Baron 1998; Wagner et al. 2004; Wan-Mohtar et al. 2016), an increase in pellet amounts within cultures may be due to pellet breakage, shaving off outer hyphae and release of appendages from the pellets. All mechanisms might occur in C. cinerea cultures. Already at day 2 of cultivation, independently of the temperature of cultivation, there were about 1200 pellets more in the cultures of the pCc1001 control transformant than in the parallel cultures of the pYSK7-26 transformant (Fig. 1d) and there was more dispersed mycelium (Fig. 2a). Pellets of the pCc1001 control transformant were overall more fringy on the surface than the pellets of the pYSK7-26 transformant (Fig. 2b) and they had more and larger loosely connected protuberances in the outer zone to break off as a nucleus for producing new pellets. For both strains, production of such appendances was more pronounced at 37 than at 25 °C (Figs. 2b and 4).
The two C. cinerea transformants differed first from each other in the projected area of their pellets but eventually had similar sizes at the two distinct cultivation temperatures. Regardless of the temperature, during the logarithmic growth phase most pellets of the pCc1001 control transformant showed a projected area of approximately 2–4 mm2 and most pellets of the pYSK7-26 transformant a projected area of 4–6 mm2, respectively. Cultures of both transformants at 37 °C approached the main absolute pellet size (in diameter and projected area) already after 2 days of cultivation, cultures at 25 °C at day 4 of cultivation. Afterwards, both parameters were more or less stable over the whole cultivation period of both transformants (Fig. 3a, b). However, the biomass and the pellet concentration still increased until day 4 and day 6 for the lcc1 transformant pYSK7-26 and the pCc1001 control transformant, respectively (Fig. 1a), and with time of cultivation also the convexity of the pellets (Fig. 3c).
One reason for the strain differences in pellet numbers and sizes was probably a slightly faster logarithmic growth of the pCc1001 control transformant resulting in a faster accumulation of total biomass (Fig. 1a) and a higher increase in the amount of pellets per culture (Fig. 1d). The increase in dry weight is probably also due to the more dense and packed nature of the pellets at day 4 compared to day 2 of cultivation (Figs. 1a and 2b). Moreover, although pre-cultures of the transformants were handled exactly the same, more mycelial debris could have been produced for the pCc1001 control transformant during maceration of the pre-culture. In addition, germination tests on YMG agar plates indicated higher amounts of active oidia for the pCc1001 control transformant (9.8 ± 0.8% germination rate) compared to the lcc1 transformant pYSK7-26 (5.1 ± 1.5% germination rate) in spore samples used for inoculation of pre-cultures. Also, pre-cultures in mKjalke medium showed higher biomass yields for the pCc1001 control transformant (4.2 ± 0.4 g/l DW) compared to the lcc1 transformant pYSK7-26 (3.1 ± 0.8 g/l) (experiments not further shown).
In pellets of older culture age, we observed formation of chlamydospores, singly and in clusters (Fig. 5). Chlamydospore production has also been reported in pellets of Phanerochaete chrysosporium (Jiménez-Tobon et al. 2003) and Agaricus blazei (Hamedi et al. 2012). Chlamydospores in P. chrysosporium pellets are the main places of Mn(II) production, whereas apices of hyphae in the outer zone are minor places of Mn(II) production (Jiménez-Tobon et al. 2003). Large nutrient-hoarding chlamydospores of Agaricomycetes function typically in duration after active fungal growth phases ended (Kües et al. 2016).
Observations on pellets in other Agaricomycetes
For cultures of the agaricomycetes Dichomitus squalens and Lentinula edodes, pellet size (radius), structure and surface properties (smooth, fluffy-fringy) were demonstrated to be strongly influenced by agitation speed and aeration as well as in L. edodes by amounts of inocula (Babič and Pavko 2012; Tepwong et al. 2012). Agitation reduced pellet sizes in P. ostreatus under higher laccase production because of more metabolically active cells while responsible laccase gene (poxc) expression remained unaltered. On the contrary, strong aeration influenced fungal growth but not pellet sizes and had negative effects on laccase gene expression and resulting enzyme production (Fernández-Alejandre et al. 2016). In cultures of G. lucidum and Grifola frondosa, aeration promoted hyphal branching and agitation influenced the release of outermost hyphae from pellets (Cui et al. 2016; Fazenda et al. 2010). Agitation schemes therefore affect yields of biomass (Cui et al. 2016; Yang et al. 2009). For Phellinius, species-specific effects of culture pH on pellet morphology were established and morphology was related to exopolysaccharide (EPS) production (Hwang et al. 2004) similar as in G. frondosa (Lee et al. 2004), Schizophyllum commune (Shu et al. 2005) and G. lucidum (Kim et al. 2006). Microparticle talc added in distinct concentration to G. frondosa cultures can well control pellet sizes and shapes for optimum biomass and EPS production (Tao et al. 2018).
A one-factor at a time analysis in A. blazei showed that types of C-sources and types of N-sources differentially influenced pellet compactness as well as EPS production but the nutritional effects were not paralleled. Variable effects of C-sources, of N-sources and of the pH on pellet size and EPS production were in addition observed (Hamedi et al. 2012). Different pellet surface morphologies have been reported to link to laccase (smooth pellets) and manganese peroxidase (MnP) production (fluffy pellets) in D. squalens, respectively (Babič and Pavko 2012) and also for MnP production (fluffy pellets) in P. chrysosporium (Jaspers et al. 1994). Furthermore in P. chrysosporium, distinct pellet sizes differentially favoured MnP production (Jiménez-Tobon et al. 1997) and influenced lignin peroxidase production (Michel et al. 1990, 1992; Žmak et al. 2006). Different pellet sizes correlate to C/N ratios and best EPS and ganoderic acid yields of G. lucidum, respectively (Fang et al. 2002; Ding et al. 2012). Larger pellets were better for ganoderic acid production which is favoured by oxygen limitation in the pellets (Fang and Zhong 2002; Tang and Zhong 2003), whereas dispersed and medium-sized pellets gave higher yields of EPS upon a pH shift from 3.0 to 6.0 (Kim et al. 2006; Ding et al. 2012). Possibly by generating anaerobic stress within larger pellets, the pellet size, form and structure also influenced antibiotic production of Cyathus striatus (Gehrig et al. 1998) and pellet size correlated with yields of the antioxidant ergothionine in L. edodes (Tepwong et al. 2012).
Further, MALDI MS imaging revealed differential patterns of localization of cyathane-type diterpenoids in pellets of C. striatus and Hericium erinaceus. Distributions of striatins in C. striatus pellets were homogeneous, distribution of erinacines in H. erinaceus pellets gradual with highest intensities in pellet centres. Distribution of compounds possibly reflected a combination of effects by production place and of specific diffusible characters of compounds (Bhandari et al. 2014).
Although pellet morphology in Agaricomycetes is only little studied and understood, the so far available examples point to its significance in production processes and to the contrasting needs and effects that different production processes and conditions can have. Nevertheless, a generalising final conclusion on required pellet morphology and interconnected functions of pellet zones cannot be provided from the available reports even for same product groups of Agaricomycetes, be it EPS, enzymes or secondary metabolites. The examples from literature presented above indicate that pellet architecture and culture productivity (in biomass, proteins, polysaccharides or metabolites) are not two simple interlinked parameters, with each case being distinctive from others (further discussed in the recent review by Veiter et al. 2018).
In line, our study did not bring conclusive proof for a link of laccase production to a specific pellet morphology of C. cinerea. Nevertheless, our work gives for the first time insight into pellet formation of this important species considered for natural and recombinant enzyme production by a basidiomycete (Cheng et al. 2009; Dutt et al. 2013; Han et al. 2010; Kikuchi et al. 2004; Kilaru et al. 2006; Ogawa et al. 1998; Pan et al. 2014; Yang et al. 2011). The study improves further the restricted knowledge we hitherto have on pellet formation in filamentous basidiomycetes. Most reports on pellet formation of Agaricomycetes are still on flasks cultures but such can give useful information for submerged cultivation at larger scale, with needs for specific products in bioreactor design and agitation schemes (Elisashvili 2012; Lee et al. 2004; Petre et al. 2010; Shu et al. 2005; Yang and Yang 2005).
References
Alves AMCR, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JGH, Wösten HAB (2004) Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol 70:6379–6384
Antecka A, Blatkiewicz M, Bizukojć M, Ledakowicz S (2016) Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotechnol Lett 38:667–672
Arimoto M, Yamagishi K, Wang JQ, Tanaka K, Miyoshi T, Kamei I, Kondo R, Moro T, Kawagishi H, Hirai H (2015) Molecular breeding of lignin-degrading brown-rot fungus Gloeophyllum trabeum by homologous expression of laccase gene. AMB Express 5:81
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Babič J, Pavko A (2012) Enhanced enzyme production with the pelleted form of D. squalens in laboratory bioreactors using added natural lignin inducer. J Ind Microbiol Biotechnol 39:449–457
Bentil JA, Thygesen A, Mensah M, Lange L, Meyer AS (2018) Cellulase production by white-rot basidiomycetous fungi: solid-state versus submerged cultivation. Appl Microbiol Biotechnol 102:5827–5839
Bertrand B, Martínez-Morales F, Trejo-Hernández MR (2017) Upgrading laccase production and biochemical properties: strategies and challenges. Biotechnol Prog 33:1015–1034
Bhandari DR, Shen T, Römpp A, Zorn H, Spengler B (2014) Analysis of cyathane-type diterpenoids from Cyathus striatus and Hericium erinaceus by high-resolution MALDI MS imaging. Anal Bioanal Chem 406:695–704
Cannatelli MD, Ragauskas AJ (2016) Conversion of lignin into value-added materials and chemicals via laccase-assisted copolymerization. Appl Microbiol Biotechnol 100:8685–8691
Cheng SJ, Yang PZ, Guo LQ, Lin JF, Lou NN (2009) Expression of multi-functional cellulase gene mfc in Coprinus cinereus under control of different basidiomycete promoters. Bioresour Technol 100:4475–4480
Coconi-Linares N, Ortiz-Vázquez E, Fernández F, Loske AM, Gómez-Lim MA (2015) Recombinant expression of four oxidoreductases in Phanerochaete chrysosporium improves degradation of phenolic and non-phenolic substrates. J Biotechnol 209:76–84
Conesa A, Punt PJ, van Luijk N, van den Hondel CAMJ (2001) The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol 33:155–171
Cui FJ, Chen XX, Liu WM, Sun WJ, Huo S, Yang Y (2016) Control of Grifola frondosa morphology by agitation and aeration for improving mycelia and exo-polymer production. Appl Biochem Biotechnol 179:459–473
de Bekker C, van Veluw GJ, Vinck A, Wiebenga A, Wösten HAB (2011) Heterogeneity of Aspergillus niger micro-colonies in liquid shaken cultures. Appl Environ Microbiol 77:1263–1267
Dekker RFH, Barbosa AM (2001) The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzym Microb Technol 28:81–88
Dhakar K, Pandey A (2013) Laccase production from a temperature and pH tolerant fungal strain of Trametes hirsuta (MTCC 11397). Enzyme Res 2013:869062
Ding Z, Wang Q, Peng L, Zhang L, Gu Z, Shi G, Zhang K (2012) Relationship between mycelium morphology and extracellular polysaccharide production of medicinal mushroom Ganoderma lucidum in submerged culture. J Med Plant Res 6:2868–2874
Domingos M, Brasil de Souza-Cruz P, Ferrau A, Ramalho Prata AM (2017) A new bioreactor design for culturing basidiomycetes: mycelial biomass production in submerged cultures of Ceriporiopsis subvermispora. Chem Eng Sci 170:670–676
Dörnte B, Kües U (2012) Reliability in transformation of the basidiomycete Coprinopsis cinerea. Curr Trends Biotechnol Pharm 6:340–355
Dörnte B, Kües U (2016) Paradoxical performance of tryptophan synthase gene trp1 + in transformations of the basidiomycete Coprinopsis cinerea. Appl Microbiol Biotechnol 100:8789–8807
Dutt D, Tyagi CH, Singh RP, Gautam A, Agnohotri S, Kumar A (2013) Isolation and biochemical characterization of crude xylanase from Coprinus cinereus AT-1 MTCC 9695 and its effectiveness in biodeinking of SOP. Cellul Chem Technol 47:203–217
Elisashvili V (2012) Submerged cultivation of medicinal mushrooms: bioprocesses and products (review). Int J Med Mushrooms 14:211–239
Fang QH, Zhong JJ (2002) Two-stage culture process for improved production of ganoderic acid by liquid fermentation of higher fungus Ganoderma lucidum. Biotechnol Prog 18:51–54
Fang QH, Tang YJ, Zhong JJ (2002) Significance of inoculation density control in production of polysaccharide and ganoderic acid by submerged culture of Ganoderma lucidum. Process Biochem 37:1375–1379
Fazenda ML, Harvey LM, McNeil B (2010) Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum. J Microbiol Biotechnol 20:844–851
Fernández-Alejandre KI, Flores N, Tinoco-Valencia R, Caro M, Flores C, Galindo E, Serrano-Carreón L (2016) Diffusional and transcriptional mechanisms involved in laccases production by Pleurotus ostreatus CP50. J Biotechnol 223:42–49
Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002a) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148:2159–2169
Galhaup C, Wagner H, Hinterstoisser B, Haltrich D (2002b) Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzym Microb Technol 30:529–536
Gehrig I, Bart HJ, Anke T, Germerdonk R (1998) Influence of morphology and rheology on the production characteristics of the basidiomycete Cyathus striatus. Biotechnol Bioeng 59:525–533
Grimm LH, Kelly S, Krull R, Hempel DC (2005) Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol 69:375–384
Hamedi A, Ghanati F, Vahidi H (2012) Study on the effects of different culture conditions on the morphology of Agaricus blazei and the relationship between morphology and biomass or EPS production. Ann Microbiol 62:699–707
Han F, Liu Y, Guo LQ, Zeng XL, Liu ZM, Lin JF (2010) Heterologous expression of the immunomodulatory protein gene from Ganoderma sinense in the basidiomycete Coprinopsis cinerea. J Appl Microbiol 109:1838–1844
Harmsen MC, Schuren FHJ, Moukha SM, Vanzuilen CM, Punt PJ, Wessels JGH (1992) Sequence analysis of the glyceraldehyde-3-phosphate dehydrogenase genes from the basidiomycetes Schizophyllum commune, Phanerochaete chrysosporium and Agaricus bisporus. Curr Genet 22:447–454
Hublik G, Schinner F (2000) Characterization and immobilization of the laccase from Pleurotus ostreatus and its use for the continuous elimination of phenolic pollutants. Enzym Microb Technol 27:330–336
Hwang HJ, Kim SW, Xu CP, Choi JW, Yun JW (2004) Morphological and rheological properties of the three different species of basidiomycetes Phellinus in submerged cultures. J Appl Microbiol 96:1296–1305
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzym Microb Technol 52:1–12
Jaspers CJ, Jimenez G, Penninckx MJ (1994) Evidence for a role of manganese peroxidase in the decolorization of Kraft pulp bleach plant effluent by P. chrysosporium: effects of initial culture conditions on enzyme production. J Biotechnol 37:229–234
Jiménez-Tobon GA, Penninckx MJ, Lejeune R (1997) The relationship between pellet size and production of Mn(II) peroxidase by Phanerochaete chrysosporium ATCC 24725 in submerged culture. Enzym Microb Technol 21:537–542
Jiménez-Tobon G, Kurzatkowski W, Rozbicka B, Solecka J, Pocsi I, Penninckx MJ (2003) In situ localization of manganese peroxidase production in mycelial pellets of Phanerochaete chrysosporium. Microbiology 149:3121–3127
Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C (2005) Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl Microbiol Biotechnol 66:450–456
Kajita S, Sugawara S, Miyazaki Y, Nakamura M, Katayama Y, Shishido K, Iimura I (2004) Overproduction of recombinant laccase using a homologous expression system in Coriolus versicolor. Appl Microbiol Biotechnol 66:194–199
Kertesz-Chaloupková K, Walser PJ, Granado JD, Aebi M, Kües U (1998) Blue light overrides repression of asexual sporulation by mating type genes in the basidiomycete Coprinus cinereus. Fungal Genet Biol 23:95–109
Kikuchi M, Kitamoto N, Shishido K (2004) Secretory production of Aspergillus oryzae xylanase XynF1, xynF1 cDNA product, in the basidiomycete Coprinus cinereus. Appl Microbiol Biotechnol 63:728–733
Kilaru S (2006) Identification of fungal multi-copper oxidase gene families: overexpression and characterization of Coprinopsis cinerea laccases for applications in biotechnology. Dissertation, University of Goettingen
Kilaru S, Kües U (2005) Comparison of gpd genes and their protein products in basidiomycetes. Fungal Genet News Lett 52:18–24
Kilaru S, Hoegger PJ, Majcherczyk A, Burns C, Shishido K, Bailey A, Foster GD, Kües U (2006) Expression of laccase gene lcc1 in Coprinopsis cinerea under control of various basidiomycetous promoters. Appl Microbiol Biotechnol 71:200–210
Kim HM, Park MK, Yun JW (2006) Culture pH affects exopolysaccharide production in submerged mycelial culture of Ganoderma lucidum. Appl Biochem Biotechnol 134:249–262
Krijgsheld P, Altelaar AF, Post H, Ringrose JH, Müller WH, Heck AJR, Wösten HAB (2012) Spatially resolving the secretome with the mycelium of the cell factory Aspergillus niger. J Proteome Res 11:2807–2818
Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, Kampen I, Kwade A, Wittmann C (2013) Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol 163:112–123
Kudanga T, Nemadziva B, Le Roes-Hill M (2017) Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol 101:13–33
Kües U (2000) Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Rev 64:316–353
Kües U (2015) Fungal enzymes for environmental management. Curr Opin Biotechnol 33:268–278
Kües U, Dörnte B, Gießler A, Badalyan SM (2016) Asexual sporulation in Agaricomycetes. In: Wendland J (ed) The mycota I, 3rd edn. Growth, differentiation and sexuality. Springer, Berlin, pp 269–328
Kum H, Lee S, Ryu S, Choi HT (2011) Degradation of endocrine disrupting chemicals by genetic transformants with two lignin degrading enzymes in Phlebia tremellosa. J Microbiol 49:824–827
Larrondo LF, Avila M, Salas L, Cullen D, Vicuña R (2003) Heterologous expression of laccase cDNA from Ceriporiopsis subvermispora yields copper-activated apoprotein and complex isoform patterns. Microbiology 149:1177–1182
Lee BC, Bae JT, Pyo HB, Choe TB, Kim SW, Hwang HJ, Yun JW (2004) Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible basidiomycete Grifola frondosa. Enzym Microb Technol 35:369–376
Lejeune R, Baron GV (1998) Modeling the exponential growth of filamentous fungi during batch cultivation. Biotechnol Bioeng 60:169–179
Levin AM, de Vries RP, Conesa A, de Bekker C, Talon M, Menke HH, van Peij NNME, Wösten HAB (2007) Spatial differentiation in the vegetative mycelium of Aspergillus niger. Eukaryot Cell 6:2311–2322
Madzak C, Otterbein L, Chamkha M, Moukha S, Asther M, Gaillardin C, Beckerich JM (2005) Heterologous production of a laccase from the basidiomycete Pycnoporus cinnabarinus in the dimorphic yeast Yarrowia lipolytica. FEMS Yeast Res 5:635–646
Martani F, Beltrametti F, Porro D, Branduardi P, Lotti M (2017) The importance of fermentative conditions for the biotechnological production of lignin modifying enzymes from white-rot fungi. FEMS Microbiol Lett 364:fnx134
Mate DM, Alcalde M (2017) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Ecol 10:1457–1467
Matsumura E, Yamamoto E, Numata A, Kawano T, Shin T, Murao S (1986) Structures of the laccase-catalyzed oxidation products of hydroxybenzoic acids in the presence of ABTS [2,2′-azino-di-(3-ethylbenzothiazoline-6-sulfonic acid)]. Agric Biol Chem 50:1355–1357
Michel FC Jr, Grulke EA, Reddy CA (1992) A kinetic model for the fungal pellet lifecycle. AICHE J 38:1449–1460
Michel FC, Grulke EA, Reddy CA (1990) Development of a stirred tank reactor system for the production of lignin peroxidases (ligninases) by Phanerochaete chrysosporium BKM-F-1767. J Ind Microbiol 5:103–112
Moukha SM, Wösten HAB, Asther M, Wessels JGH (1993) In situ localization of the secretion of lignin peroxidases in colonies of Phanerochaete chrysosporium using a sandwiched mode of culture. J Gen Microbiol 139:969–978
Nevalainen H, Peterson R (2014) Making recombinant proteins in filamentous fungi – are we expecting too much? Front Microbiol 5:75
Nyanhongo GS, Gomes J, Gübitz G, Zvauya R, Read JS, Steiner W (2002) Production of laccase by a newly isolated strain of Trametes modesta. Bioresour Technol 84:259–263
Ogawa K, Yamazaki T, Hasebe T, Kajiwara S, Watanabe A, Asada Y, Shishido K (1998) Molecular breeding of the basidiomycete Coprinus cinereus strains with high lignin-decolorization and -degradation activities using novel heterologous protein expression vectors. Appl Microbiol Biotechnol 49:285–289
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Uses of laccases in the food industry. Enzyme Res 2010:918761
Pan K, Zhao N, Yin Q, Zhang T, Xu X, Fang W, Hong Y, Fang Z, Xiao Y (2014) Induction of a laccase Lcc9 from Coprinopsis cinerea by fungal coculture and its application on indigo dye decolorization. Bioresour Technol 162:45–52
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial process. Biotechnol Adv 22:189–259
Petre M, Teodorescu A, Tiluca E, Bejan C, Andronescu A (2010) Biotechnology of mushroom pellets producing by controlled submerged fermentation. Rom Biotechnol Lett 15(S2):50–55
Piscitelli A, Pezzella C, Giardina P, Faraco V, Giovanni S (2010) Heterologous laccase production and its role in industrial applications. Bioeng Bugs 1:252–262
Rao PS, Niederpruem DJ (1969) Carbohydrate metabolism during morphogenesis of Coprinus lagopus (sensu Buller). J Bacteriol 100:1222–1228
Rühl M (2010) Laccases and other ligninolytic enzymes of the basidiomycetes Coprinopsis cinerea and Pleurotus ostreatus. Dissertation. Georg-August-University, Göttingen
Rühl M, Kües U (2009) Automated image analysis to observe pellet morphology in liquid cultures of filamentous fungi such as the basidiomycete Coprinopsis cinerea. Curr Trends Biotechnol Pharm 3:241–253
Rühl M, Majcherczyk A, Kües U (2013) Lcc1 and Lcc5 are the main laccases secreted in liquid cultures of Coprinopsis cinerea strains. Antonie Van Leeuwenhoek 103:1029–1039
Ryu SH, Cho MK, Kim M, Jung SM, Seo JH (2013) Enhanced lignin biodegradation by a laccase-overexpressed white-rot fungus Polyporus brumalis in the pretreatment of wood chips. Appl Biochem Biotechnol 171:1525–1534
Shu CH, Chou PF, Hsu IC (2005) Effects of morphology and oxygen supply on schizophyllan formation by Schizophyllum commune using a pellet size controlling bioreactor. J Chem Technol Biotechnol 80:1383–1388
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, van den Hondel CAMJ, Fournel A, Sigoillot JC, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Šnajdr J, Baldrian P (2007) Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbiol 52:498–502
Sun W, Xu M, Xia C, Li A, Sun C (2013) Enhanced production of laccase by Coriolus hirsutus using molasses distillery wastewater. Front Environ Sci Eng 7:200–210
Tang YJ, Zhong JJ (2003) Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enzym Microb Technol 32:478–484
Tang YJ, Zhu LW, Li HM, Li DS (2007) Submerged culture of mushrooms in bioreactors – challenges, current state-of-the-art, and future prospects. Food Technol Biotechnol 45:221–229
Tao TL, Cui FJ, Chen YX, Sun WJ, Huang DM, Zhang YY, Wu D, Liu WM (2018) Improved mycelia and polysaccharide production of Grifolia frondosa by controlling morphology with microparticle talc. Microb Cell Factories 17:1
Tavares APM, Coelho MAZ, Coutinho JAP, Xavier AMRB (2005) Laccase improvement in submerged cultivation: induced production and kinetic modelling. J Chem Technol Biotechnol 80:669–676
Tepwong P, Giri A, Ohshima T (2012) Effect of mycelial morphology on ergothioneine production during liquid fermentation of Lentinula edodes. Mycoscience 53:102–112
Tišma M, Žnidaršič-Plazl P, Vasić-Raćki D, Zelić B (2012) Optimization of laccase production by Trametes versicolor cultivated on industrial waste. Appl Biochem Biotechnol 166:36–46
van Veluw GJ, Teertstra WR, de Bekker C, Vinck A, van Beck N, Muller WH, Arentshorst M, van der Mei HC, Ram AFJ, Dijksterhius J, Wösten HAB (2013) Heterogeneity in liquid shaken cultures of Aspergillus niger inoculated with melanised conidia or conidia of pigmentation mutants. Stud Mycol 74:47–57
Veiter L, Rajamanickam V, Herwig C (2018) The filamentous fungal pellet-relationship between morphology and productivity. Appl Microbiol Biotechnol 102:2997–3006
Wagner R, Mitchell DA, Sassaki GL, Amazonas MALA (2004) Links between morphology and physiology of Ganoderma lucidum in submerged culture for the production of exopolysaccharide. J Biotechnol 114:153–164
Wan-Mohtar WAAQI, Kadir SA, Saaro N (2016) The morphology of Ganoderma lucidum mycelium in a repeated-batch fermentation for exosaccharode production. Biotech Rep 11:2–11
Wösten HAB, Moukha SM, Sietsma JH, Wessels JGH (1991) Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol 137:2017–2023
Wösten HAB, Veluw GJ, Bekker C, Krijgsheld P (2013) Heterogeneity in the mycelium: implications for the use of fungi as cell factories. Biotechnol Lett 35:1155–1164
Wucherpfennig T, Lakowitz A, Krull R (2013) Comprehension of viscous morphology - evaluation of fractal and conventional parameters for rheological characterization of Aspergillus niger culture broth. J Biotechnol 163:124–132
Yang FC, Yang MJ (2005) Influence of agitation intensity on mycelium aggregation of Ganoderma lucidum. J Chin Inst Chem Eng 36:669–674
Yang FC, Yang MJ, Cheng SH (2009) A novel method to enhance the mycelia production of Ganoderma lucidum in submerged cultures by polymer additives and agitation strategies. J Taiwan Inst Chem Eng 40:148–154
Yang PZ, Gui LQ, Cheng SJ, Lou NN, Lin JF (2011) Recombinant multi-functional cellulose activity in submerged fermentation of lignocellulosic wastes. Renew Energy 36:3268–3272
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8:832
Žmak PM, Podgornik A, Podgornik H, Koloini T (2006) Impact of pellet size on growth and lignin peroxidase activity of Phanerochaete chrysosporium. World J Microbiol Biotechnol 22:1243–1249
Acknowledgements
We thank Zemin Fang for sharing his unpublished results with us and Gisbert Langer for his excellent technical support in measuring the C/N ratio of the culture supernatants. Work on recombinant laccase production in C. cinerea was supported within the framework of a Common Lower Saxony-Israel-Project (ZN 2043) by the Ministry of Science and Culture of Lower Saxony.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Rühl and U. Kües declare that they have no conflict of interest. K. Lange approved a firstly reviewed manuscript and agreed to submission but died prior to a resubmission of the manuscript upon improvements made based on appreciated good reviewer comments. The paper is dedicated to her memory.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Karin Lange is deceased.
Rights and permissions
About this article
Cite this article
Rühl, M., Lange, K. & Kües, U. Laccase production and pellet morphology of Coprinopsis cinerea transformants in liquid shake flask cultures. Appl Microbiol Biotechnol 102, 7849–7863 (2018). https://doi.org/10.1007/s00253-018-9227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9227-7