Abstract
The morphological type of a microorganism generally influences its metabolite production. In the present study, we investigated the effects of the mycelial morphology of shiitake (Lentinula edodes) on the production of 2-mercaptohistidine trimethylbetaine (ergothioneine, ESH) during liquid fermentation. Analyses of the distribution of ESH in mycelial cells of different morphological types revealed that the ESH content of pellets obtained from the liquid fermentation media was much greater than the content in the free mycelia and clumps. The concentration of ESH in pellets on day 15 of liquid fermentation reached 0.79 mg/g dry weight (DW), which is approximately three times the concentration found in mycelia clumps (0.28 mg/g DW) and free mycelia (0.31 mg/g DW). Macroscopic image analysis of the development and morphological changes of the pellets during a liquid fermentation period of up to 25 days indicated that pellet growth showed a highly positive correlation with the increase in ESH concentration (r 2 = 0.9851). A reduced agitation rate of 50 rpm for the culture medium was suitable for pellet formation and size enlargement. The results obtained in this work would be helpful in guiding the intentional manipulation of the distribution and enrichment of ESH in L. edodes through changes in liquid fermentation conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The morphology of filamentous fungi in liquid fermentation has been shown to play a critical role in many industrial fermentation processes for the production of secondary metabolites (Berovic et al. 1991; Park et al. 1993; Nielsen et al. 1995; Gibbs and Seviour 1996). The physicochemical conditions during mycelial cultivation have a variety of effects on certain characteristics of microorganisms, which may include rupture of the cell wall, changes in the morphology of filamentous microorganisms, variation in the efficiency and rate of growth, and variation in the rate of formation of the desired product (Gibbs and Seviour 1996; Riscaldati et al. 2000). During liquid fermentation, many filamentous fungi grow as either free mycelia, clumps, or pellets, and the growth form is determined by factors including growth medium, size of inoculum, and physical environmental conditions (Berovic et al. 1991; Sinha et al. 2001). Pellets are characterized by the mycelia developing into stable, spherical aggregates consisting of a more or less dense, branched, and partially intertwined network of hyphae (Berovic and Cimerman 1982). Many investigators have characterized the mycelial pellets by means of image analysis (Packer and Thomas 1990; Nielsen et al. 1995). Previous studies on image analyses of Paecilomyces japonicus have reported that mycelial morphology markedly affects mycelial growth and biopolymer production (Sinha et al. 2001). However, extensive studies on the relationship between morphological parameters and productivity of fermentation products are still scarce (Zorn et al. 2005; Alvim et al. 2009; Rühl 2009).
The shiitake (Lentinula edodes) mushroom is renowned as a food product and a substance used in traditional medicine (Turło et al. 2008). It has a high nutritional value and contains many bioactive compounds, including 2-mercaptohistidine trimethylbetaine (ergothioneine, ESH), polysaccharides, amino acids, phenolics, dietary fiber, ergosterol, vitamins, and minerals (Mattila et al. 2002). ESH is a naturally occurring amino acid analogue that is usually biosynthesized in mycobacteria and fungi, including certain species of mushrooms (Melville et al. 1955). In addition, ESH is a stable antioxidant and is nonautoxidizable at physiological pH (Hartman 1990). ESH has several physiological benefits, including enhancement of metabolic energy, protection against formation of cataracts, and molecular regulation of antiinflammatory mechanisms in the lungs (Rahman et al. 2003). Moreover, a recent study showed that ESH depletion in mammalian cells causes severe oxidative stress, suggesting that ESH may represent a new vitamin as a consequence of its dietary origin and the toxicity associated with its depletion (Paul and Snyder 2010).
Several studies involving the manipulation of culture media constituents have been conducted to improve the accumulation of ESH in fruiting bodies and mycelia (Dubost et al. 2007; Estrada et al. 2009; Lee et al. 2009). However, the relationship between the morphology of mycelia and ESH production, which might assist in designing the physicochemical parameters of a liquid fermentation system to facilitate the enriched production of ESH, has not yet been elucidated. Therefore, in the present study, the effects of changes in mycelial morphology on ESH production in L. edodes were investigated during liquid fermentation.
Materials and methods
Microorganisms
The mycelial strain H-601 of shiitake mushroom was kindly donated by Hokken Co. (Tochigi, Japan). Shiitake mycelium was maintained on potato dextrose agar (Becton-Dickinson, Sparks, MD, USA) plates and subsequently subcultured every 2 months. The plates were incubated at 25°C for 8 days and subsequently stored at 4°C.
To evaluate the distribution of ESH in the free mycelia, clump, and pellet forms, the mycelial strains of various mushrooms were obtained from Hokken Co. (Tochigi, Japan) and a mushroom farm (Nakhon Pathom District, Thailand). A list of the fungi analyzed for the accumulation of ESH in their mycelia is summarized in Table 1. Each mycelium was maintained under the same conditions as described above.
Inoculum preparation
The shiitake mycelial strain was initially grown on sterilized potato dextrose agar (Difco; Becton-Dickinson, Sparks, MD, USA) in Petri dishes (15 mm × 60 mm i.d.) for 8 days; the potato dextrose agar medium was then cut into approximately 5 × 5 mm squares with a sterilized blade and transferred to the seed culture medium. The inoculated medium was further homogenized at 13,500 rpm for 10 s with an Ultra Turrax T25 homogenizer (Janke and Kunkel IKA-Labortechnik, Staufen im Breisgau, Germany). The seed culture medium comprised 25 g/l glucose, 2 g/l yeast extract (lot code: BCBB7893; BioChemika Fluka, Sigma-Aldrich, St. Louis, MO, USA), 1 g/l glutamic acid, 0.5 mg/l biotin, 0.1 g/l thiamin, 2 g/l KH2PO4, 0.5 g/l MgSO4, 5 ml 0.1 M FeCl3, and 5 ml 0.1 M MnSO4. The initial pH of the medium was adjusted to 5.5 before autoclaving at 121°C for 30 min. The seed culture was cultivated for 10 days at 25°C in a shaker at 140 rpm (EYELA Multi Shaker MMS-3000; Tokyo Rikakikai, Tokyo, Japan). A cyclic oscillation mode was employed at an amplitude of 25 mm. The chemical compounds used in the present study, d (+)-glucose, glutamic acid, biotin, thiamin, and KH2PO4, were purchased from Wako Pure Chemical Industries (Osaka, Japan); MgSO4, FeCl3, and MnSO4 were purchased from Sigma.
Fermentation conditions
Different concentrations of inoculum
Cultivated seed cultures with different volumes of the mycelial homogenate, 0.5% and 5% (v/v), were used as inoculum to investigate the relationship between mycelial morphology and ESH production. The test culture was cultivated at 25°C for 25 days in 100-ml flask on a shaker at a rotation speed of 100 rpm. Sampling was conducted at intervals of 0, 1, 2, 5, 10, 15, 20, and 25 days.
Different agitation rates
To follow the morphological changes of free filaments to the pellets, the seed culture media were homogenated and filtered using a 0.2-mm sieve. The screened free filaments were used to inoculate the test culture medium, followed by incubation in a 100-ml glass cultivation flask at 25°C for 25 days with different agitation rates, including 50, 100, and 300 rpm, using an orbital shaker.
Harvesting of mycelia and determination of mycelial growth
At the end of each fermentation process, the cultured mycelia were isolated from the medium by centrifugation at 11,400 rpm for 20 min and subsequently washed thoroughly with 500 ml distilled water. The culture medium, separated from the mycelia as a supernatant, was further filtered through a hydrophilic polytetrafluoroethylene (PTFE) membrane filter of 0.2-μm pore size (Omnipore; Millipore, Bedford, MA, USA) to avoid removal of any tiny mycelia. The mycelia were further lyophilized and stored at −30°C until use.
Morphological measurements of the mycelia
To separate free mycelia, clumps, and pellets, a known volume sample of the fermentation broth was passed through a 420-μm sieve and a 200-μm sieve, in that order. The permeates were passed through hydrophilic PTFE membrane filters of 0.45-μm pore size (Omnipore; Millipore) and were diluted with a known volume of distilled water for the analysis of cell morphology and percentage distribution. The free filament and clump samples were fixed with an equal volume of fixative (13 ml 40% formaldehyde, 5 ml glacial acetic acid, and 200 ml 50% ethanol). A 10-μl portion of diluted sample was transferred to a glass slide, air dried, and stained on the glass slide with 10 μl methylene blue solution (3 g methylene blue dissolved in 300 ml 95% ethanol) according to the method of Packer and Thomas (1990). The image was captured with a USB digital camera (WRAYCAM G130; Wraymer, Osaka, Japan), mounted on a model CKX-41 stereoscopic microscope (Olympus, Tokyo, Japan), at 40× magnification. Image analysis was performed with WrayView software (Wraymer). Within a sample, a minimum of 10–15 images were acquired for each determination of free filaments and clumps.
For the analysis of the pellet morphology, the screened pellet samples were placed in a Petri dish with a diameter of 5.5 cm containing 6 ml distilled water, and macroscopic gray-scale images were captured with a model IXY30s digital camera (Canon, Tokyo, Japan). The pellet morphology was characterized using image analysis, performed using ImageJ v1.39d (http://imagej.nih.gov/ij/, U.S. National Institutes of Health) software. The morphology of the pellets was characterized by their mean diameter, circularity, and roughness according to Riley et al. (2000) and Sinha et al. (2001). Circularity (C) was estimated using the equation C = (pellet aggregate perimeter)2/pellet area. Roughness (R) was measured using the following equation: R = (pellet aggregate perimeter)2/(4π × pellet area).
For the measurement of dry weight of the different mycelial cells, including free filaments, clumps, and pellets, which were screened by the methods described above, samples were further washed several times with deionized water, transferred to a weighing cup, and lyophilized until the weight became constant.
Preparation of mycelial extracts for quantitative determination of ESH
Ergothioneine in the lyophilized mycelia (approximately 20 mg) was extracted using 10 ml 70% ethanol containing 100 μM of 2-mercapto-1-methyl imidazole as an internal standard (IS) by homogenization at 9,500 rpm for 5 min with the Ultra Turrax T25 homogenizer. The homogenate was centrifuged at 4,500 rpm for 5 min, and the resulting supernatant was collected. The extract from 70% ethanol was then evaporated to dryness at 40°C in vacuo. The extract thus obtained was redissolved in 10 ml distilled water and was used for quantitative determination of ESH.
Identification and quantification of ESH in mycelia
For the identification and quantitative determination of ESH, the mycelial extract was filtered though a PTFE membrane of 0.45-μm pore size before high performance liquid chromatography-mass spectrometric analysis (LC–MS). LC–MS was carried out using a Shimadzu LCMS-2010EV instrument (Kyoto, Japan) equipped with three Develosil C30-UG-5 reversed-phase columns (4.6 mm i.d. × 250 mm, 5-μm particle size; Nomura Chemical, Aichi, Japan) arranged in a series. The mobile phase consisted of 10% methanol in deionized water containing 0.1% acetic acid with a flow rate of 0.2 ml/min. Injection volume was 20 μl; column oven temperature was set at 40°C. ESH eluted from the column was monitored by absorbance measurement at 254 nm with a photodiode array detector (PDA) model SPD-M20A (Shimadzu, Kyoto, Japan). Conditions of mass spectrometry were as follows: curved desolvation line temperature, 250°C; voltage, 1.5 kV; nebulizer nitrogen gas flow, 1.5 l/min. The analytical mode was electrospray ionization (ESI)-positive scan, and the MS scan range of m/z was between 50 and 1,000. For selected ion monitoring, m/z 230 and m/z 115 were chosen for ESH and IS, respectively. A calibration curve was obtained by using different concentrations of authentic l-(+) ergothioneine solutions (Bachem, Bubendorf, Switzerland).
The ESH content was quantified by the peak area ratio (ESH/IS). All ESH concentration data were expressed as milligrams of ESH per gram of the mycelia in dry weight (mg ESH/g DW). Extractions were performed from one crop of sample, tested in triplicate for each sample, and used for the ESH analysis.
Statistical analyses
All analyses were performed in triplicate. The data were expressed as means (standard deviations) (n = 3). Statistical analyses were performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA). Data sets were evaluated by analysis of variance (ANOVA). A statistically significant difference was evaluated at the 95% confidence level by post hoc test; comparisons were made on the basis of the P value (α = 0.05) by using Duncan’s new multiple range test. Microsoft Excel 2003 was used to analyze the coefficient of determination (r 2) and evaluate the relationship between ESH content and mean diameter, circularity, and roughness.
Results and discussion
Identification of ESH in mycelia
For identification and quantification of ESH, lyophilized shiitake mycelia were extracted using 70% ethanol and analyzed by LC–ESI–MS (Fig. 1). The HPLC chromatogram monitored at 254 nm and ESI–MS selected ion monitored at m/z 230 confirmed that ESH was eluted at 45.6 min (Fig. 1a,b). The results of ESI–MS also revealed a prominent molecular ion [M+H]+ of m/z 230 in the mycelial extract (Fig. 1c). Identification of the fragment ions, m/z 186 for trimethylammonioethyl-imidazole-sulfhydryl (C8H15N3S), m/z 127 for ethyl-imidazole-sulfhydryl (C5H6N2S), m/z 252 for the sodiated cluster ion [M+Na]+, and m/z 459 for the cluster ion [2M+H]+, assigned the compound as ESH (Fig. 1c). Further isolation of the compound through several chromatographic steps, including ion exchange, silica gel chromatography, as well as reversed-phase HPLC and analysis through high-resolution time–of-flight mass spectroscopy, also provided the molecular ion of m/z 230.0976 in agreement with the calculated exact molecular ion mass of 230.0959 (data not shown). Therefore, LC–ESI–MS was employed for routine monitoring of ESH in mycelia by using [M+H]+ of m/z 230 to recognize that the retention time coincided with that of the authentic ESH.
High-performance liquid chromatography and mass spectrometry of ergothioneine in shiitake mushroom mycelia produced by liquid fermentation. a Chromatogram of shiitake mushroom mycelial extract monitored at 254 nm. b Selected ion monitoring profiles of ergothioneine at m/z 230 and 1-methyl imidazole used as an internal standard at m/z 115. c Electrospray ionization (ESI) mass spectrum of ergothioneine obtained from shiitake mycelial extract
Effects of inoculum volume on the morphological changes of mycelia and distribution of ESH
The percentages of free filaments, clumps, and pellets at inoculation doses of 0.5% and 5% are illustrated in Fig. 2. During the fermentation period of 25 days, the numerical percentage of pellets remained at low levels at both inoculations. Free filaments dominated at about 90% in the early growth phase until 10 days. The free filaments and clumps accounted for about 45% each in the following late growth phase, after 10 days of the liquid fermentation period. A remarkable increase in the percentage of clumps and a corresponding decrease in that of free filaments occurred between 5 and 10 days of fermentation with the 5% inoculation dose. However, at the lower inoculation dose of 0.5%, the decrease in the percentage of free filaments and the subsequent increase in clumps were much lower and more gradual than those at the higher (5%) inoculation dose. At the lower inoculation dose (0.5%), the propagation of free filaments was higher than the conversion of free filaments to clumps. These results suggested that the morphological changes might be caused by depletion of one or more of the limiting components in the culture media; it can also be speculated that this phenomenon is associated with the secretion of autoregulators by the mycelial cells. Indeed, in the case of L. edodes, morphological differentiation was started by the depletion of one or more of the limiting components contained in the culture media, which further stimulated the secretion of autoregulators by the mycelial cells (Yang et al. 1995). This speculation requires further investigation to confirm its validity and to identify the limiting substances. A decrease in the percentage of clumps with the advancement of the fermentation process (see Fig. 2) suggested the transformation of clumps to pellets, as well as the enlargement of the pellets themselves.
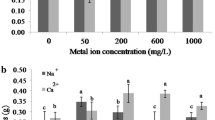
Free mycelia, clumps, and pellets were separated and analyzed for concentrations of ESH (Fig. 3). ESH content of the pellets obtained from the liquid fermentation media after 15 days of fermentation was much higher than that of free mycelia and in clumps. With prolongation of the fermentation period beyond 15 days, no significant increase in ESH content was observed. Based on these results, we selected samples after 15 days of fermentation for further analyses. The concentration of ESH in the pellets on the 15th day of the fermentation period amounted to 0.79 mg/g DW, approximately three times as much as those of the mycelia clumps and free mycelia. ESH concentrations in the mycelia clumps and free mycelia were 0.28 and 0.31 mg/g DW, respectively. Based on the dry cell weights of 4.9, 1.1, and 0.5 g/l in the pellets, clumps, and free mycelia, respectively, after 15 days of fermentation, the distribution of total ESH amount in pellets, clumps, and free mycelia was 3.92, 0.34, and 0.14 mg/l, respectively. From these results, it was suggested that pellet formation was highly associated with the accumulation of ESH during the liquid fermentation.
In order to confirm the association of ESH with the pellet form of mycelia, several edible mushroom mycelia species were evaluated, as depicted in Table 1. Due to the fact that most of the edible mushroom mycelia can not form spores in liquid culture, an inoculum of vegetative mycelium had to be used after the treatment of homogenizing. Where no more pellets were present in seed culture and the broth apparently looked homogenous. With the prolongation of the submerged cultivation the mycelia form into rather large visible pellets. The formation of large pellets was usual in the mycelia submerged-culture of medicinal mushroom (Yang and Liau 1998). As the size of the pellets increases, the diffusion of nutrients, particularly, oxygen into the center of the pellets becomes limited. Critical oxygen limited radius was about 200–400 µm (Schügerl et al. 1983; Prosser and Tough 1991; Gibbs et al. 2000). At the oxygen depleted region of the pellet the cells differentiate and the synthesis of the secondary metabolites was induced (Gehrig et al. 1998). Considering this it could be assumed that a lower agitation rate enhanced the pellet size and thus promoted the ESH accumulation. The results of the present study also clearly indicated that the accumulation of ESH was mainly limited to the pellet forms for most of the mushroom species rather than clumps or free filaments.
Effects of different agitation rates on development of mycelial pellets and ESH production
Typical morphological changes in L. edodes during the entire fermentation period under different agitation conditions are presented in Fig. 4. After 5 days of fermentation, the formation of mycelial clumps and pellets with a spherical and dense core appeared in the culture media (Fig. 4a). However, as the fermentation proceeded, the outer hairy region of the pellets became fluffier and there was a corresponding increase in pellet roughness. The cells mainly formed pellets, and their size increased continuously during the 25 days of the entire fermentation period, irrespective of agitation intensity. However, significant variation in mycelial morphology was observed under different agitation conditions. The pellet size increased as agitation intensity was reduced. The rates of pellet formation were faster at the lower agitation intensities, 50 and 100 rpm (Fig. 4a,b), whereas reduced pellet size and increased circularity were observed at a higher agitation intensity of 300 rpm (Fig. 4c). During the later stages of fermentation, after day 10, at lower agitation intensities (50 and 100 rpm), the pellets lost their rigidity and formed looser mycelial clumps. These bulkier and looser pellets were probably caused by depletion of dissolved oxygen in the pellet cores, leading to faster pellet autolysis and the formation of hyphal fragments and flocks. Metz and Kossen (1977) as well as Gibbs and Seviour (1996) reported that the decrease in the length of the pellets found in cultures agitated more vigorously was a direct result of breakage of the hyphae and was not caused by acceleration of the aging process, leading to autolysis.
A number of factors are known to influence pellet formation from free filaments. Inoculum size is often critical, with higher spore concentrations favoring dispersed growth and lower concentrations favoring aggregation and pellet formation. Medium composition and impeller agitation of the culture medium also play major roles in overall morphology during submerged growth. In some cases, a culture medium with a low pH has been shown to favor dispersed growth without formation of pellets (Moore et al. 2011). In the present study, it is also evident that pellet formation was favored by the low agitation intensities. However, mycelial growth in terms of the mycelial dry weight per liter was significantly increased by shifting the agitation speed from 50 to 300 rpm as a result of enhanced mixing of the fermentation broth, thereby increasing uptake of oxygen and nutrients (Fig. 5a). Similar results have been reported stating that an increased agitation speed enhances yields of mycelial biomass in other liquid fermentations of macrofungi (Baets et al. 2002; Sobczuk et al. 2005). In contrast, a higher yield of ESH production (1.10 mg/g DW) was achieved in the fermentation with a lower agitation rate for 25 days of liquid fermentation (Fig. 5b). With an increase in the agitation rate, a significant decrease was observed in the ESH concentration per gram dry weight, which was closely related to the development of compact morphological characteristics, particularly for the pellet-forming trend (see Fig. 4). An increase in the percentage of the pellet form in mycelial biomass in response to the lower agitation rate might have contributed to the increase in the total ESH content. Gibbs et al. (2000) and Papagianni (2004) indicated an inverse relationship between agitation intensity and pellet size. They demonstrated for several filamentous fungi that vigorous agitation appears to prevent pellet formation; increased agitation resulted in small and more compact pellets. In contrast, a lower agitation rate increased the pellet size. With the increasing size of the pellet the oxygen transfer to the center of the pellet was restrained. In the oxygen-limited regions, the cells differentiate and synthesis of the secondary metabolites is induced. Considering this, it could be assumed that a lower agitation rate enhanced the pellet size, thus promoting ESH accumulation. A similar observation was also reported by Gehrig et al. (1998). They mentioned that the influence of the pellet morphology of the basidiomycete Cyathus striatus on the production of the antibiotics striatals A, B, and C was highly associated with the increasing size and the oxygen-limited regions of the pellets. Static condition of the flask shaker, however, demonstrated the least growth of mycelia as well as less ESH production. It is obvious that loose mycelium aggregation occurred under no agitation, and in combination with low oxygen availability, further destruction and decomposition occurred during the 15-day fermentation period. However, loosely aggregated mycelium can easily disrupt to mycelium fragments again under low agitation intensity although the oxidative stress remains. Although few reports have been available on ESH production by mushroom mycelia, Gehrig et al. (1998) mentioned that the influence of the pellet morphology of the basidiomycete Cyathus striatus on the production of the antibiotics striatals A, B, and C was highly associated with the increasing size and oxygen-limited regions of the pellets. Working on Ganoderma lucidum in submerged culture in shaken flasks on a medium containing peptone, yeast extract, and glucose, Wagner et al. (2004) concluded that manipulating the physiological events and subsequent morphological characteristics of pellets during the culture of G. lucidum will allow the proposal of culture strategies to improve exopolysaccharide production. However, Fazenda et al. (2010) concluded that fungal morphology and broth rheology influenced the extracellular polysaccharide, wherein the clump form was the most productive morphology in terms of EPS production, compared with the free filamentous mycelial form. Investigating the morphological and rheological properties in submerged culture of three different species of the basidiomycete Phellinus (P. baumii, P. gilvus, and P. linteus), Hwang et al. (2004) reported that morphological change in the pellets of the three species of Phellinus was a good indicator for identifying cell activity for pharmacologically important exopolysaccharide production. Alvim et al. (2009) also demonstrated a relationship between mycelial morphology and secretion of necrosis-inducing proteins in Moniliophthora perniciosa (Basidiomycetes) in carbon source-induced media. Results shown in Fig. 5 also showed that a lower agitation rate of 50 rpm is superior for the enrichment of the ESH/g DW of mycelia; however, for total production of ESH/l of media, an agitation rate of 100 rpm is considerably more productive. ESH-enriched mycelia might be a suitable choice for the isolation of ESH for pharmaceutical or other industrial uses. On the other hand, total production of ESH/L of media will be required for crude ESH application. The fermentation process can be manipulated based on industry prerequisites.
In the present study, we reached an important conclusion, namely, agitation is a critical factor in the liquid fermentation of L. edodes, particularly for the production of ESH. The yield of ESH at the lower agitation rate of 50 rpm was approximately two times that at a higher agitation rate of 300 rpm. However, cumulative studies revealed that the high agitation rate of 300 rpm significantly increased mycelial growth, whereas the highest production of ESH was achieved at a lower agitation rate of 50 rpm, in liquid fermentation of mycelial L. edodes. Other investigators have also stressed the importance of agitation in liquid fermentation of fungi; however, their results are controversial. Hsieh et al. (2006) demonstrated that vigorous agitation inhibited both cell growth and polysaccharide production, with a higher rate of glucose consumption in liquid fermentation of the mushroom Grifola frondosa in a 5-l stirred tank fermenter. Similar results were found in liquid fermentation of mycelia for the macrofungus Ganoderma lucidum (Yang and Liau 1998). Although higher speeds of agitation enhanced mixing efficiency and polysaccharide release, higher shear stress had a detrimental effect on mycelial growth and secondary metabolite formation. Indeed, high aeration rate and high agitation intensity are important, but are not always required for enhanced production of fungal growth and metabolites (Tang and Zhong 2003; López et al. 2005).
Morphological changes in mycelial pellets and ESH production during fermentation
Morphological changes of pellets and the association of these changes with ESH production, during liquid fermentation at an inoculation dose of 5% and moderate agitation rate of 100 rpm, were further investigated. Pellet morphology was usually characterized by mean diameter, circularity, and roughness, which are adequate parameters to evaluate the mycelial morphology (Cui et al. 1998; Riley et al. 2000). Results of the simplified image analysis of mycelial morphology are depicted in Fig. 6. Reichl et al. (1992) have used image analysis to investigate the shapes and sizes of Streptomyces tendae pellets at a low magnification at which individual hyphae were not distinct. The chosen morphological parameters in their study were the occurrence percentage based on the number of pellets, the projected area of all morphological forms, the percentage of pellet area, as well as the shape of pellet (circularity). In the present study, on the other hand, macroscopic studies were carried out on the development and morphological changes of pellets during the fermentation period. Typical binary images of pellets, representing their morphological changes throughout the fermentation period, are depicted in Fig. 7. The binary images were further analyzed for the estimation of mean diameter, circularity, and roughness of the pellets (Fig. 8a–c). The results indicated that pellet diameter increased rapidly from the first day until the end of the fermentation period of 25 days. In contrast, circularity decreased until 10 days of fermentation, then gradually increased until the end of fermentation. Roughness of the pellets, on the other hand, increased until 15 days and subsequently decreased gradually. Taking into account that circularity is a shape factor describing the deviation of the pellet image from a true circle, and roughness refers to the irregularity of the perimeter of the pellet, compact pellet formation was observed in the later phase of the fermentation period, on and after 15 days. This result is probably caused by hyphae being shaved off from the outer hairy region of the mycelial pellets during the later phase of fermentation (Packer and Thomas 1990; Park et al. 1993; Riley et al. 2000). Further investigation of the ESH accumulation (Fig. 8d) in the mycelial pellets throughout the fermentation period revealed that pellet morphology, particularly mean diameter, was highly positively correlated with ESH content (r 2 = 0.9851). However, circularity and roughness were not strongly correlated with ESH concentration (r 2 = 0.3650 and 0.7212, respectively). This result clearly indicated that development of pellets is highly associated with the formation of ESH. It is well known that as the small pellets enlarge, the center of pellets becomes progressively nutrient- and oxygen deficient. As a result, the central metabolism becomes anaerobic and fermentative, which results in the production of several secondary metabolites (Moore et al. 2011). Mycelial microorganisms are exploited extensively in the commercial production of a wide range of secondary metabolites. Because the morphological type can strongly influence metabolite production, the methodology for inducing pellet formation, and the type of pellets produced, are important considerations for the effective production of these metabolites (Braun and Vecht-Lifshitz 1991).
Wide distribution and heterogeneity of secondary metabolites has been observed in nature. Although challenging, it is valuable to understand how to intentionally manipulate heterogeneity by controlling the cell culture environment (Zhong and Yue 2005). At present, although the mechanisms of bioaccumulation and synthesis of ESH in pellets are yet unclear, the results of this work will be very helpful in the intentional manipulation of the distribution and enrichment of ESH in L. edodes by means of liquid fermentation.
In conclusion, we have established for the first time that mycelial morphology is associated with the production of ESH. The pellet formation of L. edodes in liquid fermentation is highly correlated with ESH production. Moreover, morphological studies of pellets indicated that the increase in ESH accumulation corresponded with the enlargement of the pellet size. The present findings are useful for promoting large-scale production of ESH through manipulation of the liquid fermentation conditions of L. edodes.
References
Alvim FC, Mattos EM, Pirovani CP, Gramacho K, Pungartnik C, Brendel M, Cascardo JCM, Vincentz M (2009) Carbon source-induced changes in the physiology of the cacao pathogen Moniliophthora perniciosa (Basidiomycetes) affect mycelial morphology and secretion of necrosis-inducing proteins. Genet Mol Res 8:1035–1050
Baets SD, Laing SD, Francois C, Vandamme E (2002) Optimization of exopolysaccharide production by Tremella mesenterica NRRL Y-6158 through implementation of fed-batch fermentation. J Ind Microbiol Biotechnol 29:181–184
Berovic M, Cimerman A (1982) Redox potential in submerged citric acid fermentation. Appl Microbiol Biotechnol 16:185–188
Berovic M, Cimerman A, Steiner W, Koloni T (1991) Submerged citric acid fermentation: rheological properties of Aspersillus niger broth in a stirred tank reactor. Appl Microbiol Biotechnol 34:579–581
Braun S, Vecht-Lifshitz SE (1991) Mycelial morphology and metabolite production. Trends Biotechnol 9:63–68
Casas López JL, Pérez Sánchez JA, Sevilla Fernández JM, Rodríguez Porcel EM, Chisti YJ (2005) Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. J Biotechnol 116:61–77
Cui YQ, Okkerse WJ, van der Lans RGJM, Luyben KChAM (1998) Modeling and measurements of fungal growth and morphology in submerged fermentations. Biotechnol Bioeng 60:216–229
Dubost NJ, Beelman R, Royse D (2007) Influence of selected cultural factors and postharvest storage on ergothioneine content of common button mushroom Agaricus bisporus (J. Lge) Imbach (Agaricomycetideae). Int J Medicinal Mushrooms 9:163–176
Estrada A, Lee HJ, Beelman R, Jimenez-Gasco M, Royse D (2009) Enhancement of the antioxidants ergothioneine and selenium in Pleurotus eryngii var. eryngii basidiomata through cultural practices. World J Microbiol Biotechnol 25:1597–1607
Fazenda ML, Harvey LM, McNeil B (2010) Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum. J Microbiol Biotechnol 20:844–851
Gehrig I, Bart HJ, Anke T, Germerdonk R (1998) Influence of morphology and rheology on the production characteristics of the basidiomycete Cyathus striatus. Biotechnol Bioeng 59:525–533
Gibbs PA, Seviour RJ (1996) Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Appl Microbiol Biotechnol 46:503–510
Gibbs PA, Seviour RJ, Schmid F (2000) Growth of filamentous fungi in submerged culture: problems and possible solutions. Crit Rev Biotechnol 20:17–48
Hartman PE (1990) Ergothioneine as antioxidant. Methods Enzymol 186:310–318
Hsieh C, Liu CJ, Tseng MH, Lo CT, Yang YC (2006) Effect of olive oil on the production of mycelial biomass and polysaccharides of Grifola frondosa under high oxygen concentration aeration. Enzyme Microb Technol 39:434–439
Hwang HJ, Kim SW, Xu CP, Choi JW, Yun JW (2004) Morphological and rheological properties of the three different species of basidiomycetes Phellinus in submerged cultures. J Appl Microbiol 96:1296–1305
Lee WY, Park EJ, Ahn JK (2009) Supplementation of methionine enhanced the ergothioneine accumulation in the Ganoderma neo-japonicum mycelia. Appl Biochem Biotechnol 158:213–221
Mattila P, Suonpää K, Piironen V (2002) Functional properties of edible mushrooms. Nutrition 16:694–696
Melville DB, Horner WH, Otken CC, Ludwig ML (1955) Studies on the origin of ergothioneine in animals. J Biol Chem 213:61–68
Metz B, Kossen NWF (1977) Biotechnology review: the growth of molds in the form of pellets. A literature review. Biotechnol Bioeng 19:781–799
Moore D, Robson GD, Trinci APJ (2011) Whole organism biotechnology. In: Moore D, Robson GD, Trinci APJ (eds) 21st century guidebook to fungi. Cambridge University Press, London, pp 451–510
Nielsen J, Johansen CL, Jacobsen M, Krabben P, Villadsen J (1995) Pellet formation and fragmentation in submerged cultures of Penicillium chrysogenum and its relation to penicillin production. Biotechnol Prog 11:93–98
Packer HL, Thomas CR (1990) Morphological measurements on filamentous microorganisms by fully automatic image analysis. Biotechnol Bioeng 35:870–881
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Park YS, Ohta N, Okabe M (1993) Effect of dissolved oxygen concentration and impeller tip speed on itaconic acid production by Aspersillus terreus. Biotechnol Lett 15:583–586
Paul BD, Snyder SH (2010) The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ 17:1134–1140
Prosser JI, Tough AJ (1991) Growth mechanisms and growth kinetics of filamentous microorganisms. Crit Rev Biotechnol 10:253–274
Rahman I, Gilmour PS, Jimenez LA, Biswas SK, Antonicelli F, Aruoma OI (2003) Ergothioneine inhibits oxidative stress- and TNF-α-induced NF-κB activation and interleukin-8 release in alveolar epithelial cells. Biochem Biophys Res Commun 302:860–864
Reichl U, King R, Gilles ED (1992) Characterization of pellet morphology during submerged growth of Streptomyces tendae by image analysis. Biotechnol Bioeng 39:164–170
Riley GL, Tucker KG, Paul GC, Thomas CR (2000) Effect of biomass concentration and mycelial morphology on fermentation broth rheology. Biotechnol Bioeng 68:160–172
Riscaldati E, Moresi M, Federici F, Petruccioli M (2000) Effect of pH and stirring rate on itaconate production by Aspergillus terreus. J Biotechnol 83:219–230
Rühl M (2009) Laccases and other ligninolytic enzymes of the basidiomycetes Coprinopsis cinerea and Pleurotus ostreatus—submerged and solid state fermentation, morphological studies of liquid cultures and characterisation of new laccases. Ph.D dissertation. Georg-August-University Göttingen, Göttingen
Schügerl K, Wittler R, Lorenz T (1983) The use of molds in pellet form. Trends Biotechnol 1:120–123
Sinha J, Bae JT, Park JP, Kim KH, Song CH, Yun JW (2001) Changes in morphology of Paecilomyces japonica and their effect on broth rheology during production of exo-biopolymers. Appl Microbiol Biotechnol 56:88–92
Sobczuk TM, Camacho FG, Grima EM, Chirti Y (2005) Effects of agitation on the microalgae Phaeodactylum tricornutum and Porphyridium cruentum. Bioprocess Biosyst Eng 28:243–250
Tang YJ, Zhong JJ (2003) Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enzyme Microb Technol 32:478–484
Turło J, Gutkowskaa B, Herold F, Krzyczkowski W, Błazewicz A, Kocjan R (2008) Optimizing vitamin B12 biosynthesis by mycelial cultures of Lentinula edodes (Berk.) Pegl. Enzyme Microb Technol 43:369–374
Wagner R, Mitchell DA, Sassaki GL, Amazonas MALA (2004) Links between morphology and physiology of Ganoderma lucidum in submerged culture for the production of exopolysaccharide. J Biotechnol 114:153–164
Yang FC, Liau CB (1998) The influence of environmental conditions on polysaccharide formation by Ganoderma lucidum in submerged cultures. Process Biochem 33:547–553
Yang YK, Sbimizu H, Shioya S, Suga K, Nihira T, Yamada Y (1995) Optimum autoregulator addition strategy for maximum virginiamycin production in batch culture of Streptomyces virginiae. Biotechnol Bioeng 46:437–442
Zhong JJ, Yue CJ (2005) Plant cells: secondary metabolite heterogeneity and its manipulation. Biotechnology for the future. Adv Biochem Eng Biotechnol 100:53–88
Zorn H, Bouws H, Takenberg M, Nimtz M, Getzlaff R, Breithaupt DE, Berger RG (2005) An extracellular carboxylesterase from the basidiomycete Pleurotus sapidus hydrolyses xanthophyll esters. Biol Chem 386:435–440
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tepwong, P., Giri, A. & Ohshima, T. Effect of mycelial morphology on ergothioneine production during liquid fermentation of Lentinula edodes . Mycoscience 53, 102–112 (2012). https://doi.org/10.1007/s10267-011-0145-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10267-011-0145-0