Abstract
The demand for compounds of therapeutic value is increasing mainly because of new applications of bioactive compounds in medicine, pharmaceutical, agricultural, and food industries. This has necessitated the search for cost-effective methods for producing bioactive compounds and therefore the intensification of the search for enzymatic approaches in organic synthesis. Laccase is one of the enzymes that have shown encouraging potential as biocatalysts in the synthesis of bioactive compounds. Laccases are multicopper oxidases with a diverse range of catalytic activities revolving around synthesis and degradative reactions. They have attracted much attention as potential industrial catalysts in organic synthesis mainly because they are essentially green catalysts with a diverse substrate range. Their reaction only requires molecular oxygen and releases water as the only by-product. Laccase catalysis involves the abstraction of a single electron from their substrates to produce reactive radicals. The free radicals subsequently undergo homo- and hetero-coupling to form dimeric, oligomeric, polymeric, or cross-coupling products which have practical implications in organic synthesis. Consequently, there is a growing body of research focused on the synthetic applications of laccases such as organic synthesis, hair and textile dyeing, polymer synthesis, and grafting processes. This paper reviews the major advances in laccase-mediated synthesis of bioactive compounds, the mechanisms of enzymatic coupling, structure-activity relationships of synthesized compounds, and the challenges that might guide future research directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The search for cost-effective methods for producing bioactive compounds is a rapidly widening research niche with their market value predicted to rise by 4.71% between 2013 and 2018 (Infiniti Research Limited 2014). Bioactive compounds are compounds with nutritional benefits and are usually found in small quantities in plants (Kris-Etherton et al. 2002), sponges (Muller et al. 2004), bacteria, and fungi (Debbab et al. 2010). They are mainly secondary metabolites and can be broadly categorized into phenolic compounds, antibiotics, alkaloids, mycotoxins, food grade pigments, and growth factors (Martins et al. 2011). Industrial applications of these bioactive compounds are increasing. Apart from their application in pharmaceutical industries, bioactive compounds are now being employed in the food industry for the production of functional foods (nutraceuticals) (Gil-Chávez et al. 2013), in agrochemicals, cosmetics, geo-medicine, nano-bioscience, and in chemical industries (Guaadaoui et al. 2014).
Some of the presently used methods for extraction and production of bioactive compounds include the heat reflux extraction method, accelerated solvent method, supercritical fluid extraction, employing high-pressure protocols, use of microwave and ultrasound extraction processes, and chemical synthesis (Martins et al. 2011). Conventional physico-chemical processes employed in the production of bioactive compounds are generally long, energy intensive, low yielding, and associated with excessive amounts of wastes which have a negative impact on the environment. Metrics such as the E factor have highlighted the inefficiencies of chemical synthesis; the amount of waste generated per kilogram of any fine chemical or pharmaceutical product manufactured was 5–100 times higher than the product (Li and Trost 2008). Such concerns have prompted the formation of bodies such as the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable (ACS GCIPR) to promote the adoption of green technologies in pharmaceutical industries (Constable et al. 2007). Thus, newer, economically feasible, and environmentally benign processes have become a priority in a bid to meet the rising demand for bioactive compounds.
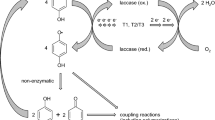
Biocatalysis is gaining notable attention in organic synthesis. This is because biocatalysts are environmentally benign and involve less process steps for the synthesis of valuable compounds. Unlike conventional means, enzymes are characteristically selective, a trait which is of importance when producing compounds of therapeutic value (Maugh 1984). However, laccases are an exception in this respect. Their catalytic mechanism leads to the formation of organic radicals as primary products, which frequently pose a challenge for biosynthesis purposes. While the highest possible yield of just one enantiomerically pure product would ideally be desired, radical processes typically lead to a range of different (and sometimes many) products appearing at rather low concentrations and as racemic mixtures. Laccases are one group of enzymes that have shown encouraging potential as biocatalysts in organic synthesis. Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) belong to the multicopper oxidase family of enzymes, and their role in nature involves both synthetic and degradative reactions (Riva 2006). Laccases are generally regarded as “green catalysts” because of their ability to oxidize a diverse range of compounds (including phenols, diphenols, methoxy-substituted phenols, phenolic, and alkyl amines) to corresponding radicals in the presence of molecular oxygen, concomitantly producing water as the only by-product (Kudanga et al. 2011a). Their catalytic mechanism generally involves the abstraction of a single electron from substrates to produce reactive free radicals (Kudanga and Le Roes-Hill 2014). These free radicals are vital intermediates which undergo coupling reactions to produce dimeric, oligomeric, polymeric, or cross-coupling products (Fig. 1). Therefore, the ability of laccases to catalyze oxidative coupling reactions makes them relevant in organic synthesis. Coupling of naturally existing bioactive compounds can result in novel products with enhanced bioefficacy. As a result, in the past two decades, there has been an increase in research activity exploiting laccases in the synthesis of bioactive compounds. Although extensive reviews on the enzymology of laccases (Claus 2004; Madhavi and Lele 2009; Mayer and Staples 2002; Morozova et al. 2007a) and their industrial application potential (Cañas and Camarero 2010; Jeon et al. 2012; Kudanga and Le Roes-Hill 2014; Kudanga et al. 2011a,b; Mikolasch and Schauer 2009; Riva 2006; Rodríguez Couto and Toca Herrera 2006; Witayakran and Ragauskas 2009) have already been published, their application in the synthesis of compounds of therapeutic value has not been comprehensively reviewed in recent articles. This paper provides a consolidated review of the work that has been covered so far in the laccase-catalyzed production of bioactive compounds mainly in the synthesis or modification of phenolic anti-oxidants, antibiotics, and alkaloids. In addition, the reaction mechanisms, structure-activity relationships, and directions for future research are also provided.
Laccase synthetic mechanism of action which involves a laccase-catalyzed oxidation of substrate to form radicals, b radicals undergo oxidative coupling to produce dimers, c further coupling results in the formation of polymers through polymerization, and d coupling with a non-laccase substrate to form cross-coupling products. Adapted from Abdel-Mohsen et al. (2014) and De Regil and Sandoval (2013), with permission from Royal Society of Chemistry and MDPI
Laccase-catalyzed production of bioactive compounds
Laccase applications in organic synthesis have been increasing in recent years mainly because the enzyme has a broad substrate specificity. Phenolic compounds, amino-phenols, polyamines, anilines, aromatic and alkyl amines, and benzenethiols all fall under the laccase substrate range (Kunamneni et al. 2008a; Madhavi and Lele 2009). Compounds carrying these functional groups have therefore become targets for biocatalytic reactions using laccases. The product range is further widened by coupling reactions involving a laccase substrate and a non-laccase substrate (variable reaction partner) to create new heteromolecular hybrid molecules (Mikolasch and Schauer 2009). The most frequently investigated compounds are phenolic anti-oxidants, alkaloids, and antibiotics.
Phenolic compounds
Phenolic compounds are widely distributed in the plant kingdom as secondary metabolites. They have been described as the “first line in plant defense against infection” (Matern and Kneusel 1988) because of their physiological role in the protection of plants from infections, harsh environments, and as a response to stress (Bhattacharya et al. 2010). Because of their bioactivity, phenolic compounds present a wide range of nutritional and therapeutic benefits ranging from anti-inflammatory, anti-allergenic, anti-artherogenic, anti-microbial, anti-oxidant, and anti-thrombotic activities and protection against several cardiovascular diseases (Balasundram et al. 2006; Pasha et al. 2013). Therefore, they have been obvious targets for researchers interested in bioactive compounds. Consequently, extensive research has focused on the application of laccase in the synthesis of phenolic compounds.
Laccase oxidation of substrates to their respective radicals is a pre-requisite for the production of dimeric, oligomeric, or polymeric compounds (through homomolecular coupling reactions) or cross-coupling products (through heteromolecular coupling of the radicals) (Kudanga et al. 2011b). Phenolic compounds have been modified mainly through homomolecular coupling (Table 1). Several studies have focused on producing novel anti-oxidant compounds through laccase-mediated dimerization of phenolic compounds. Adelakun et al. (2012a,b) used monomeric natural phenolic compounds as laccase substrates for the production of new anti-oxidants. Using ferulic acid as starting material, two derivatives, β-5 and β-β dimers, were successfully produced (Adelakun et al. 2012a). The β-5 dimers showed enhanced anti-oxidant activity, while β-β dimers had lower activity compared to ferulic acid. The enhanced activity of the β-5 dimer was attributed to the increase in electron-donating groups on the compound and the carboxylic acid group with an adjacent unsaturated C–C double bond, which can provide additional sites of attack for free radicals (Srinivasan et al. 2007). In related studies, 2,6-dimethoxyphenol (2,6-DMP) was also used in a laccase-oxidized reaction that resulted in the formation of a symmetrical C–C-linked 2,6-DMP dimer, 3,3′,5,5′-tetramethoxy biphenyl-4,4-diol, with approximately twice the anti-oxidant activity of 2,6-DMP (Adelakun et al. 2012b). During laccase catalysis, 2,6-DMP is oxidized to phenoxy radical species which form para-radical species through resonance stabilization; the dimer is subsequently formed through radical coupling of two para-radical species (Fig. 2). The superior anti-oxidant activity of the dimer was attributed to the increased functional groups with electron-donating capacity (Matsuura and Ohkatsu 2000), the reduction in the O-H bond dissociation energy, and increased stability of radical due to resonance delocalization (Sánchez-Moreno et al. 1998).
Proposed reaction mechanism for the homomolecular coupling of 2,6-DMP to produce the C–C dimer (3,3′,5,5′-tetramethoxy biphenyl-4,4′-diol) (Adelakun et al. 2012b). Reprinted with permission from Elsevier
Laccase has been successfully used as catalyst for improving the properties of natural phenolic compound rutin. Rutin is naturally a hardly water-soluble flavonoid glycoside. Myceliophthora laccase was used as the catalyst to synthesize polymerized rutin (poly(rutin)), which showed significantly improved solubility and radical scavenging properties (Kurisawa et al. 2003a). Rutin is commonly found on the market as a dietary supplement with remarkable anti-oxidant activity. Recent research has revealed rutin as an effective anti-thrombotic agent (Jasuja et al. 2012). Rutin act as an excellent inhibitor of protein disulfide isomerase (PDI), the enzyme which, when secreted rapidly from platelets and endothelial cells, is responsible for thrombosis (blood clotting). The production of poly(rutin), which has already proved to have enhanced properties such as improved solubility, may potentially enhance its biological properties.
Lignans are dimeric forms of phenylpropanoid units that have been identified as one of the primary active groups of Eucommia ulmoides, a Chinese traditional medicine that is recognized for its anti-cancer activities (Li and Zhang 2008), anti-oxidant activity (Zhang et al. 2013), antibiotic properties (JI and SU 2008), blood pressure reduction (Greenway et al. 2011), and anti-hypertensive activity (Luo et al. 2004). Wan et al. (2007) used crude Rhus laccases (CRL) and purified Rhus laccases (PRL) derived from the Rhus vernicifera plant in a domino oxidation of phenylpropanoids to produce bioactive compounds. Even though Rhus laccases are often marginalized for their low activity, the investigation resulted in the formation of several compounds of therapeutic importance. Two compounds that were identifiable include pinoresinol (8 and 23.5% yield using CRL and PRL, respectively) and dehydrodiisoeugenol (24.5 and 25% yield using CRL and PRL, respectively) (Wan et al. 2007). Pinoresinol has proven to be an effective anti-inflammatory drug (During et al. 2012; Jung et al. 2010). Research also showed that pinoresinol-rich olive oil had chemopreventive properties (Fini et al. 2008). Dehydrodiisoeugenol is popularly used in treating gastrointestinal disorders (Li and Yang 2012) and can be applied as an anti-oxidant or anti-inflammatory agent (Murakami et al. 2005b).
Myceliophthora thermophila laccase was used as an oxidant in the synthesis of aminonaphthoquinones (Wellington and Kolesnikova 2012). The enzyme catalyzed the amination of 1,4-dihydroxy-2-naphthoic acid with primary aromatic amines by facilitating C–N bond formation. Aminonaphthoquinones are a class of phenolic compounds that are known to have anti-cancer activity. The process resulted in the synthesis of 11 compounds with varying physiological properties. Some of the compounds exhibited high potency when tested against TK10 (renal), UACC62 (melanoma), and MCF7 (breast) cancer cell lines. The compounds also recorded a weak cytotoxicity on HeLa cell lines, highlighting their importance as potential anti-cancer drugs (Wellington and Kolesnikova 2012).
Catechol thioethers have been produced by reacting laccase-oxidized catechol with thiols. Laccase oxidation of catechol produces o-benzoquinone, which subsequently reacts with a thiol by nucleophilic conjugate addition to produce a catechol thioether (Fig. 3) (Abdel-Mohsen et al. 2014). Using 2-mercaptobenzoxazole and 2-mercaptobenzothiazole as thiols, thioester yields in the range of 74–96% were produced at room temperature, atmospheric pressure, and a pH of 6.0 (Abdel-Mohsen et al. 2014). Catechol thioethers have potential application as anti-microbial and anti-oxidant agents (Adibi et al. 2011).
Proposed reaction mechanism for the heteromolecular coupling of catechol and 2-mercaptobenzoxazole to produce catechol thioethers. Adapted from Abdel-Mohsen et al. (2014), with permission from Royal Society of Chemistry
Laccase has also been used in the synthesis of 2,3-ethylenedithio-1,4-quinones by cross-coupling 1,2-ethanedithiol with substituted hydroquinones (Cannatelli and Ragauskas 2015a). The reaction proceeds via sequential oxidation and addition reactions initiated by laccase-catalyzed oxidation of a hydroquinone into the corresponding 1,4-quinone derivative. The highly reactive 1,4-quinones then undergo nucleophilic addition by 1,2-ethanedithiol followed by further oxidation and addition steps to produce the respective 2,3-ethylenedithio-1,4-quinone products (Fig. 4). It was argued that the products are similar to several quinone-containing derivatives of natural compounds which have exhibited anti-tumor and anti-microbial activities (Abraham et al. 2011; Bozic et al. 2010). In related studies, Trametes villosa laccase was employed in the α-arylation of benzoylacetonitrile by hydroquinones to produce benzylic nitriles (Cannatelli and Ragauskas 2015b). Benzylic nitriles are primary ingredients in the production of several pharmaceutical products such as anti-helmintic drugs and analgesics (Kermanshai et al. 2001; Vardanyan and Hruby 2006).
Proposed reaction mechanism for the laccase-catalyzed reaction of 1,2-ethanedithiol (1) with substituted hydroquinones (2) to produce 2,3-ethylenedithio-1,4-quinones (3) (Cannatelli and Ragauskas 2015a). Reprinted with permission from Elsevier
The synthetic reactions of phenolic bioactive compounds as with the ones described below for alkaloids and antibiotics are carried out in appropriate buffers usually in combination with miscible or immiscible organic cosolvents in monophasic or biphasic systems, respectively. Solvents are required to keep the substrates in solution (most are insoluble in aqueous environments), as well as minimize formation of polymeric products which are difficult to characterize. Ethyl acetate is frequently used in biphasic systems (Adelakun et al. 2012a,b; Gažák et al. 2008), while chloroform has also been used in a few studies (Agematu et al. 1993). In monophasic systems, methanol appears to be the most frequently used solvent (Abdel-Mohsen et al. 2014; Mikolasch et al. 2008a; Kurisawa et al. 2003a,b; Burton and Davids 2012; Zwane et al. 2012; Anthoni et al. 2010), while other miscible solvents such as acetone, methanol, dioxane, ethanol, 2-propanol, and n-butanol have also been used in some synthetic reactions (Nicotra et al. 2004a; Kurisawa et al. 2003b). Dimethyl formamide (DMF) can also be used for substrates that are difficult to dissolve but usually at low concentration (due to its high boiling point) in combination with other solvents (Gavezzotti et al. 2014).
Alkaloids
Although laccases have mostly been employed in the development of bioactive compounds of phenolic origin, inroads are being made in other areas such as in alkaloid synthesis. Alkaloids are organic compounds consisting of a nitrogenous moiety and are usually heteocyclic in nature (Pelletier 1983). They are naturally found in organisms as secondary metabolites and are essential for diverse physiological functions such as analgesic, anti-hypertensive, and anti-cancer activities (Roberts and Wink 1998). The ability of laccase to oxidize amines has been exploited in the modification of alkaloids to products with high bioactivities. A Trametes pubescens laccase has been used in the coupling of catharanthine and vindoline to produce anhydrovinblastine (Sagui et al. 2009), an anti-neoplastic bisindole alkaloid which is reportedly useful in production of anti-tumor and anti-cancer drugs (van der Heijden et al. 2004). To date, the 56% yield obtained is the highest, compared to chemical synthesis methods and enzyme cocktail biocatalysis protocols previously employed. The low yields and costly production of bisindole alkaloids have hindered their commercial production, which has resulted in their replacement by semisynthetic analogs. The utilization of laccase thus comes as a welcome alternative that could provide a cost-efficient process that can potentially be scaled-up for industrial production.
Ergot alkaloid (EA) is a class of bioactive alkaloids of therapeutic value and find application as anti-Parkinson drugs, anti-hypertensive agents, cerebral dysfunction therapy, migraine treatment, and anti-prolactin drugs, among other uses (Gerhards et al. 2014). At the turn of the millennium, it was considered rather impossible to engineer a biocatalytic means of producing natural EA derivatives. However, recently, Chirivì et al. (2012) have, for the first time, reported the addition of a hydroxyl group at the C-4 position of the tetracyclic ergoline ring using a laccase obtained from Trametes versicolor. Because of the relatively low redox potential of the laccase, a mediator compound would be required for the oxidation of clavine EA with hydroxyl moieties. Surprisingly, the reaction also proceeded in the absence of the 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) mediator. This is because instead of oxidation to occur at the expected terminal CH2OH site of trans-dihydrolysergol, a mild hydroxylation reaction occurred at the C-4 site, resulting in a 34% yield of the monohydroxylated derivative. The functionalization of EA at this position has not been achieved before even by chemical means (Chirivì et al. 2012). This is of particular importance, considering that many researchers developing EA-derived drugs have been striving to produce EA derivatives with narrowed biospecificity and therefore predictable bioactivity (Mantegani et al. 1999).
Antibiotics
Although there was already a general awareness of the presence of anti-microbial compounds among the scientific community, much interest emanated from the success of penicillin in treating various infectious diseases such as gangrene during the Second World War (Jones and Ricke 2003). Massive bioprospecting for new anti-microbials then led to the discovery of many antibiotics that helped treat diseases that were deemed incurable at the time. One challenge faced in the use of antibiotics is the development of resistance mechanisms by microorganisms, which results in the antibiotic losing its potency. This has become a global concern, with strains such as Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (commonly referred to as the ESKAPE pathogens) notorious for devising mechanisms to “escape” the potency of antibiotics (Lewis 2013). Cases involving multidrug-resistant tuberculosis (MDR-TB) have become increasingly recurrent, and according to the World Health Organization, 480,000 cases were reported in the year 2013. Such cases of resistance, coupled with a decrease in discovery of new antibiotics, have caused scientists to consider the option of modifying existing antibiotics to their bioactive derivatives (Aminov 2010; France et al. 2004). Apparently, antibiotic modification dates as far back as the 1970s (Aminov 2010).
Laccase-catalyzed modification of antibiotics was first reported by Agematu et al. (1993). They reported the laccase-catalyzed dimerization of penicillin X. Penicillin X is generally oxidizable by laccase because of a hydroxyl group it possesses. An initial attempt to dimerize penicillin X was unsuccessful because the products were not stable. Subsequently, the acetylation of the antibiotic resulted in the formation of stable dimers. Although the resulting dimers had no significant improvement in anti-microbial activity and stability, the work opened new avenues of antibiotic research (Agematu et al. 1993).
Subsequent work on laccase-mediated modification of antibiotics has focused mainly on heterocoupling as a way of improving efficacy of antibiotics (Table 2). Mikolasch and coworkers have carried out extensive work on the application of laccase in the production of novel antibiotics (Hahn et al. 2009a,b; Manda et al. 2006; Mikolasch et al. 2012, 2008a,b, 2007, 2006). Unlike the conventional modifications which generally explore the reactivity of moieties to form antibiotic derivatives with improved activity and lower cytotoxicity, they adopted the approach of coupling the existing antibiotics with other bioactive compounds to produce novel compounds with potentially improved therapeutic properties. Using derivatives of gentisic acid to cross-couple amoxicillin or ampicillin, eight novel penicillins were synthesized (Mikolasch et al. 2006). This approach appeared to be highly efficient, with yields of around 98% achieved within 3 h. The produced derivatives showed interesting bioactivity, particularly in vivo efficacy; they were able to protect mice infected with S. aureus (ATCC 6538 and 3841) without any signs of intoxication. Although the derivatives did not show a significant improvement in activity compared to amoxicillin or ampicillin, some coupling products were stable against β-lactamases that reduce activity of amoxicillin and ampicillin.
The presence of catechol groups on β-lactam-based antibiotics has been demonstrated to improve antibiotic activity by enhancing antibiotic penetration through the bacterial cell wall (Erwin et al. 1991). Using a laccase-catalyzed amination process, novel antibiotics were obtained by cross-coupling catechols and amino β-lactams such as cefadroxil, amoxicillin, and ampicillin (Mikolasch et al. 2008b). Several novel derivatives of N-analogous corollosporine (Mikolasch et al. 2008a), morpholines (Hahn et al. 2009b), and cephalosporins (Mikolasch et al. 2007) have also been reported (Table 2).
Synthesis of bioactive polymers
Laccase has been used for the functionalization of polymers through grafting reactions. For example, extensive work has been performed on the functionalization of lignocellulose material (Kudanga et al. 2011b, 2010a,b, 2009, 2008; Widsten et al. 2010). Recently, research activities have also focused on functionalization of polymers for the production of bioactive polymers (Table 3). Natural polymers, mainly chitosan, have been extensively investigated for possible grafting with several phenolics as bioactive compounds.
Chitosan is a readily available biopolymer that is usually produced from the deacetylation of crustacean shells such as shrimp and crab shells. It has recently received much attention as a potentially useful bioactive polymer. Although chitosan has been applied in food industry (Shahidi et al. 1999), medical industries (as antibiotics) (Raafat et al. 2008), and in wine industries (to prevent spoilage) (Bagder Elmaci et al. 2015), its application has been limited because of its poor solubility and poor anti-oxidant capacity (Aljawish et al. 2014b; Božič et al. 2013). The poor anti-oxidant property of chitosan is a result of limited number of hydroxyl groups on the biopolymer (Božič et al. 2012b). Grafting of phenolic compounds has been used to enhance the bioactive properties of chitosan. For example, grafting of laccase-oxidized ferulic acid (FA) and ethyl ferulate (EF) onto a chitosan backbone resulted in chitosan derivatives with superior anti-oxidant activity compared to natural chitosan (Aljawish et al. 2014b). Bozic and coworkers also performed a series of studies on chitosan functionalization, using several phenolic compounds such as tannic acid and quercetin (Božič et al. 2012a), gallic acid, and caffeic acid (Božič et al. 2013, 2012b). The resulting chitosan derivatives exhibited enhanced anti-oxidant activity. The derivatives also showed improved anti-microbial activity against Escherichia coli and Listeria monocytogenes (in the case of gallic and caffeic acid-functionalized chitosan derivatives) compared to the natural biopolymer.
Structure-activity relationships of enzymatically synthesized bioactive compounds
In laccase-catalyzed synthesis of bioactive compounds, the main aim is to produce coupling products exhibiting improved bioactive properties compared to the starting materials. Depending on the intended purpose of the bioactive compound, several structural factors determine the efficacy of the coupling products. In this section, some of these factors are discussed, with reference to the type of bioactive compounds produced.
Anti-oxidants
The bioefficacy of anti-oxidants is usually determined by the structure and stability of the synthesized compound (Table 4). Several researchers have analyzed the structure-activity relationship (SAR) of anti-oxidants (Bendary et al. 2013; Rice-Evans et al. 1996). Firstly, the anti-oxidant must have active groups (e.g., hydroxyl, alkyl, or aniline) (Bendary et al. 2013) attached to the aromatic ring, and the more active groups are present, the more bioactive the anti-oxidant can be (Bendary et al. 2013; Lien et al. 1999). For example, hydroxytyrosol consists of two hydroxyl groups attached to its aromatic ring; however, after a laccase-catalyzed oxidation process, a hydroxytyrosol dimer with four hydroxyl groups is produced (Zwane et al. 2012) (Table 4). This dimer showed a threefold increase in anti-oxidant activity when tested using the ferric-reducing anti-oxidant power (FRAP) assay (Zwane et al. 2012). Adelakun et al. (2012a) attributed the enhanced activity of the dimeric form of ferulic acid (β-5) to increased electron-donating groups. Functional groups such as alkyl, aniline, or hydroxyl groups enhance anti-oxidant activity (Bendary et al. 2013), while bulky alkyl groups contribute towards the stability of phenoxyl radicals (Decker 2008; Eskin and Przybylski 2000). On the other hand, compounds containing moieties such as nitro group or halogens, which are electron withdrawing groups, have poor anti-oxidant activity (Rakesh et al. 2015). The position of the active groups on the aromatic ring also determines the activity of the product. Enhanced activity of a phenolic anti-oxidant can be achieved when active groups occupy the ortho or para position to the hydroxyl group (Decker 2008). Recently, Najafi (2014) investigated the relationship between the position of active substituents on the daidzein aromatic ring and the compound’s anti-oxidant activity. It was concluded that the ortho position can result in production of useful bioactive compounds (Najafi 2014).
The bond dissociation enthalpies (BDEs) of the active groups will also determine the inertia of the anti-oxidant in releasing the electron. The anti-oxidants containing active groups with lower BDE are better anti-oxidants because they readily release electrons to radical species (Szymusiak and Zielinski 2003). Adelakun et al. (2012a) showed that the β-β dimers of ferulic acid had a lower anti-oxidant activity than the monomeric ferulic acid. This is consistent with earlier findings, which showed that bis-ferulic acid (β-β dimers) had a higher BDE (85.76 kcal/mol) than ferulic acid (84.70 kcal/mol) (Murakami et al. 2005a). The determination of BDE varies with compounds and also experimental conditions; thus, many researchers focusing on the BDE of phenolic compounds have published contrasting results (Chandra and Uchimaru 2002; dos Santos and Simoes 1998; Klein and Lukeš 2006; Szymusiak and Zielinski 2003). However, in general, hydroxyl moieties have lower BDE than other active groups such as alkyl and aniline groups (Bendary et al. 2013), which probably explains why phenolics are frequently used as anti-oxidants.
An ideal anti-oxidant must also produce a stable radical, which will not facilitate the propagation of the oxidation chain bubble (Alov et al. 2015). The stability results from the resonance delocalization of lone electrons into the aromatic ring and absence of groups prone to attack by oxygen (Flora 2009; Shahidi and Naczk 2004). Although bulky groups on the ortho positions of the aromatic ring help stabilize anti-oxidant radicals (Shahidi and Naczk 2004), the bulky groups may also reduce anti-oxidant activity by steric masking of the phenolic hydroxyl group (Murakami et al. 2005a).
Hydrophobicity is also another attribute which affects anti-oxidant activity especially in a multicellular environment. Hydrophobicity increases the bioavailability of the anti-oxidant at sites where free radicals are generated (Ishige et al. 2001). For instance, hydrophobic anti-oxidants would be effective scavengers of free radicals generated from lipid peroxidation on the lipid bilayer because of their lipophilic properties (Lu et al. 2006). An ideal anti-oxidant would thus consist of a balance of electron-donating groups such as hydroxyl groups, which will ensure the free radical scavenging ability of the anti-oxidant, and also hydrophobic moieties which will enable the bioavailability of the anti-oxidant in multicellular systems. Research that focused on improving the hydrophilicity of silybin resulted in its compromised anti-oxidant activity in lipophilic environments (Gažák et al. 2010; Gažák et al. 2004), highlighting hydrophobicity as an important factor in the function of anti-oxidants in cell medium.
Antibiotics
Generally, microbial resistance to antibiotics is through three mechanisms: (i) enzymatic inactivation of the antibiotic, for example, hydrolysis of β-lactam-based antibiotics by β-lactamase enzymes; (ii) alteration of the targets; and (iii) reduced penetration of the antibiotic into the microorganism (Watanabe et al. 1987). Laccases have been used in developing antibiotics with enhanced penetration into host cell. These antibiotics have been produced by coupling catechols and β-lactam-based antibiotics (Mikolasch et al. 2008b). The produced β-lactam derivatives expressed significant activity against gram-positive bacteria, including drug-resistant S. aureus and enterococci. It has been demonstrated that the availability of a catechol moiety on the antibiotic improves its penetration into the bacterial cell through the iron transport system (Fung-Tomc et al. 1997; Silley et al. 1990). The incorporation of catechol groups onto antibiotic compounds thus enhances antibiotic activity of the antibiotic through effective drug delivery towards the targeted site. The coupling of monomeric antibiotic units to dimeric forms can result in enhanced efficacy. Some reports have highlighted the efficacy of dimeric vancomycin in inhibiting bacteria resistant to monomeric vancomycin units (Yoshida et al. 2011). Dimerization of monomeric antibiotics can also prevent enzymatic hydrolysis of the antibiotic since the dimerization alters the compound in such a way that hydrolyzing enzymes fail to recognize it.
Directions for future research
The use of laccases as biocatalysts offer economically viable domino processes for the synthesis of bioactive compounds. However, the translation of this green technology into a feasible industrial process requires several factors to be considered. For example, there is a need to develop a robust enzyme with properties that are ideal for industrial application. Specific research areas could include heterologous expression so as to produce enough enzyme with improved activity, thermostability, and ability to withstand organic solvents and inhibitors which are frequently encountered in industrial applications (Mate and Alcalde 2015; Kudanga and Le Roes-Hill 2014; Kunamneni et al. 2008b). Laccase-catalyzed reactions generally result in low product yield (see for example, Adelakun et al. 2012b; Wan et al. 2007). While the creation of radicals is a pre-requisite for laccase synthesis, there are also some negative implications for biosynthesis. With radical processes, there is a possibility of many different radical forms of the oxidized molecule mainly due to resonance stabilization and non-specific radical-mediated reactions. Therefore, such processes usually result in a wide range of different racemic mixtures of products appearing at rather low concentrations. Frequently high concentrations of organic solvents are used to minimize radical proliferation, reduce polymerization reactions, and therefore increase yield, but the same solvents also inactivate enzymes. Therefore, reaction engineering to increase product yield remains a major challenge in laccase-mediated synthesis of bioactive compounds. However, other key research areas that need particular attention could include (i) the search for cheap substrate sources coupled with the bioprospection of natural laccase mediator systems (LMSs) and (ii) production of enantiomericaly pure products.
Biopolymers as substrate sources
The potential of laccases can be extended beyond the oxidation of its natural substrates through the LMS (Riva 2006). This involves the generation of radicals from small compounds within laccase’s redox potential range (viz. 0.5–0.8 mV against a standard hydrogen electrode) (Witayakran and Ragauskas 2009). The generated radicals can then act as redox shuttles, oxidizing substrates with higher redox potentials and those too large to fit the enzyme active site (Zhu et al. 2014). The LMS technology has been extensively used in the textile industry, pulp and paper industry, alcohol oxidation, and lignin degradation (D’Alfonso et al. 2014; Morozova et al. 2007b; Fabbrini et al. 2002). Some researchers are of the opinion that the use of LMS presents an opportunity to mine the plethora of low-molecular-weight phenolics and other bioactive compounds entrapped within biopolymers such as lignin (Christopher et al. 2014; Rich et al. 2016). The degradation of lignin, which is the second most abundant biopolymer, and is laden with bioactive functional groups such as phenolic hydroxyls, benzyl alcohols, carbonyls, and methoxyls (Boeriu et al. 2004; El Mansouri and Salvadó 2007), can present a wealthy source of substrates for the synthesis of valuable bioactive compounds (Barclay et al. 1997; Božič et al. 2012a). The widely available artificial LMSs such as ABTS and 1-hydroxybenzotriazole (HBT) remain expensive and are potential contaminants when applied in the synthesis of compounds of therapeutic value (Cañas and Camarero 2010). Therefore, bioprospecting for more efficient natural mediator systems for the degradation of biopolymers also remains a key research area.
Towards the production of enantiomerically pure compounds
To date, much of the research on the exploitation of laccase for organic synthesis has only produced racemic mixtures of oligomeric and cross-coupling products. This is a limitation especially in the synthesis of therapeutic drugs, which in most cases requires enantiomerically pure compounds. It has also been observed that enantiomers can have significantly different bioactivities (Davis-Searles et al. 2005; Plíšková et al. 2005). Therefore, research is now also focusing on synthesizing enantiomerically pure compounds (Girol et al. 2012; Kim et al. 2012). Strikingly, in vivo laccase-catalyzed coupling reactions are highly stereospecific, leading to the formation of compounds such as lignans, lignins, and suberins (Orlandi et al. 2001; Zoia et al. 2008). Development of a protocol that can mimic the same specificity in vitro will be valuable in industrial processes. Research towards production of pure final products thus represents a primary focus area for future research. A number of studies have laid a foundation for future studies in this respect as explained below.
Davin and colleagues demonstrated the role played by a 78-kDa protein (dubbed “dirigent” protein) isolated from Forsythia intermedia in the in vivo synthesis of stereospecific dimers of E-coniferyl alcohol, which are building units for lignin polymers in plants (Davin et al. 1997). Coupling reactions carried out in the absence of the dirigent protein resulted in the racemic dimers (±)-dehydrodiconiferyl alcohols, (±)-pinoresinols, and (±)-guaiacylglycerol 8-O-4-(coniferyl alcohol) ethers (Davin and Lewis 2005). However, in the presence of the dirigent protein, stereospecific coupling reaction occurred, resulting in (+)-pinoresinol as the only product (Fig. 5) (Davin et al. 1997; Davin and Lewis 2000; Halls et al. 2004). This trend was reproducible when either laccase, flavin mononucleotide (FMN), flavin adenine dinucleotide (FDN), ammonium peroxydisulfate, or an oxidase native to F. intermedia was used as oxidant, proving that stereoselectivity in the reaction was not promoted by the oxidant employed. The substrate specificity of the dirigent protein from F. intermedia restricts it only to the production of (+)-pinoresinol. This knowledge has already opened fresh avenues of enquiry, allowing scientists to bioprospect for their homologous proteins in nature (Präg et al. 2014; Pickel and Schaller 2013; Girol et al. 2012; Umezawa 2003) as well as taking advantage of the modern day tools such as molecular technology (Kazenwadel et al. 2013; Kim et al. 2012) to design modified proteins of such ilk that can control directed coupling to produce desired bioactive products.
The proposed in vivo stereoselective synthesis of (+)-pinoresinol in Forsythia intermedia involving dirigent protein-facilitated binding and orientation of coniferyl alcohol radicals. Adapted from Davin et al. (1997), with permission from the American Association for the Advancement of Science
In related studies, stereospecific bioactive lignans were synthesized by attaching chiral auxiliary compounds to the substrates (Orlandi et al. 2001). Riva and coworkers have also carried out extensive research on protecting functional groups of laccase substrates as a strategy for reducing the diversity of products formed in the oxidation reactions. A benzyl group was added to protect the OH group on the C′7 of silybin A, resulting in the 87% yield of its symmetric dimer (Gavezzotti et al. 2014) (Fig. 6). As shown by earlier studies, benzylation of functional groups seemed to be better than adding methyl groups, which made deprotection impossible (Gažák et al. 2008).
Protection of the hydroxyl group on the C′7 of silybin A by benzylation to reduce the product range of the reaction. Adapted from Gavezzotti et al. (2014), with permission from Elsevier
It has also been reported that regioselectivity can be influenced by the reaction conditions such as pH and solvents (Chioccara et al. 1993; Orlandi et al. 2001). Horseradish peroxidase-catalyzed coupling of lignans (isoeugenol, methyl ferulate, or coniferyl alcohol) under acidic pH resulted in dimer formation; neutral pH promoted the formation of oligomers, while a racemic β-O-4 product was formed in the presence of methanol solvent (Chioccara et al. 1993).
The emergence and subsequent advances in the field of molecular biology have opened a host of opportunities in developing biocatalysts better equipped for industrial application. Besides the improved expression of proteins in heterologous hosts, molecular techniques also allow bioprospecting in unculturable microorganisms as well as database mining. Using bioinformatic databases, it is now possible to profile the sequence of the polypeptide chain. With this information, predictions can be made on how alteration of the amino acid sequence can affect the characteristics of the enzyme. Usually, these alterations are performed at or near the enzyme’s catalytic core (Mate and Alcalde 2015; Prins et al. 2015; Turner 2009). However, in addition to carrying out these modifications with the goal of improving enzyme activity and/or robustness, genetic manipulation could also focus on improving stereoselectivity and facilitating the production of pure compounds (Robert et al. 2011).
Concluding remarks
The potential of laccase as a green biocatalyst in the synthesis of bioactive compounds is vast. Many studies have increased our understanding of reaction mechanisms involved, desired reaction conditions, and structure-activity relationships. Future research should highlight not only synthetic properties of the enzyme but also reaction engineering to optimize synthesis of specifically desired products of economic value. This could possibly facilitate transfer of the technology from bench scale to industrial application processes.
References
Abdel-Mohsen HT, Conrad J, Beifuss U (2014) Laccase-catalyzed synthesis of catechol thioethers by reaction of catechols with thiols using air as an oxidant. Green Chem 16:90–95
Abraham I, Joshi R, Pardasani P, Pardasani RT (2011) Recent advances in 1,4-benzoquinone chemistry. J Braz Chem Soc 22:385–421
Adelakun OE, Kudanga T, Parker A, Green IR, le Roes-Hill M, Burton SG (2012a) Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J Mol Catal B Enzym 74:29–35
Adelakun OE, Kudanga T, Green IR, le Roes-Hill M, Burton SG (2012b) Enzymatic modification of 2,6-dimethoxyphenol for the synthesis of dimers with high antioxidant capacity. Process Biochem 47:1926–1932
Adibi H, Rashidi A, Khodaei MM, Alizadeh A, Majnooni MB, Pakravan N, Abiri R, Nematollahi D (2011) Catechol thioether derivatives: preliminary study of in-vitro antimicrobial and antioxidant activities. Chem Pharm Bull 59:1149–1152
Agematu H, Tsuchida T, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Shin T, Murao S (1993) Enzymatic dimerization of penicillin X. J Antibiot (Tokyo) 46:141–148
Aljawish A, Chevalot I, Jasniewski J, Paris C, Scher J, Muniglia L (2014a) Laccase-catalysed oxidation of ferulic acid and ethyl ferulate in aqueous medium: a green procedure for the synthesis of new compounds. Food Chem 145:1046–1054
Aljawish A, Chevalot I, Jasniewski J, Revol-Junelles AM, Scher J, Muniglia L (2014b) Laccase-catalysed functionalisation of chitosan by ferulic acid and ethyl ferulate: evaluation of physicochemical and biofunctional properties. Food Chem 161:279–287
Alov P, Tsakovska I, Pajeva I (2015) Computational studies of free radical-scavenging properties of phenolic compounds. Curr Top Med Chem 15:85–104
Aminov RI (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:134
Anthoni J, Humeau C, Maia E, Chebil L, Engasser JM, Ghoul M (2010) Enzymatic synthesis of oligoesculin: structure and biological activities characterizations. Eur Food Res Technol 231:571–579
Anyanwutaku IO, Petroski RJ, Rosazza JPN (1994) Oxidative coupling of mithramycin and hydroquinone catalyzed by copper oxidases and benzoquinone. Implications for the mechanism of action of aureolic acid antibiotics. Bioorg Med Chem 2:543–551
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Baldelli E, Danieli B, Fontana G, Riva S (2009) Process for the preparation of bisindole alkaloid derivatives. Google Patents
Barclay LRC, Xi F, Norris JQ (1997) Antioxidant properties of phenolic lignin model compounds. J Wood Chem Technol 17:73–90
Bendary E, Francis RR, Ali HMG, Sarwat MI, El Hady S (2013) Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci 58:173–181
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crop Prod 20:205–218
Božič M, Gorgieva S, Kokol V (2012a) Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr Polym 89:854–864
Božič M, Gorgieva S, Kokol V (2012b) Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr Polym 87:2388–2398
Božič M, Štrancar J, Kokol V (2013) Laccase-initiated reaction between phenolic acids and chitosan. React Funct Polym 73:1377–1383
Bozic T, Novakovic I, Gasic MJ, Juranic Z, Stanojkovic T, Tufegdzic S, Kljajić Z, Sladić D (2010) Synthesis and biological activity of derivatives of the marine quinone avarone. Eur J Med Chem 45:923–929
Burton SG, Davids LM (2012) Hydroxytyrosol compounds. Google Patents
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Cannatelli MD, Ragauskas AJ (2015a) Laccase-catalyzed synthesis of 2,3-ethylenedithio-1,4-quinones. J Mol Catal B Enzym 119:85–89
Cannatelli MD, Ragauskas AJ (2015b) Laccase-catalyzed α-arylation of benzoylacetonitrile with substituted hydroquinones. Chem Eng Res Des 97:128–134
Chakroun H, Bouaziz M, Yangui T, Blibech I, Dhouib A, Sayadi S (2013) Enzymatic transformation of tyrosol by Trametes trogii laccases: identification of the product and study of its biological activities. J Mol Catal B Enzym 87:11–17
Chandra AK, Uchimaru T (2002) The O-H bond dissociation energies of substituted phenols and proton affinities of substituted phenoxide ions: a DFT study. Int J Mol Sci 3:407–422
Chioccara F, Poli S, Rindone B, Pilati T, Brunow G, Pietikäinen P, Setala H (1993) Regio- and diastereo-selective synthesis of dimeric lignans using oxidative coupling. Acta Chem Scand 47:610–616
Chirivì C, Fontana G, Monti D, Ottolina G, Riva S, Danieli B (2012) The quest for new mild and selective modifications of natural structures: laccase-catalysed oxidation of ergot alkaloids leads to unexpected stereoselective C-4 hydroxylation. Chem Eur J 18:10355–10361
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res 2:12
Chung J, Kurisawa M, Uyama H, Kobayashi S (2003) Enzymatic synthesis and antioxidant property of gelatin-catechin conjugates. Biotechnol Lett 25:1993–1997
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JL Jr, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY (2007) Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem 9:411–420
Constantin MA, Conrad J, Beifuss U (2012a) Laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives. Green Chem 14:2375–2379
Constantin MA, Conrad J, Beifuss U (2012b) An unprecedented oxidative trimerization of sesamol catalyzed by laccases. Tetrahedron Lett 53:3254–3258
D’Alfonso C, Lanzalunga O, Lapi A, Vadalà R (2014) Comparing the catalytic efficiency of ring substituted 1-hydroxybenzotriazoles as laccase mediators. Tetrahedron 70:3049–3055
Davin LB, Lewis NG (2000) Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol 123:453–462
Davin LB, Lewis NG (2005) Dirigent phenoxy radical coupling: advances and challenges. Curr Opin Biotechnol 16:398–406
Davin LB, Wang HB, Crowell AL, Bedgar DL, Martin DM, Sarkanen S, Lewis NG (1997) Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 275:362–367
Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ (2005) Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 65:4448–4457
Debbab A, Aly AH, Lin WH, Proksch P (2010) Bioactive compounds from marine bacteria and fungi. Microb Biotechnol 3:544–563
de Regil R, Sandoval G (2013) Biocatalysis for biobased chemicals. Biomolecules 3:812–847
Decker EA (2008) Antioxidant mechanisms. In: Akoh CC, Min DB (eds) Food lipids: chemistry, nutrition, and biotechnology, 3rd edn. CRC Press, Boca Raton, pp. 475–492
dos Santos RMB, Simoes JAM (1998) Energetics of the O-H bond in phenol and substituted phenols: a critical evaluation of litrature data. J Phys Chem Ref Data 27:707–737
During A, Debouche C, Raas T, Larondelle Y (2012) Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J Nutr 142:1798–1805
Eggert C (1997) Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol Res 152:315–318
Eggert C, Temp U, Dean JF, Eriksson KE (1995) Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett 376:202–206
El Mansouri NE, Salvadó J (2007) Analytical methods for determining functional groups in various technical lignins. Ind Crop Prod 26:116–124
Elmaci BS, Gulgor G, Tokatli M, Erten H, Isci A, Ozcelik F (2015) Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 107:675–686
Emirdağ-Öztürk S, Hajdok S, Conrad J, Beifuss U (2013) Laccase-catalyzed reaction of 3-tert-butyl-1H-pyrazol-5(4H)-one with substituted catechols using air as an oxidant. Tetrahedron 69:3664–3668
Erwin ME, Jones RN, Barrett MS, Briggs BM, Johnson DM (1991) In vitro evaluation of GR69153, a novel catechol-substituted cephalosporin. Antimicrob Agents Chemother 35:929–937
Eskin M, Przybylski R (2000) Antioxidants and shelf life of foods. In: Eskin NAM, Robinson DS (eds) Food shelf life stability: chemical, biochemical, and microbiological changes. CRC Press, Boca Raton, p. 194
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B Enzym 16:231–240
Fini L, Hotchkiss E, Fogliano V, Graziani G, Romano M, De Vol EB, Qin H, Selgrad M, Boland CR, Ricciardiello L (2008) Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 29:139–146
Flora SJS (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2:191–206
France S, Weatherwax A, Taggi AE, Lectka T (2004) Advances in the catalytic, asymmetric synthesis of β-lactams. Acc Chem Res 37:592–600
Fung-Tomc J, Bush K, Minassian B, Kolek B, Flamm R, Gradelski E, Bonner D (1997) Antibacterial activity of BMS-180680, a new catechol-containing monobactam. Antimicrob Agents Chemother 41:1010–1016
Gavezzotti P, Vavříková E, Valentová K, Fronza G, Kudanga T, Kuzma M, Riva S, Biedermann D, Kren V (2014) Enzymatic oxidative dimerization of silymarin flavonolignans. J Mol Catal B Enzym 109:24–30
Gažák R, Purchartová K, Marhol P, Živná L, Sedmera P, Valentová K, Kato N, Matsumura H, Kaihatsu K, Kren V (2010) Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur J Med Chem 45:1059–1067
Gažák R, Sedmera P, Marzorati M, Riva S, Křen V (2008) Laccase-mediated dimerization of the flavonolignan silybin. J Mol Catal B Enzym 50:87–92
Gažák R, Svobodová A, Psotová J, Sedmera P, Přikrylová V, Walterová D, Kren V (2004) Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg Med Chem 12:5677–5687
Gerhards N, Neubauer L, Tudzynski P, Li SM (2014) Biosynthetic pathways of ergot alkaloids. Toxins 6:3281–3295
Gil-Chávez JG, Villa JA, Ayala-Zavala FJ, Heredia JB, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci F 12:5–23
Girol GC, Fisch KM, Heinekamp T, Gunther S, Huttel W, Piel J, Brakhage AA, Müller M (2012) Regio- and stereoselective oxidative phenol coupling in Aspergillus niger. Angew Chem Int Ed Engl 51:9788–9791
Greenway F, Liu Z, Yu Y, Gupta A (2011) A clinical trial testing the safety and efficacy of a standardized Eucommia ulmoides Oliver bark extract to treat hypertension. Altern Med Rev 16:338–347
Guaadaoui A, Benaicha S, Elmajdoub N, Bellaoui M, Hamal A (2014) What is a bioactive compound? A combined definition for a preliminary consensus. Int J Food Sci Nutr 3:174–179
Hahn V, Mikolasch A, Manda K, Gordes D, Thurow K, Schauer F (2009a) Laccase-catalyzed carbon-nitrogen bond formation: coupling and derivatization of unprotected L-phenylalanine with different para-hydroquinones. Amino Acids 37:315–321
Hahn V, Mikolasch A, Wende K, Bartrow H, Lindequist U, Schauer F (2009b) Synthesis of model morpholine derivatives with biological activities by laccase-catalysed reactions. Biotechnol Appl Biochem 54:187–195
Halls SC, Davin LB, Kramer DM, Lewis NG (2004) Kinetic study of coniferyl alcohol radical binding to the (+)-pinoresinol forming dirigent protein. Biochemistry 43:2587–2595
Infiniti Research Limited (2014) Global antioxidants market 2014–2018. Sandler Research. http://www.sandlerresearch.org/global-antioxidants-market-2014-2018.html.2014. Accessed 28 June 2015
Ishige K, Schubert D, Sagara Y (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30:433–446
Jadhav SB, Singhal RS (2014) Laccase–gum Arabic conjugate for preparation of water-soluble oligomer of catechin with enhanced antioxidant activity. Food Chem 150:9–16
Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R (2012) Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest 122:2104–2113
Jeon JR, Baldrian P, Murugesan K, Chang YS (2012) Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microb Biotechnol 5:318–332
Ji Z, Su Y (2008) Study on antimicrobial activities of extracts from Eucommia ulmoides Oliv. leaves. J Chem Ind Forest Prod 2:16
Jones F, Ricke S (2003) Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci 82:613–617
Jung HW, Mahesh R, Lee JG, Lee SH, Kim YS, Park YK (2010) Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci Lett 480:215–220
Kazenwadel C, Klebensberger J, Richter S, Pfannstiel J, Gerken U, Pickel B, Schaller A, Hauer B (2013) Optimized expression of the dirigent protein AtDIR6 in Pichia pastoris and impact of glycosylation on protein structure and function. Appl Microbiol Biotechnol 97:7215–7227
Kermanshai R, McCarry BE, Rosenfeld J, Summers PS, Weretilnyk EA, Sorger GJ (2001) Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts. Phytochemistry 57:427–435
Kim KW, Moinuddin SGA, Atwell KM, Costa MA, Davin LB, Lewis NG (2012) Opposite stereoselectivities of dirigent proteins in Arabidopsis and Schizandra species. J Biol Chem 287:33957–33972
Klein E, Lukeš V (2006) Study of gas-phase O–H bond dissociation enthalpies and ionization potentials of substituted phenols—applicability of ab initio and DFT/B3LYP methods. J Chem Phys 330:515–525
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 113:71–88
Kudanga T, Burton S, Nyanhongo GS, Guebitz GM (2011a) Versatility of oxidoreductases in the remediation of environmental pollutants. Front Biosci (Elite Ed) 4:1127–1149
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98:6525–6542
Kudanga T, Prasetyo EN, Sipilä J, Eberl A, Nyanhongo GS, Guebitz GM (2009) Coupling of aromatic amines onto syringylglycerol β-guaiacylether using Bacillus SF spore laccase: a model for functionalization of lignin-based materials. J Mol Catal B Enzym 61:143–149
Kudanga T, Nyanhongo GS, Guebitz GM, Burton S (2011b) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzym Microb Technol 48:195–208
Kudanga T, Prasetyo EN, Sipilä J, Guebitz GM, Nyanhongo GS (2010a) Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol 149:81–87
Kudanga T, Prasetyo EN, Sipilä J, Nousiainen P, Widsten P, Kandelbauer A, Nyanhongo GS (2008) Laccase-mediated wood surface functionalization. Eng Life Sci 8:297–302
Kudanga T, Prasetyo EN, Sipilä J, Nyanhongo GS, Guebitz GM (2010b) Enzymatic grafting of functional molecules to the lignin model dibenzodioxocin and lignocellulose material. Enzym Microb Technol 46:272–280
Kunamneni A, Camarero S, Garcia-Burgos C, Plou F, Ballesteros A, Alcalde M (2008a) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:32
Kunamneni A, Plou FJ, Ballesteros A, Alcalde M (2008b) Laccases and their applications: a patent review. Recent Pat Biotechnol 2:10–24
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003a) Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 4:1394–1399
Kurisawa M, Chung JE, Uyama H, Kobayashi S (2003b) Laccase-catalyzed synthesis and antioxidant property of poly (catechin). Macromol Biosci 3:758–764
Leutbecher H, Constantin MA, Mika S, Conrad J, Beifuss U (2011) A new laccase-catalyzed domino process and its application to the efficient synthesis of 2-aryl-1H-benzimidazoles. Tetrahedron Lett 52:605–608
Lewis K (2013) Platforms for antibiotic discovery. Nat Rev Drug Discov 12:371–387
Li CJ, Trost BM (2008) Green chemistry for chemical synthesis. Proc Natl Acad Sci U S A 105:13197–13202
Li F, Yang XW (2012) Analysis of anti-inflammatory dehydrodiisoeugenol and metabolites excreted in rat feces and urine using HPLC-UV. Biomed Chromatogr 26:703–707
Li XJ, Zhang HY (2008) Western-medicine-validated anti-tumor agents and traditional Chinese medicine. Trends Mol Med 14:1–2
Lien EJ, Ren S, Bui HH, Wang R (1999) Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26:285–294
Lu Z, Nie G, Belton PS, Tang H, Zhao B (2006) Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 48:263–274
Luo X, Ma M, Chen B, Yao S, Wan Z, Yang D, Hang H (2004) Analysis of nine bioactive compounds in Eucommia ulmoides Oliv. and their preparation by HPLC-photodiode array detection and mass spectrometry. J Liq Chromatogr Relat Technol 27:63–81
Madhavi V, Lele SS (2009) Laccase: properties and applications. Bioresources 4:1694–1717
Manda K, Hammer E, Mikolasch A, Gördes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid L-tryptophan by coupling with p-hydroquinone 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31:409–419
Mantegani S, Brambilla E, Varasi M (1999) Ergoline derivatives: receptor affinity and selectivity. Farmaco 54:288–296
Martins S, Mussatto SI, Martínez-Avila G, Montañez-Saenz J, Aguilar CN, Teixeira JA (2011) Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol Adv 29:365–373
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33:25–40
Matern U, Kneusel RE (1988) Phenolic compounds in plant disease resistance. Phytoparasitica 16:153–170
Matsuura T, Ohkatsu Y (2000) Phenolic antioxidants: effect of o-benzyl substituents. Polym Degrad Stab 70:59–63
Maugh TH (1984) Semisynthetic enzymes are new catalysts. Science 223:154–156
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mikolasch A, Hessel S, Salazar MG, Neumann H, Manda K, Gordes D, Schmidt E, Thurow K, Hammer E, Lindequist U, Beller M, Schauer F (2008a) Synthesis of new N-analogous corollosporine derivatives with antibacterial activity by laccase-catalyzed amination. Chem Pharm Bull 56:781–786
Mikolasch A, Manda K, Schlüter R, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Jülich WD, Lindequist U (2012) Comparative analyses of laccase-catalyzed amination reactions for production of novel β-lactam antibiotics. Biotechnol Appl Biochem 59:295–306
Mikolasch A, Niedermeyer TH, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Jülich WD, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes species. Chem Pharm Bull 54:632–638
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell Salazar M, Hessel S, Jülich WD, Lindequist U (2007) Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases II. Chem Pharm Bull 55:412–416
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Mikolasch A, Wurster M, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Jülich WD, Lindequist U (2008b) Novel β-lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull 56:902–907
Morozova O, Shumakovich G, Gorbacheva M, Shleev S, Yaropolov A (2007a) “Blue” laccases. Biochem Mosc 72:1136–1150
Morozova O, Shumakovich G, Shleev S, Yaropolov YI (2007b) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Muller WEG, Grebenjuk VA, Le Pennec G, Schroder HC, Brummer F, Hentschel U, Müller IM, Breter H (2004) Sustainable production of bioactive compounds by sponge-cell culture and gene cluster approach: a review. Mar Biotechnol 6:105–117
Murakami Y, Ito S, Atsumi T, Fujisawa S (2005a) Theoretical prediction of the relationship between phenol function and COX-2/AP-1 inhibition for ferulic acid-related compounds. In Vivo 19:1039–1044
Murakami Y, Shoji M, Hirata A, Tanaka S, Yokoe I, Fujisawa S (2005b) Dehydrodiisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. Arch Biochem Biophys 434:326–332
Najafi M (2014) On the antioxidant activity of the ortho and meta substituted daidzein derivatives in the gas phase and solvent environment. J Mex Chem Soc 58:36–45
Nicotra S, Cramarossa MR, Mucci A, Pagnoni UM, Riva S, Forti L (2004a) Biotransformation of resveratrol: synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophthora thermophila and from Trametes pubescens. Tetrahedron 60:595–600
Nicotra S, Intra A, Ottolina G, Riva S, Danieli B (2004b) Laccase-mediated oxidation of the steroid hormone 17β-estradiol in organic solvents. Tetrahedron Asymmetry 15:2927–2931
Orlandi M, Rindone B, Molteni G, Rummakko P, Brunow G (2001) Asymmetric biomimetic oxidations of phenols: the mechanism of the diastereo-and enantioselective synthesis of dehydrodiconiferyl ferulate (DDF) and dehydrodiconiferyl alcohol (DDA). Tetrahedron 57:371–378
Osiadacz J, Al-Adhami AJH, Bajraszewska D, Fischer P, Peczyñska-Czoch W (1999) On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol 72:141–149
Pasha I, Saeed F, Waqas K, Anjum FM, Arshad MU (2013) Nutraceutical and functional scenario of wheat straw. Crit Rev Food Sci Nutr 53:287–295
Pelletier SW (1983) Alkaloids: chemical and biological perspectives. Wiley, New York
Pickel B, Schaller A (2013) Dirigent proteins: molecular characteristics and potential biotechnological applications. Appl Microbiol Biotechnol 97:8427–8438
Pietruszka J, Wang C (2012) Laccase-catalysed [small alpha]-arylation of cyclic [small beta]-dicarbonyl compounds. Green Chem 14:2402–2409
Plíšková M, Vondráček J, Křen V, Gažák R, Sedmera P, Walterová D, Psotová J, Šimánek V, Machala M (2005) Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology 215:80–89
Präg A, Grüning BA, Häckh M, Lüdeke S, Wilde M, Luzhetskyy A, Richter M, Luzhetska M, Günther S, Müller M (2014) Regio- and stereoselective intermolecular oxidative phenol coupling in Streptomyces. J Am Chem Soc 136:6195–6198
Prins A, Kleinsmidt L, Khan N, Kirby B, Kudanga T, Vollmer J, Pleiss J, Burton S, Le Roes-Hill M (2015) The effect of mutations near the T1 copper site on the biochemical characteristics of the small laccase from Streptomyces coelicolor A3(2). Enzym Microb Technol 68:23–32
Raafat D, von Bargen K, Haas A, Sahl HG (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74:3764–3773
Rakesh KP, Manukumar HM, Gowda DC (2015) Schiff’s bases of quinazolinone derivatives: synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg Med Chem Lett 25:1072–1077
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rich JO, Anderson AM, Berhow MA (2016) Laccase-mediator catalyzed conversion of model lignin compounds. Biocatal Agric Biotechnol 5:111–115
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Robert V, Mekmouche Y, Pailley PR, Tron T (2011) Engineering laccases: in search for novel catalysts. Curr Genomics 12:123–129
Roberts MF, Wink M (1998) Alkaloids: biochemistry, ecology, and medicinal applications, 1st edn. Springer Science & Business Media, New York
Rocasalbas G, Francesko A, Touriño S, Fernández-Francos X, Guebitz GM, Tzanov T (2013) Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr Polym 92:989–996
Rodríguez Couto S, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Sagui F, Chirivì C, Fontana G, Nicotra S, Passarella D, Riva S, Danieli B (2009) Laccase-catalyzed coupling of catharanthine and vindoline: an efficient approach to the bisindole alkaloid anhydrovinblastine. Tetrahedron 65:312–317
Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998) A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76:270–276
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Tech 10:37–51
Shahidi F, Naczk M (2004) Antioxidant properties of food phenolics. In: Shahidi F, Naczk M (eds) Phenolics in food and nutraceuticals. CRC Press, Boca Raton, pp. 403–436
Silley P, Griffiths JW, Monsey D, Harris AM (1990) Mode of action of GR69153, a novel catechol-substituted cephalosporin, and its interaction with the tonB-dependent iron transport system. Antimicrob Agents Chemother 34:1806–1808
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40:92–100
Szymusiak H, Zielinski R (2003) Bond dissociation enthalpy of phenolic antioxidants. Pol J Food Nutr Sci 53:129–135
Turner NJ (2009) Directed evolution drives the next generation of biocatalysts. Nat Chem Biol 5:567–573
Umezawa T (2003) Diversity in lignan biosynthesis. Phytochem Rev 2:371–390
van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Vardanyan R, Hruby V (2006) Synthesis of essential drugs. Elsevier, Amsterdam
Wan Y, Lu R, Akiyama K, Miyakoshi T, Du Y (2007) Enzymatic synthesis of bioactive compounds by Rhus laccase from Chinese Rhus vernicifera. Sci China Ser B 50:179–182
Watanabe NA, Nagasu T, Katsu K, Kitoh K (1987) E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother 31:497–504
Wellington KW, Qwebani-Ogunleye T, Kolesnikova NI, Brady D, de Koning CB (2013) One-pot laccase-catalysed synthesis of 5,6-dihydroxylated benzo[b]furans and catechol derivatives, and their anticancer activity. Arch Pharm Chem Life Sci 346:266–277
Wellington KW, Kolesnikova NI (2012) A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorg Med Chem 20:4472–4481
Widsten P, Heathcote C, Kandelbauer A, Guebitz G, Nyanhongo GS, Prasetyo EN, Kudanga T (2010) Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Process Biochem 45:1072–1081
Witayakran S, Ragauskas AJ (2009) Synthetic applications of laccase in green chemistry. Adv Synth Catal 351:1187–1209
Yoshida O, Nakamura J, Yamashiro H, Miura K, Hayashi S, Umetsu K, Xu S, Maki H, Arimoto H (2011) New insight into the mode of action of vancomycin dimers in bacterial cell wall synthesis. Med Chem Commun 2:278–282
Zhang Q, Su Y, Zhang J (2013) Seasonal difference in antioxidant capacity and active compounds contents of Eucommia ulmoides Oliver leaf. Molecules 18:1857–1867
Zhu C, Zhang Z, Ding W, Xie J, Chen Y, Wu J, Chen X, Ying H (2014) A mild and highly efficient laccase-mediator system for aerobic oxidation of alcohols. Green Chem 16:1131–1138
Zoia L, Bruschi M, Orlandi M, Tolppa EL, Rindone B (2008) Asymmetric biomimetic oxidations of phenols: the mechanism of the diastereo-and enantioselective synthesis of thomasidioic acid. Molecules 13:129–148
Zwane RE, Parker A, Kudanga T, Davids LM, Burton SG (2012) Novel, biocatalytically produced hydroxytyrosol dimer protects against ultraviolet-induced cell death in human immortalized keratinocytes. J Agric Food Chem 60:11509–11511
Acknowledgements
Financial support from the National Research Foundation (South Africa) is gratefully acknowledged. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s), and therefore, the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kudanga, T., Nemadziva, B. & Le Roes-Hill, M. Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol 101, 13–33 (2017). https://doi.org/10.1007/s00253-016-7987-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7987-5