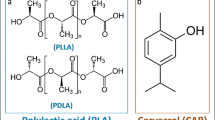
Abstract
Poly(lactic acid)-based antimicrobial materials received considerable attention as promising systems to control microbial growth. The remarkable physicochemical properties of PLA such as renewability, biodegradability, and US Food and Drug Administration (FDA) approval for clinical use open up interesting perspectives for application in food packaging and biomedical materials. Nowadays, there is an increasing consumer demands for fresh, high-quality, and natural foods packaged with environmentally friendly materials that prolong the shelf life. The incorporation of antimicrobial agents into PLA-based polymers is likely to lead to the next generation of packaging materials. The development of antimicrobial PLA materials as a delivery system or coating for biomedical devices is also advantageous in order to reduce possible dose-dependent side effects and limit the phenomena of antibiotic resistance. This mini-review summarizes the most recent advances made in antimicrobial PLA-based polymers including their preparation, biocidal action, and applications. It also highlights the potential of PLA systems as efficient stabilizers-carriers of various kinds of antimicrobial additives including essential oils and other natural compounds, active particles and nanoparticles, and conventional and synthetic molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial contamination is a great concern in several fields, ranging from food packaging to medical devices (Lau and Wong 2000; Darouiche 2004). Various kinds of polymers are usually sterilized by means of either dry/wet heat or ionizing radiation (Kenawy et al. 2007). However, these materials are able to be colonized by microbial cells (Sousa et al. 2011) and give rise to infection if they are exposed to the atmosphere or other contaminating environments. For instance, they can come into contact with microorganisms usually present on foods or wounds (Lau and Wong 2000). Therefore, there is a definite need for new antimicrobial materials able to inhibit the microbial growth and to prevent the subsequent colonization and proliferation (Nostro et al. 2010, 2012, 2013; Liu et al. 2016; Scaffaro and Lopresti 2018).
In this context, poly(lactic acid) (PLA) can be considered one of the most attractive biopolymers due to its physical properties, renewability, biodegradability, and biocompatibility (Tawakkal et al. 2016; Scaffaro et al. 2017a). The great advantages of PLA are due in part to its ability to degrade into the naturally occurring lactic acid under physiological conditions, but other exceptional qualities such as low immunogenicity and good mechanical properties must also be considered (Llorens et al. 2015). Moreover, PLA can be processed adopting a large number of techniques and it is commercially available in a wide range of grades making it suitable for several applications (Scaffaro et al. 2016, 2017a, c).
Over recent years, several additives, including natural compounds, peptides, enzymes, metals, chelating agents, and antibiotics, were incorporated into PLA polymeric matrix to provide antimicrobial activity (Tawakkal et al. 2014). The incorporation of antimicrobial additives into PLA is a promising way to overcome microbial proliferation (Scaffaro et al. 2018). The most common methods to prepare PLA-based antimicrobial materials can be divided in two main approaches: melt processing and wet processing, each one presenting its own advantages and disadvantages. Melt processing has the advantage to involve equipment commonly used to process thermoplastic materials, thus ensuring easy scale up of the production volumes and solventless environments. These features reduce the overall environmental impact and the production costs; furthermore, they minimize the presence of solvents in the final device (Scaffaro et al. 2013). On the other hand, high temperature (PLA requires melting and molding temperature of 160–190 °C) can be a problem for those drugs that undergo thermodegradation or in presence of highly volatile compounds (Nostro et al. 2015). In these cases, wet processing can be preferred since it is carried out at ambient temperature (Scaffaro and Lopresti 2018). Furthermore, polymer solutions used in wet processing can enhance the dispersion in case of active particles or insoluble drugs, i.e., dispersed as separated phase.
This review focuses on the recent advances (from 2015 to date) on antimicrobial additives for PLA-based materials including their preparation, biocidal action, and application, thus updating previous reviews released on the same (Jamshidian et al. 2010; Pawar et al. 2014; Tawakkal et al. 2014) or different polymer (Appendini and Hotchkiss 2002; Kuorwel et al. 2011; Palza 2015; Huang et al. 2016). Among the reported studies, some evaluated the antimicrobial activity by the in vitro test in solid and liquid media; some others investigated the efficacy in a food model. Conversely, the antibiofilm efficacy and the in vivo assays received little attention. The potential of PLA was particularly investigated for use in antimicrobial food packaging and biomedical applications. The first section will be devoted to the essential oils (EOs), their components, and other compounds of natural origin that are the most investigated additives for PLA. Another section will be dedicated to active particles and nanoparticles such as silver or zinc oxide. Finally, the last section will focus on conventional and synthetic molecules added into PLA polymer. Figure 1 reports the schematic representation of the topic that will be discussed in the following sections.
Essential oils, their components, and other compounds of natural origin
The EOs are complex mixtures of plant secondary metabolites with high inhibitory potential against a wide spectrum of microorganisms. The most important limitations of their direct use, namely high hydrophobicity and volatility, can be overcome by their incorporation into polymeric materials. Table 1 reports the processing for the incorporation of different EOs and other compounds of natural origin into PLA polymer such as melt mixing (Chieng et al. 2015; Llana-Ruiz-Cabello et al. 2016; Râpă et al. 2016; Moustafa et al. 2017; Tawakkal et al. 2017), extrusion (Llana-Ruiz-Cabello et al. 2015; Moreno-Vásquez et al. 2017; Wang et al. 2017), solvent casting (Bonan et al. 2015; Qin et al. 2015; Ahmed et al. 2016a; Javidi et al. 2016; Liu et al. 2016; Yahyaoui et al. 2016; Yang and Song 2016; Ahmed et al. 2016b, c; George et al. 2017; Muller et al. 2017; Rezaeigolestani et al. 2017; Shavisi et al. 2017; Arfat et al. 2018; Milovanovic et al. 2018; Niu et al. 2018), and electrospinning (Jiang et al. 2015; Wen et al. 2016; Adomavičiute et al. 2017; Gomaa et al. 2017; Liu et al. 2017; Scaffaro et al. 2018). In some cases, the presence of additives such as β-cyclodextrin (β-CD) (Wen et al. 2016; Wang et al. 2017), chitosan (CS), nanoparticles (Liu et al. 2017), cellulose nanocrystals (George et al. 2017), maleic anhydride (Moreno-Vásquez et al. 2017), and nanoclays (Moustafa et al. 2017) were proposed in order to achieve higher thermal stability; reduce volatility of the active compound, masking unpleasant odors in the case of food packaging applications; and to control the release of drugs and flavors. In other cases, additives such as graphene oxide (GO) (Arfat et al. 2018) and kenaf (Tawakkal et al. 2017) were used to enhance the tensile strength of the material. In other circumstances, polymers such as tributyl o-acetyl citrate (ATBC) (Râpă et al. 2016), trimethylene carbonate (Qin et al. 2015), polyethylene glycol (PEG) (Chieng et al. 2015; Ahmed et al. 2016a, b; Muller et al. 2017; Arfat et al. 2018; Nepomuceno et al. 2018), poly(ε-caprolactone) (PCL) (Milovanovic et al. 2018), and cellulose acetate (Gomaa et al. 2017) were used as plasticizers to improve the processability and the ductility of the final material.
Different antimicrobial EOs such as cinnamon (Ahmed et al. 2016a, b, c; Wen et al. 2016; Liu et al. 2017), garlic (Ahmed et al. 2016a), clove (Ahmed et al. 2016a; Arfat et al. 2018), copaiba (Bonan et al. 2015), epoxidized palm oil (Chieng et al. 2015), lemongrass (Yang and Song 2016), rosemary, myrtle, thyme (Yahyaoui et al. 2016), and oregano (Javidi et al. 2016; Liu et al. 2016; Llana-Ruiz-Cabello et al. 2016) or their major active constituents including carvacrol (Scaffaro et al. 2018), cinnamaldehyde (Qin et al. 2015; Muller et al. 2017; Villegas et al. 2017), terpinen-4-ol (Nepomuceno et al. 2018), thymol (Tawakkal et al. 2017; Milovanovic et al. 2018), and thymoquinone (Gomaa et al. 2017) were incorporated in PLA. Considering the high number of variables such as kind and amount of EO, processing method, microbial strain, and antimicrobial test, it is very difficult to compare the different data.
Several papers documented the antimicrobial properties of oregano essential oil (OEO) added to PLA (Liu et al. 2016; Javidi et al. 2016; Llana-Ruiz-Cabello et al. 2016). Specifically, PLA/poly (trimethylene carbonate) films containing OEO exhibited strong antioxidant and antimicrobial activity against Escherichia coli and Listeria monocytogenes (log reduction of 3.5–3.6) (Liu et al. 2016; Javidi et al. 2016; Llana-Ruiz-Cabello et al. 2016). Javidi et al. (2016) reported the higher inhibition area of PLA films containing 1.5 wt% OEO detected by direct contact than that observed by vapor phase assay and described the significant delay of bacterial growth (reduction of colony-forming units/g) on rainbow trout fillets. Llana-Ruiz-Cabello et al. (2016) studied the greater antimicrobial activity of PLA films containing OEO 5–10 wt% against yeasts and molds and suggested a new active packaging for use in ready-to-eat salads. Similarly, a significant decrease in different microbial counts was observed in lettuce packaged in active ethylene-vinyl alcohol copolymer (EVOH)-coated polypropylene (PP) films containing OEO 7.5 wt% (Muriel-Galet et al. 2013). Regarding the OEO constituents, composite PLA films containing kenaf fibers (20 wt%) and thymol (30 wt%) significantly killed E. coli on chicken slice samples by direct food contact and also were effective against naturally occurring fungi by indirect food contact (Tawakkal et al. 2017). The death rate of E. coli in the presence of the PLA/kenaf/thymol was related to the concentration of thymol in the formulation and was higher than that detected for the PLA/thymol films. The population of E. coli decreased upon increasing the thymol concentration from 10 to 30 wt%, with a death rate of ca. 0.19/day. Recently, innovative supercritical fluid technology was employed to impregnate PLA/PCL films with thymol and thyme extract for potential use in packaging against Bacillus subtilis and E. coli (Villegas et al. 2017; Milovanovic et al. 2018) or to impregnate PLA films with cinnamaldehyde against E. coli and Staphylococcus aureus (Villegas et al. 2017). This technology exploits supercritical carbon dioxide allowing the addition of volatile compounds avoiding the limitations of the conventional methods such as the evaporation of the active substance (Milovanovic et al. 2018).
Among the EOs, also cinnamon essential oil (CEO) (Ahmed et al. 2016a, b, c; Wen et al. 2016; Liu et al. 2017) or its major component cinnamaldehyde (Qin et al. 2015; Muller et al. 2017; Villegas et al. 2017) incorporated into PLA-based materials showed antimicrobial activity. Liu et al. (2017) successfully encapsulated cinnamon essential oil into CS nanoparticles subsequently added in a PLA solution and electrospun together for active packaging applications. The nanoparticles enhanced the EO stability and retained the antimicrobial activity of the compound. Overall, 75% more cinnamon essential oil was released from the fiber with the highest concentration exhibiting a diffusion-swelling controlled process. Muller et al. (2017) developed antibacterial monolayer and bilayer films with PLA (NatureWorks® Ingeo4060D)/cinnamaldehyde and starch by compression molding of previously solvent casted films with a loading efficiency of 87%. The authors studied the release kinetics of the active compound into food simulants of differing polarities finding that Fick’s model fitted to the experimental points in each simulant. Occasionally, the inclusion of β-CD stabilized and improved the antimicrobial activity of PLA polymers containing CEO or allyl isothiocyanate (AITC) (Wen et al. 2016; Wang et al. 2017) despite the high polymer-processing temperature. Ahmed et al. (2016b, c) documented the efficacy of PLA (NatureWorks® Ingeo4043D)/CEO composite films also in a real food system such as chicken samples. The efficacy of PLA/CEO films was measured by evaluation of general indicators of microbial quality by the poultry industry such as total viable counts (TVC), lactic acid bacteria (LAB), Pseudomonas spp., and total coliform. The TVC, LAB, Pseudomonas, and total coliforms in the chicken samples wrapped with antimicrobial PLA/CEO films were less than 1.0 log colony-forming unit (CFU)/g during the entire storage period (day 0 to day 20). The efficacy of PLA/CEO films was also evaluated by performing a challenge test in chicken sample inoculated with L. monocytogenes and Salmonella enterica Typhimurium and storage at 4 °C for 17 days. The counts were reduced by 1.5 and 3 log cycles for L. monocytogenes and S. enterica Typhimurium, respectively. Additionally, a synergistic effect was observed between high-pressure treatment and the PLA/CEO films on survival of L. monocytogenes. CEO and clove oil-based PLA films exhibited higher activity against Campylobacter jejuni (approximately 7 log reduction) compared to the garlic oil-based films suggesting their use for preservation of poultry meats (Ahmed et al. 2016a). In a recent paper, Arfat et al. (2018) developed composite PLA (NatureWorks® Ingeo4043D) films with excellent antibacterial activity against S. aureus and E. coli by incorporating clove EO (CLO) (15–30 wt%) and graphene oxide nanosheets (1 wt%). After 7 days of incubation, about 7 log reductions of S. aureus and 6 log reductions of E. coli were achieved for films containing 30% CLO.
Other antimicrobial natural compounds such as plant extracts (Llana-Ruiz-Cabello et al. 2015), epigallocatechin gallate (Moreno-Vásquez et al. 2017), propolis (Rezaeigolestani et al. 2017; Shavisi et al. 2017), and rosin (Moustafa et al. 2017; Niu et al. 2018) were used to develop PLA polymeric materials for food packaging applications. Llana-Ruiz-Cabello et al. (2015) demonstrated the inhibiting activity of PLA (NatureWorks® Ingeo2003D) films containing Proallium®, a commercial product based on Allium spp. extract, against Enterobacteriaceae, aerobic bacteria, yeasts, and molds on ready-to-eat lettuce salads. Moreno-Vásquez et al. (2017) prepared antimicrobial PLA (NatureWorks® Ingeo4042D) films through extrusion incorporating a certain amount of PLA grafted maleic anhydride (PLA-gr) as a compatibilizing agent to increase the miscibility between neat PLA and epigallocatechin gallate (EGCG). EGCG diffusion from PLA films followed a Fickian behavior and after 7 days, the release of EGCG for PLA–EGCG and PLA-gr–EGCG was 2.40 and 3.01 wt%, respectively. For the authors, this result could indicate that the EGCG distribution in PLA-gr–EGCG was more homogeneous than PLA–EGCG, such that the surface contact between EGCG and deionized water was higher. Also, propolis in association with EOs or nanoparticles was successfully incorporated in PLA (FkuR kunststoffm GmbH, 197,000 g/mol) materials. In particular, combinations of propolis ethanolic extract (PEE) with Zataria multiflora Bioss. essential oil (ZME 1% v/v) (Rezaeigolestani et al. 2017) or Ziziphora clinopodioides essential oil (ZEO 1–2 wt%) (Shavisi et al. 2017), containing carvacrol and thymol as the most abundant constituents, included into PLA polymer showed higher antibacterial effects against Gram-positive and Gram-negative bacteria than those obtained with each single ZEO or PEE (Shavisi et al. 2017) or increased the shelf life in vacuum-packed cooked sausages (Rezaeigolestani et al. 2017).
Interestingly, novel perspectives in biomedical area such as drug release systems, treatment of periodontitis, and wound healing were suggested by PLA materials containing PPE and silver nanoparticles (Adomavičiute et al. 2017), copaiba oil (Bonan et al. 2015), epoxidized palm oil (Chieng et al. 2015), or thymoquinone (Gomaa et al. 2017). Combination of PPE and silver nanoparticles loaded in PLA (NatureWorks® Ingeo6202D) provided efficient antimicrobial protection and maintained viability of HaCaT cells indicating a possible application for wound healing (Adomavičiute et al. 2017). Bonan et al. (2015) added polyvinylpyrrolidone (PVP) in order to prepare PLA (NatureWorks®, 66,000 g/mol)/PVP electrospun blends containing copaiba oil. The authors demonstrated that the EO increased the diameter of the fibers, reduced the contact angle, and showed activity against S. aureus (inhibition zone of 20.3–21.5 mm) suggesting a potential use in controlled drug system. In addition, plasticized PLA-based (NatureWorks® Ingeo4042D) nanocomposites filled with graphene nanoplatelets and containing PEG and epoxidized palm oil exhibited potentiated antimicrobial activity (log reduction enhancing by adding the graphene nanoplatelets) against E. coli, S. typhimurium, S. aureus, and L. monocytogenes (Chieng et al. 2015). Gomaa et al. (2017) proposed thymoquinone (TQ)-loaded PLA (NatureWorks® Ingeo4043D)/cellulose acetate nanofibers for wound dressing applications. The authors demonstrated a loading efficiency of TQ in PLA ranging from 80 to 90.5% and the efficacy of this system to prevent bacterial infection and to accelerate the rate of in vivo wound closure reepithelialization.
Microbial biofilms represent a serious problem because microorganisms embedded in a self-produced extracellular polymeric substance are less susceptible to conventional treatment (Fux et al. 2005). Although several publications focused on PLA as a suitable matrix for the incorporation of antimicrobial compounds, there are limited reports on the effects of PLA containing natural compounds against microbial biofilm. As reported in Fig. 2, Scaffaro et al. (2018) studied the efficacy of PLA (NatureWorks® Ingeo2002D)/carvacrol electrospun membranes against S. aureus and Candida albicans up to 144 h and suggested the potential of nanofibers as new tools for skin and wound polymicrobial infections. The gradual release of carvacrol from PLA membranes (up to 90% of carvacrol released after 144 h with respect of the nominal CAR loaded in PLA) resulted in the antimicrobial activity for all the investigated time and reduced the biofilm production of S. aureus and C. albicans in single and mixed cultures (> 80%). Nepomuceno et al. (2018) proposed solution blow spinning as a particular approach to prepare PLA/poly(ethylene glycol) nanofibers containing terpinen-4-ol (up to 40 wt%) or chlorhexidine gluconate (up to 0.12 wt% used as control) and demonstrated their antimicrobial and antibiofilm activity (> 80–90%) against Aggregatibacter actinomycetemcomitans for potential treatment of aggressive periodontitis.
Antimicrobial peptides are another broad class of naturally occurring molecules that can be incorporated into PLA polymer. In particular, nisin is a bacteriocin approved as a food preservative because of its negligible toxicity and antibacterial effectiveness (Nostro et al. 2010; Scaffaro 2012). Jiang et al. (2015) described the S. aureus inhibition by nisin loaded into phosphorylated soybean protein isolate/PLA/zirconium dioxide nanofibrous membranes and suggested their use as a potential material in drug delivery, food active packaging, and wound dressing. Notably, PLA fortified with cellulose nanocrystals and E14LKK (a 14 residue, magainin-class peptide) or silver nanoparticles (control) were studied for their inhibitory effects against microorganisms (log reduction ≥ 8) commonly encountered in the food industry (George et al. 2017).
Active particles and nanoparticles
The processing approaches for the preparation of antimicrobial PLA-based polymer containing active particles and nanoparticles are reported in Table 2. For these systems, the particle dispersion is a crucial parameter for the performances of the material such as biocidal efficacy, mechanical properties, and barrier properties. In order to improve filler dispersion in solvent-based processing such as solvent casting (De Silva et al. 2015; Huang et al. 2015; Chu et al. 2017; Li et al. 2017) and electrospinning (Quirós et al. 2015; Adomavičiute et al. 2017), sonication of the polymeric solution is usually proposed (Huang et al. 2015; Quirós et al. 2015; Adomavičiute et al. 2017; Li et al. 2017). On the other hand, improvement of the particles dispersion in melt processing such as melt mixing (Tsou et al. 2017; Nootsuwan et al. 2018) and extrusion (Marra et al. 2016; Yang et al. 2016) is generally achieved by using masterbatch of PLA and particles (Marra et al. 2016) or by PLA functionalization (Yang et al. 2016).
Silver is known to have antibacterial effects since ancient times (Silver and Phung 1996) and its use in antimicrobial packaging is attracting intense interest in recent times. In this context, silver nanoparticles were incorporated into PLA polymer in order to provide antimicrobial efficacy (Adomavičiute et al. 2017; Chu et al. 2017; Li et al. 2017; Tsou et al. 2017; Nootsuwan et al. 2018). Li et al. (2017) described PLA (NatureWorks®, 280 kDa) nanocomposite films with different amounts of nanosilver (0.5 wt%) and nanotitanium dioxide (1–5 wt%) particles and demonstrated their good antimicrobial activity (CFU reduction > 4.5) toward E. coli and L. monocytogenes. The author also studied the migration of the nanoparticles into different media. For Ti nanoparticles, the maximum migration ratios for 3% (w/v) aqueous acetic acid were 2.19, 2.36, 3.12, and 3.5 μg/kg for PLA/Ti1%, PLA/Ti1%/Ag, PLA/Ti5%, and PLA/Ti5%/Ag, respectively. For 50% (v/v) aqueous ethanol, the maximum migration ratio amounts were 0.593, 0.72, 0.80, and 0.99 μg/kg. For the 50% (v/v) aqueous ethanol, the 3% (w/v) aqueous acetic acid shows a higher amount of Ti migration. This result was explained by dissolution experiments, which show that an acidic solution could more easily dissolve Ti or TiO2, compared to an organic solution. Tsou et al. (2017) added nanosilver-doped multiwall carbon nanotube (MWCNT-Ag) as active PLA (Cargill-Dow Biopolymer 4032D) filler to avoid the use of organic solvents, to improve tensile strength, thermostability, and antimicrobial activity in order to obtain novel materials for biomedical applications (Tsou et al. 2017). In a recent study, Nootsuwan et al. (2018) developed biodegradable hybrid materials between PLA- (NatureWorks® Ingeo2003D) and nanosilver-coated carbon black with good mechanical properties, electrical conductivity, and antimicrobial activity (inhibition zone 13–25 mm) toward S. aureus (CFU reduction 99.86%), B. subtilis, Micrococcus luteus, E. coli, Pseudomonas aeruginosa, and C. albicans and suggested a different application as novel materials for computer keyboards.
Other metal particles were proposed as additives in PLA-based antimicrobial food packaging. Marra et al. (2016) prepared PLA (NatureWorks® Ingeo4032D) filled with nano zinc oxide (ZnO) particles in a twin-screw extruder. PLA/ZnO masterbatch was first prepared with the aim to improve the dispersion of the filler within the PLA matrix. The addition of ZnO increased the Young modulus and the stress at yield point and decreased the O2 and CO2 permeability. Specifically, PLA film with 5 wt% of ZnO showed excellent activity against E. coli, with a bacterial reduction of 99.99% after 24 h. Chu et al. (2017) compared the effect of nano-Ag and nano-ZnO on PLA (NatureWorks®, 280 kDa) films prepared by solvent casting and demonstrated that the higher the content of nano-ZnO, the more white particles aggregated. This phenomenon affected the mechanical properties that resulted in lower elastic modulus and maximum strength than that of neat PLA as well as the water vapor permeability and opacity that were enhanced with respect to neat PLA film. Nevertheless, nano-Ag and particles nano-ZnO added alone and in combination into PLA films significantly improved the antimicrobial activity against E. coli. Again, ZnO nanoparticles deposited halloysite nanotubes incorporated into PLA matrix (NatureWorks® Ingeo3051D) as a reinforcing filler simultaneously increased the mechanical and the antimicrobial properties, reducing E. coli and S. aureus counts by more than 99% (De Silva et al. 2015). Huang et al. (2015) reported that graphene oxide loaded with ZnO nanoparticles (0.2 wt%) was mixed with PLA (Zhejiang Haizheng Biological Materials PLA290) to prepare nanocomposite films with strong ultraviolet resistance and antibacterial activity against S. aureus and E. coli, both in dark conditions and under light irradiation. In addition, the release of metal contained in the structure of metal-organic frameworks (MOF) gave rise to antimicrobial materials. In this context, a cobalt-MOF, Co-SIM-1, successfully embedded in PLA (NatureWorks® Ingeo2002D) electrospun matrix decreased the bacterial colonization and biofilm formation up to 30–40% of the surface of mats by P. putida and S. aureus (Quirós et al. 2015). The authors also found that the time profile for metal release took place during the first 24 h. For mats loaded with a lower amount of Co-SIM-1, the metal was released at a higher rate (Quirós et al. 2015).
Particular efficacy against plant pathogens, namely Xanthomonas axonopodis pv. vesicatoria and X. arboricola pv. pruni, was demonstrated by using a ternary system composed of cellulose and lignin filled into PLA (NatureWorks® Ingeo3251D) grafted with glycidyl methacrylate (Yang et al. 2016). The effectiveness of the reactive melt grafting and the high value of disintegration rate of the composites after 10 days revealed the potential to prevent the hazard of microbial contamination from post-harvest phases to the final users.
Conventional and synthetic molecules
The local treatment of microbial infections is clinically advantageous as it could reduce systemic drug administration and then avoid widespread harmful effects. The development of antimicrobial delivery systems based on localized antibiotic release at the site of infection is claimed as a way to limit antibiotic resistant strains, to prevent the appearance of biofilm and avoid secondary infection (Luo et al. 2017).
As reported in Table 3, PLA compounding with conventional and synthetic drugs is often carried out by incorporation of the drug during electrospinning (Llorens et al. 2015; Jiang et al. 2016; Moslem et al. 2016; Luo et al. 2017; Shahi et al. 2017). Electrospinning process permits the fabrication of non-woven mats composed of continuous fibers ranging from micro to nanometer diameters. The remarkable physicochemical properties of nanofibers such as high levels of flexibility, porosity, gas permeation, and surface-to-volume ratio make them ideal materials to be applied in the biomedical field. Another interesting approach is 3D printing that focuses on the on-demand production of anti-infective and chemotherapeutic filaments that can be used to create discs, beads, catheters, or any medical construct using a 3D printing system (Weisman et al. 2015; Hall Barrientos et al. 2017) and solvent casting(Weisman et al. 2015). Solvent casting approach was adopted for preparing both dense and porous antimicrobial films by eventually, addition of PEG into PLA as a water-soluble porogen agent (Concilio et al. 2015; Chitrattha and Phaechamud 2016). Moslem et al. (2016) reported that electrospun membranes of chitosan/PLA (Sigma-Aldrich, 59,800 g/mol)/imipenem were effective against the growth of E. coli (inhibition zone of 10–14 mm), allowed good proliferation of the fibroblast cells, and maintained up to 1 week the released imipenem. The system containing imipenem was indicated as a novel biocompatible and antibacterial scaffold used for wound and burns dressing. PLA matrix (Sigma-Aldrich, GF45989881) loaded and electrospun with levofloxacin or irgasan (triclosan) and collagen type I were examined. PLA systems were effective in inhibiting the growth of E. coli and S. aureus (inhibition zone equal to 21 mm for levofloxacin and 10 mm for irgasan) except PLA-collagen-levofloxacin which showed a regrowth of bacteria after 48 h (Hall Barrientos et al. 2017). Weisman et al. (2015) proposed a new class of bioactive 3D printing filaments using gentamicin sulfate (GS) for bone infection treatment and methotrexate (MTX) for inhibition of osteosarcoma. The author found that both molecules retained the antibacterial activity (inhibition zone 12.9–21.35 mm for GS) and the cancer growth-inhibiting cytostatic activity (inhibition of 65% of osteosarcoma cells proliferation for MTX) throughout the manufacturing process despite the heat required for this method. Moreover, the composite showed superior combination of strength, versatility, and enhanced drug delivery. Chitrattha and Phaechamud (2016) also documented the efficacy of PLA (NatureWorks® Ingeo2002D) film loaded with gentamicin sulfate against a wide variety of Gram-positive and Gram-negative microorganisms (inhibition zone of 27.17–35.67 mm) whereas PLA with metronidazole inhibited only Bacteroides fragilis (inhibition zone of 54–55 mm). They sustained the antimicrobial activity for a week indicating that PEG 400 filled in PLA enhanced the drug release of films. The authors explained this result considering the porous structure of the films and the high water solubility of PEG likely able to enhance the diffusivity of water and drug into the drug-loaded films. Scaffaro et al. (2017b) prepared antimicrobial PLA (NatureWorks® Ingeo2002D) sheets containing ciprofloxacin (CFX), chosen as model molecule since its melting temperature is higher than that of PLA processing temperature. The incorporation of graphene nanoplatelets (GnPs) improved the stiffness of the system and affected the release of ciprofloxacin without hindering the antimicrobial activity (inhibition zone of 42 mm for CFX and 35 mm for CFX/GnPs). In particular, the presence of GnPs reduced the burst release effect thus suggesting the potential ability of GnP for controlled drug release applications (Scaffaro et al. 2017b). PLA-based materials containing chitosan were also carriers for tetracycline with activity against S. aureus (inhibition zone of 11–35 mm) (Jiang et al. 2016). The concentration of S. aureus decreased rapidly (absorbance values from 0.9 to 0.04) with increasing Tet content (20%) at first, and then decreased slightly at Tet content beyond 20% (absorbance values from 0.04 to 0.02).
Microbial infections associated with medical devices represent a significant public health challenge (Darouiche 2004). The presence of biofilm-forming microorganisms increases this problem (Fux et al. 2005). Antibiotic-containing fibers hold great potential as an antibacterial and antibiofilm implant coating. Innovative biodegradable PLA-based films, containing different percentages of antimicrobial azo dyes, showed qualitative colorimetric biofilm inhibition against S. aureus and C. albicans and were indicated for biomedical and antimicrobial active packaging applications (Concilio et al. 2015). Shahi et al. (2017) deposited antibiotic-containing PLA (NatureWorks® Ingeo4060D) nanofibers on titanium dental implants. The authors first studied the in vitro antimicrobial properties against a multispecies peri-implantitis-relevant biofilm such as Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, and Aggregatibacter actinomycetemcomita, and then evaluated its effects on a pre-clinical animal model.
PLA electrospun microfibers filled with triclosan (TCS), ketoprofen (KTP), or their combination to obtain multifunctional scaffolds with anti-inflammatory and bactericide activities against E. coli and M. luteus were prepared (Llorens et al. 2015). Specifically, the authors studied the influence of different ratios between L- and D-lactide units on the polymer matrix crystallinity and on the release behavior (NatureWorks® Ingeo4032D and Ingeo2002D). In particular, release of TCS and KTP was found to be dependent on the stereoregularity of the polymer matrix and also on the intermolecular interactions potentially established in dual drug-loaded scaffolds. More in detail, PLA 2002D microfibers showed the highest release percentages, probably as a consequence of the decrease in trapping efficiency caused by their lower molecular orientation and less dense molecular arrangement if compared with PLA 4032D. In fact, TCS and KTP from PLA 2002D scaffolds after 8 h of exposure to PBS medium rose to 40 and 30%, respectively, while these percentages decreased to 30 and 5% for a similar exposure time when PLA 4032D (Fig. 3). Furthermore, a decrease of the release percentage and the release rate for both drugs was detected in the binary system. This feature demonstrates the potential interest of the studied binary system since the intrinsic cytotoxicity of TCS could be suppressed while the bactericide activity could be maintained (growth inhibition of 80–95%).
Release curves in PBS medium of TCS (□) and KTP (○) from single drug-loaded (a, c) and dual drug-loaded (b, d) PLA 2002D (a, b) and PLA 4032D (c, d) electrospun scaffolds. Reprint with permission of Springer Nature (Llorens et al. 2015)
Luo et al. (2017) proposed a novel no cytotoxic system consisting of electrospun PLA (NatureWorks® Ingeo2002D) fiber with sustained antibacterial properties (inhibition zone equal to 35 mm) filled with uncoated or encapsulated chlorhexidine (0.5 and 1% wt/wt). The encapsulation of chlorhexidine spheres by polyelectrolytes had a fundamental influence on the chlorhexidine release kinetics in H2O lowering the diffusion of the drug. The use for the treatment of persistent infections in medicine and dentistry was suggested.
Conclusion and future perspectives
PLA-based antimicrobial systems received considerable attention in both academic and industrial research. This mini-review summarizes the recent advances made in antimicrobial PLA-based polymers and highlights the potential of PLA systems as efficient stabilizers–carriers of various kinds of molecules.
Nowadays, there is an increasing consumer demands for fresh, high-quality, and natural foods packaged in environmentally friendly materials that prolong the shelf life. The physicochemical properties of PLA coupled to beneficial properties of incorporated molecules open up interesting perspectives and are likely to lead to the next generation of food packaging materials.
The antimicrobial PLA materials offer novel perspectives also in biomedical area such as drug release systems, wound healing, or coating for medical devices. The antimicrobial-releasing systems can be advantageous to reduce possible dose-dependent side effects and limit the phenomena of antibiotic resistance.
Despite the substantial progress on PLA polymers, further studies on their antibiofilm activity and in vivo studies are needed in order to design promising effective antimicrobial systems. A better understanding of this information will pave the way toward more applications in the near future.
Change history
24 July 2018
The published online version contains mistake in Tables 1 and 3. instead of using "g/mol" it was presented as "g/mol mol-1" and "g/mol-1".
References
Adomavičiute E, Pupkevičiute S, Juškaite V, Žilius M, Stanys S, Pavilonis A, Briedis V (2017) Formation and investigation of electrospun PLA materials with propolis extracts and silver nanoparticles for biomedical applications. J Nanomater 2017:11. https://doi.org/10.1155/2017/8612819
Ahmed J, Hiremath N, Jacob H (2016a) Antimicrobial, rheological, and thermal properties of plasticized polylactide films incorporated with essential oils to inhibit Staphylococcus aureus and Campylobacter jejuni. J Food Sci 81:E419–E429. https://doi.org/10.1111/1750-3841.13193
Ahmed J, Hiremath N, Jacob H (2016b) Efficacy of antimicrobial properties of polylactide/cinnamon oil film with and without high-pressure treatment against Listeria monocytogenes and Salmonella typhimurium inoculated in chicken sample. Food Packag Shelf Life 10:72–78. https://doi.org/10.1016/j.fpsl.2016.10.003
Ahmed J, Mulla MZ, Arfat YA (2016c) Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control 69:196–204. https://doi.org/10.1016/j.foodcont.2016.05.013
Appendini P, Hotchkiss JH (2002) Review of antimicrobial food packaging. Innovative Food Sci Emerg Technol 3:113–126. https://doi.org/10.1016/S1466-8564(02)00012-7
Arfat YA, Ahmed J, Ejaz M, Mullah M (2018) Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int J Biol Macromol 107:194–203. https://doi.org/10.1016/j.ijbiomac.2017.08.156
Bonan RF, Bonan PRF, Batista AUD, Sampaio FC, Albuquerque AJR, Moraes MCB, Mattoso LHC, Glenn GM, Medeiros ES, Oliveira JE (2015) In vitro antimicrobial activity of solution blow spun poly(lactic acid)/polyvinylpyrrolidone nanofibers loaded with Copaiba (Copaifera sp.) oil. Mater Sci Eng C 48:372–377. https://doi.org/10.1016/j.msec.2014.12.021
Chieng BW, Ibrahim NA, Yunus WMZW, Hussein MZ, Then YY, Loo YY (2015) Reinforcement of graphene nanoplatelets on plasticized poly(lactic acid) nanocomposites: mechanical, thermal, morphology, and antibacterial properties. J Appl Polym Sci 132:1–5. https://doi.org/10.1002/app.41652
Chitrattha S, Phaechamud T (2016) Porous poly(dl-lactic acid) matrix film with antimicrobial activities for wound dressing application. Mater Sci Eng C 58:1122–1130. https://doi.org/10.1016/j.msec.2015.09.083
Chu Z, Zhao T, Li L, Fan J, Qin Y (2017) Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials (Basel) 10:1–13. https://doi.org/10.3390/ma10060659
Concilio S, Iannelli P, Sessa L, Olivieri R, Porta A, De Santis F, Pantani R, Piotto S (2015) Biodegradable antimicrobial films based on poly(lactic acid) matrices and active azo compounds. J Appl Polym Sci 132:1–8. https://doi.org/10.1002/app.42357
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. https://doi.org/10.1056/NEJMra035415
De Silva RT, Pasbakhsh P, Lee SM, Kit AY (2015) ZnO deposited/encapsulated halloysite-poly (lactic acid) (PLA) nanocomposites for high performance packaging films with improved mechanical and antimicrobial properties. Appl Clay Sci 111:10–20. https://doi.org/10.1016/j.clay.2015.03.024
Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. https://doi.org/10.1016/j.tim.2004.11.010
George M, George M, Shen W, Qi Z, Bhatnagar A (2017) Characterization of cellulose nanocrystals and PLA based thin films with either silver or antimicrobial peptide. JOSR J Polym Text Eng 4:8–24. https://doi.org/10.9790/019X-04030824
Gomaa SF, Madkour TM, Moghannem S, El-Sherbiny IM (2017) New polylactic acid/ cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int J Biol Macromol 105:1148–1160. https://doi.org/10.1016/j.ijbiomac.2017.07.145
Hall Barrientos IJ, Paladino E, Szabó P, Brozio S, Hall PJ, Oseghale CI, Passarelli MK, Moug SJ, Black RA, Wilson CG, Zelkó R, Lamprou DA (2017) Electrospun collagen-based nanofibres: a sustainable material for improved antibiotic utilisation in tissue engineering applications. Int J Pharm 531:67–79. https://doi.org/10.1016/j.ijpharm.2017.08.071
Huang Y, Wang T, Zhao X, Wang X, Zhou L, Yang Y, Liao F, Ju Y (2015) Poly(lactic acid)/graphene oxide-ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J Chem Technol Biotechnol 90:1677–1684. https://doi.org/10.1002/jctb.4476
Huang KS, Yang CH, Huang SL, Chen CY, Lu YY, Lin YS (2016) Recent advances in antimicrobial polymers: a mini-review. Int J Mol Sci 17. https://doi.org/10.3390/ijms17091578
Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S (2010) Poly-lactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf 9:552–571. https://doi.org/10.1111/j.1541-4337.2010.00126.x
Javidi Z, Hosseini SF, Rezaei M (2016) Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT - Food Sci Technol 72:251–260. https://doi.org/10.1016/j.lwt.2016.04.052
Jiang S, Wang H, Chu C, Ma X, Sun M, Jiang S (2015) Synthesis of antimicrobial Nisin-phosphorylated soybean protein isolate/poly(l-lactic acid)/ZrO2 membranes. Int J Biol Macromol 72:502–509. https://doi.org/10.1016/j.ijbiomac.2014.08.041
Jiang S, Lv J, Ding M, Li Y, Wang H, Jiang S (2016) Release behavior of tetracycline hydrochloride loaded chitosan/poly(lactic acid) antimicrobial nanofibrous membranes. Mater Sci Eng C 59:86–91. https://doi.org/10.1016/j.msec.2015.10.005
Kenawy ER, Worley SD, Broughton R (2007) The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 8:1359–1384. https://doi.org/10.1021/bm061150q
Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2011) Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J Food Sci 76:R164–R177
Lau O-W, Wong S-K (2000) Contamination in food from packaging material. J Chromatogr A 882:255–270
Li W, Zhang C, Chi H, Li L, Lan T, Han P, Chen H, Qin Y (2017) Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 22:1170. https://doi.org/10.3390/molecules22071170
Liu D, Li H, Jiang L, Chuan Y, Yuan M, Chen H (2016) Characterization of active packaging films made from poly(lactic acid)/poly(trimethylene carbonate) incorporated with oregano essential oil. Molecules 21:695–709. https://doi.org/10.3390/molecules21060695
Liu Y, Wang S, Zhang R, Lan W, Qin W (2017) Development of poly(lactic acid)/chitosan fibers loaded with essential oil for antimicrobial applications. Nanomaterials 7:194–207. https://doi.org/10.3390/nano7070194
Llana-Ruiz-Cabello M, Pichardo S, Bãnos A, Núñez C, Bermúdez JM, Guillamón E, Aucejo S, Cameán AM (2015) Characterisation and evaluation of PLA films containing an extract of Allium spp. to be used in the packaging of ready-to-eat salads under controlled atmospheres. LWT - Food Sci Technol 64:1354–1361. https://doi.org/10.1016/j.lwt.2015.07.057
Llana-Ruiz-Cabello M, Pichardo S, Bermúdez JM, Baños A, Núñez C, Guillamón E, Aucejo S, Cameán AM (2016) Development of PLA films containing oregano essential oil (Origanum vulgare L. virens) intended for use in food packaging. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33:1374–1386. https://doi.org/10.1080/19440049.2016.1204666
Llorens E, del Valle LJ, Puiggalí J (2015) Electrospun scaffolds of polylactide with a different enantiomeric content and loaded with anti-inflammatory and antibacterial drugs. Macromol Res 23:636–648. https://doi.org/10.1007/s13233-015-3082-5
Luo D, Zhang X, Shahid S, Cattell MJ, Gould DJ, Sukhorukov GB (2017) Electrospun poly(lactic acid) fibers containing novel chlorhexidine particles with sustained antibacterial activity. Biomater Sci 5:111–119. https://doi.org/10.1039/C6BM00646A
Marra A, Silvestre C, Duraccio D, Cimmino S (2016) Polylactic acid/zinc oxide biocomposite films for food packaging application. Int J Biol Macromol 88:254–262. https://doi.org/10.1016/j.ijbiomac.2016.03.039
Milovanovic S, Hollermann G, Errenst C, Pajnik J, Frerich S, Kroll S, Rezwan K, Ivanovic J (2018) Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res Int 107:486–495. https://doi.org/10.1016/j.foodres.2018.02.065
Moreno-Vásquez MJ, Plascencia-Jatomea M, Sánchez-Valdes S, Castillo-Yáñez FJ, Ocaño-Higuera VM, Rodríguez-Félix F, Rosas-Burgos EC, Graciano-Verdugo AZ (2017) Preparation and characterization of films made of poly(l -lactic acid)/poly(l -lactic acid) grafted maleic anhydride/epigallocatechin gallate blends for antibacterial food packaging. J Plast Film Sheeting 33:10–34. https://doi.org/10.1177/8756087916631602
Moslem Z, Sadri M, Pebdeni AB (2016) Antimicrobial and cellular activity of poly(L-lactide)/chitosan/Imipenem antibiotic composite nanofibers. Fibers Polym 17:1336–1342. https://doi.org/10.1007/s12221-016-6425-8
Moustafa H, El Kissi N, Abou-Kandil AI, Abdel-Aziz MS, Dufresne A (2017) PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl Mater Interfaces 9:20132–20141. https://doi.org/10.1021/acsami.7b05557
Muller J, Casado Quesada A, González-Martínez C, Chiralt A (2017) Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur Polym J 96:316–325. https://doi.org/10.1016/j.eurpolymj.2017.09.009
Muriel-Galet V, Cerisuelo JP, López-Carballo G, Aucejo S, Gavara R, Hernández-Muñoz P (2013) Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 30:137–143
Nepomuceno NC, Barbosa MA, Bonan RF, Oliveira JE, Sampaio FC, Medeiros ES (2018) Antimicrobial activity of PLA/PEG nanofibers containing terpinen-4-ol against Aggregatibacter actinomycetemcomitans. J Appl Polym Sci 135:1–9. https://doi.org/10.1002/app.45782
Niu X, Liu Y, Song Y, Han J, Pan H (2018) Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr Polym 183:102–109. https://doi.org/10.1016/j.carbpol.2017.11.079
Nootsuwan N, Wattanathana W, Jongrungruangchok S, Veranitisagul C, Koonsaeng N, Laobuthee A (2018) Development of novel hybrid materials from polylactic acid and nano-silver coated carbon black with distinct antimicrobial and electrical properties. J Polym Res 25:90–104. https://doi.org/10.1007/s10965-018-1484-8
Nostro A, Scaffaro R, Ginestra G, D’Arrigo M, Botta L, Marino A, Bisignano G (2010) Control of biofilm formation by poly-ethylene-co-vinyl acetate films incorporating nisin. Appl Microbiol Biotechnol 87:729–737. https://doi.org/10.1007/s00253-010-2598-z
Nostro A, Scaffaro R, D’Arrigo M, Botta L, Filocamo A, Marino A, Bisignano G (2012) Study on carvacrol and cinnamaldehyde polymeric films: mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl Microbiol Biotechnol 96:1029–1038. https://doi.org/10.1007/s00253-012-4091-3
Nostro A, Scaffaro R, D’Arrigo M, Botta L, Filocamo A, Marino A, Bisignano G (2013) Development and characterization of essential oil component-based polymer films: a potential approach to reduce bacterial biofilm. Appl Microbiol Biotechnol 97:9515–9523. https://doi.org/10.1007/s00253-013-5196-z
Nostro A, Scaffaro R, Botta L, Filocamo A, Marino A, Bisignano G (2015) Effect of temperature on the release of carvacrol and cinnamaldehyde incorporated into polymeric systems to control growth and biofilms of Escherichia coli and Staphylococcus aureus. Biofouling 31:639–649. https://doi.org/10.1080/08927014.2015.1079703
Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16:2099–2116
Pawar RP, Tekale SU, Shisodia SU, Totre JT, Domb AJ (2014) Biomedical applications of poly(lactic acid). Rec Pat Regen Med 4:40–51. https://doi.org/10.2174/2210296504666140402235024
Qin Y, Yang J, Xue J (2015) Characterization of antimicrobial poly(lactic acid)/poly(trimethylene carbonate) films with cinnamaldehyde. J Mater Sci 50:1150–1158. https://doi.org/10.1007/s10853-014-8671-8
Quirós J, Boltes K, Aguado S, de Villoria RG, Vilatela JJ, Rosal R (2015) Antimicrobial metal-organic frameworks incorporated into electrospun fibers. Chem Eng J 262:189–197. https://doi.org/10.1016/j.cej.2014.09.104
Râpă M, Miteluţ AC, Tănase EE, Grosu E, Popescu P, Popa ME, Rosnes JT, Sivertsvik M, Darie-Niţă RN, Vasile C (2016) Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos Part B Eng 102:112–121. https://doi.org/10.1016/j.compositesb.2016.07.016
Rezaeigolestani M, Misaghi A, Khanjari A, Basti AA, Abdulkhani A, Fayazfar S (2017) Antimicrobial evaluation of novel poly-lactic acid based nanocomposites incorporated with bioactive compounds in-vitro and in refrigerated vacuum-packed cooked sausages. Int J Food Microbiol 260:1–10. https://doi.org/10.1016/j.ijfoodmicro.2017.08.006
Scaffaro RBLGG (2012) Photo-oxidative degradation of poly(ethylene-co-vinyl acetate)/nisin antimicrobial films. Polym Degrad Stab 97:653–660. https://doi.org/10.1016/j.polymdegradstab.2012.01.003
Scaffaro R, Lopresti F (2018) Processing, structure, property relationships and release kinetics of electrospun PLA/Carvacrol membranes. Eur Polym J 100C:165–171. https://doi.org/10.1016/j.eurpolymj.2018.01.035
Scaffaro R, Botta L, Sanfilippo M, Gallo G, Palazzolo G, Puglia AM (2013) Combining in the melt physical and biological properties of poly(caprolactone) and chlorhexidine to obtain antimicrobial surgical monofilaments. Appl Microbiol Biotechnol 97:99–109. https://doi.org/10.1007/s00253-012-4283-x
Scaffaro R, Lopresti F, Botta L, Maio A, Sutera F, Mistretta MC, La Mantia FP (2016) A facile and eco-friendly route to fabricate poly(lactic acid) scaffolds with graded pore size. J Vis Exp:1–8. https://doi.org/10.3791/54595
Scaffaro R, Botta L, Lopresti F, Maio A, Sutera F (2017a) Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 24:447–478. https://doi.org/10.1007/s10570-016-1143-3
Scaffaro R, Botta L, Maio A, Gallo G (2017b) PLA graphene nanoplatelets nanocomposites: physical properties and release kinetics of an antimicrobial agent. Compos Part B Eng 109:138–146. https://doi.org/10.1016/j.compositesb.2016.10.058
Scaffaro R, Lopresti F, Botta L (2017c) Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur Polym J 96:266–277. https://doi.org/10.1016/j.eurpolymj.2017.09.016
Scaffaro R, Lopresti F, D’Arrigo M, Marino A, Nostro A (2018) Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl Microbiol Biotechnol 102:4171–4181. https://doi.org/10.1007/s00253-018-8879-7
Shahi RG, Albuquerque MTP, Münchow EA, Blanchard SB, Gregory RL, Bottino MC (2017) Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 105:354–363. https://doi.org/10.1007/s10266-016-0268-z
Shavisi N, Akhondzadeh Basti A, Khanjari A, Misaghi A, Shahbazi Y, Hajjar Bargh A, Vanaki E (2017) In vitro antibacterial activity of polylactic acid film incorporated with ethanolic propolis extract and Ziziphora clinopodioides essential oil. J Food Qual Hazards Control 4:3–8
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Sousa C, Rodrigues D, Oliveira R, Song W, Mano JF, Azeredo J (2011) Superhydrophobic poly (L-lactic acid) surface as potential bacterial colonization substrate. AMB Express 1:34. https://doi.org/10.1186/2191-0855-1-34
Tawakkal ISMA, Cran MJ, Miltz J, Bigger SW (2014) A review of poly(lactic acid)-based materials for antimicrobial packaging. J Food Sci 79:R1477–R1490. https://doi.org/10.1111/1750-3841.12534
Tawakkal ISMA, Cran MJ, Bigger SW (2016) The influence of chemically treated natural fibers in poly(lactic acid) composites containing thymol. Polym Compos 39:1261–1272. https://doi.org/10.1002/pc.24062
Tawakkal ISMA, Cran MJ, Bigger SW (2017) Effect of poly(lactic acid)/kenaf composites incorporated with thymol on the antimicrobial activity of processed meat. J Food Process Preserv 41:1–12. https://doi.org/10.1111/jfpp.13145
Tsou CH, Yao WH, Lu YC, Tsou CY, Wu CS, Chen J, Wang RY, Su CC, Hung WS, De GM, Suen MC (2017) Antibacterial property and cytotoxicity of a poly(lactic acid)/nanosilver-doped multiwall carbon nanotube nanocomposite. Polymers (Basel) 9:100–112. https://doi.org/10.3390/polym9030100
Villegas C, Torres A, Rios M, Rojas A, Romero J, de Dicastillo CL, Valenzuela X, Galotto MJ, Guarda A (2017) Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res Int 99:650–659. https://doi.org/10.1016/j.foodres.2017.06.031
Wang J, Qiu C, Narsimhan G, Jin Z (2017) Preparation and characterization of ternary antimicrobial films of β-cyclodextrin/allyl isothiocyanate/polylactic acid for the enhancement of long-term controlled release. Materials (Basel) 10:1210–1221. https://doi.org/10.3390/ma10101210
Weisman JA, Nicholson JC, Tappa K, Jammalamadaka U, Wilson CG, Mills D (2015) Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int J Nanomedicine 10:357–370. https://doi.org/10.2147/IJN.S74811
Wen P, Zhu D-H, Feng K, Liu F-J, Lou W-Y, Li N, Zong M-H, Wu H (2016) Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem 196:996–1004. https://doi.org/10.1016/j.foodchem.2015.10.043
Yahyaoui M, Gordobil O, Herrera Díaz R, Abderrabba M, Labidi J (2016) Development of novel antimicrobial films based on poly(lactic acid) and essential oils. React Funct Polym 109:1–8. https://doi.org/10.1016/j.reactfunctpolym.2016.09.001
Yang H-J, Song KB (2016) Application of lemongrass oil-containing polylactic acid films to the packaging of pork sausages. Korean J Food Sci Anim Resour 36:421–426. https://doi.org/10.5851/kosfa.2016.36.3.421
Yang W, Fortunati E, Dominici F, Giovanale G, Mazzaglia A, Balestra GM, Kenny JM, Puglia D (2016) Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int J Biol Macromol 89:360–368. https://doi.org/10.1016/j.ijbiomac.2016.04.068
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The original version of this article was revised: The published online version contains mistake in Tables 1 and 3. instead of using “g/mol” it was presented as “g/mol mol-1” and “g/mol-1”.
Rights and permissions
About this article
Cite this article
Scaffaro, R., Lopresti, F., Marino, A. et al. Antimicrobial additives for poly(lactic acid) materials and their applications: current state and perspectives. Appl Microbiol Biotechnol 102, 7739–7756 (2018). https://doi.org/10.1007/s00253-018-9220-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9220-1