Abstract
The aim of this study was to evaluate the effect of poly-ethylene-co-vinyl acetate (EVA) films incorporating different concentrations (0.1%, 0.5% and 1%) of nisin on the biofilm-forming ability of Listeria monocytogenes ATCC 7644, Staphylococcus aureus 815 and Staphylococcus epidermidis ATCC 35984. Nisin was incorporated into two grades of EVA (EVA14 and EVA28) in the melt during a common film-blowing operation. The efficacy of EVA/nisin films was evaluated by biofilm biomass measurements and Live/Dead staining in combination with fluorescence microscopy. In order to evaluate whether the nisin incorporation could modify the film surface properties, contact angle measurements and scanning electron microscopy were performed. The results revealed the efficacy of EVA14/nisin films in reducing biofilm formation on their surfaces with more evident effect for S. epidermidis than L. monocytogenes and S. aureus strains. In contrast, EVA28/nisin films showed unsatisfactory activity. Fluorescence microscopy confirmed poor biofilm formation on EVA14/nisin films, also characterised by the presence of dead cells. The data presented in this study offer new potential applications for developing strategies aimed to improve the effect of antimicrobial agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polymeric films are widely known for their applications in biomedical, food and industrial fields. However, as many surfaces they are susceptible to bacterial colonization and biofilm formation (Hogt et al. 1986; Ji and Zhang 2009). Biofilm is the microbial lifestyle in natural and manmade environments. The initial microbial adhesion to surfaces is a complex process dependent on the non-specific interactions between bacteria and the surface, including van der Waals interactions, electrostatic forces, Lewis acid-base and hydrophobic interactions, the latter being the strongest of all long-range non-covalent forces (Doyle 2000). After initial attachment, the accumulation step in biofilm formation depends on the bacterial proliferation, exopolysaccharide matrix production and intercellular adhesion. Once firmly established, a biofilm can be very difficult to eradicate because the bacteria embedded in a self-produced extracellular polymeric substance exhibit increased resistance to sanitizers and commonly used biocides (Mah and O’Toole 2001; Smith and Hunter 2008).
Staphylococcus aureus, Staphylococcus epidermidis and Listeria monocytogenes are important pathogenic bacteria and represent a serious problem for the industrial environment and food manufactures because of their ability to adhere to different surfaces (polymers, metals, glass) and form biofilm (Le Magrex-Debar et al. 2000; Mafu et al. 1990; Norwood and Gilmour 1999; Rieu et al. 2008; Schlegelová et al. 2008). The attachment of bacteria with subsequent biofilm development in food processing environments is a potential source of contamination that may compromise food quality or cause diseases transmission with health hazards.
Recently, a keen attention has been focused on the development of antimicrobial polymeric films in order to provide efficacy in antibacterial surfaces (Ercolini et al. 2006; Joerger 2007; La Storia et al. 2008; Mauriello et al. 2004). In this context, polymers treated with natural agents preventing or at least interfering with the initial adhesion and subsequent biofilm formation, are a considerable achievement which offer an alternative to removing the adhered bacteria.
Poly (ethylene-co-vinyl acetate) copolymer (EVA) has several industrial applications. It is used typically for producing film for greenhouses covering (Scaffaro et al. 2009a), for food packaging applications (Kirwan and Strawbridge 2003) but also in systems for controlled drug release (Arnold et al. 2008). EVA is characterized by low friction coefficients and high adhesivity. For these properties, it is usually used in multilayer film as outer layer, to promote self-sealing and welding (Kirwan and Strawbridge 2003).
Nisin is a natural polypeptide, recognized as a safe (GRAS) antimicrobial. It has been studied for its antibacterial activity against Gram-positive bacteria both in laboratory media and in foods (Delves-Broughton and Gasson 1994) and for its antimicrobial activity when coated or incorporated into different polymers (Coma et al. 2001; Cutter et al. 2001; Guerra et al. 2005; Guiga et al. 2009; Jin and Zhang 2008; Jin et al. 2009; Kim et al. 2002; Lee et al. 2003, 2004; Liu et al. 2007, 2009; Mauriello et al. 2005; Neetoo et al. 2007; Siragusa et al. 1999, Tai et al. 2008).
Nisin has also been investigated for its possible therapeutic use as effective against multi-resistant Gram-positive cocci such as methicillin-resistant S. aureus and vancomycin-resistant enterococci (Brumfitt et al. 2002).
It is worth noting that although many papers deal with the use of nisin, only in a very few of these works was the nisin added to polymer by incorporating it in the melt (Cutter et al. 2001; Liu et al. 2009). The use of common processing equipments grants the possibility to produce large amounts of materials in a solventless system (for packaging applications for instance) with obvious positive implications from an environmental and economical point of view. Moreover, there is a lack of information about the antibiofilm activity of nisin when incorporated into polymeric films.
The aim of this study was to prepare films of EVA containing nisin by incorporating it into the matrix in the melt during a common film blowing operation and to evaluate the effect of these films on the bacterial biofilm-forming ability of L. monocytogenes, S. aureus and S. epidermidis strains.
Materials and methods
Polymeric films preparation
The polymers used in the frame of the present work were two grades of EVA copolymers kindly supplied by Polimeri Europa (Italy): EVA 14 (Greenflex FC 45, melt flow index 0.3 dg/min, melting temperature of 93°C), containing 14% of vinyl acetate and EVA 28 (Greenflex FC 45, melt flow index 2.5 dg/min, melting temperature of 74°C) containing 28% of vinyl acetate.
Two samples of nisin (Polichimica, Italy) were used as antimicrobial agents: nisin 1000 (nominal activity of 1,000 IU/mg) and nisin 8000 (8,000 IU/mg).
The films were prepared by film blowing. The matrix and nisin (at different concentrations, 0.1%, 0.5% and 1% by weight) were premixed at the solid state and therefore fed to a single screw extruder (Brabender, L/D = 25, D = 19) equipped with a film blowing unit. The temperature profile was 95–115–120–120°C from the first barrel zone to the dye. The screw speed was kept constant at 50 rpm for all tests. The residence time was approximately 90 s. A top processing temperature (120°C) was adopted in order to preserve the activity of nisin which can be reduced at temperature higher than 121°C although, as reported elsewhere (Scaffaro et al. 2009c), higher processing temperatures (140°C and 160°C in the dye ) seem not to influence the antimicrobial activity of the nisin likely due to the very short processing time for this kind of operation. The thickness of the obtained films was in the range of 70–100 microns.
Contact angle measurements
Contact angles measurements were performed on all samples using deionized water as a fluid by an FTA 1000 instrument.
Scanning electron microscopy
The morphology of the films was analysed by scanning electron microscopy (SEM; FEI Quanta 200 ESEM). The specimens were obtained by cutting them off directly from the films, therefore sputtering with gold to make them electrically conductive.
Experimental design
The bacteria used in this study were: L. monocytogenes ATCC 7644, S. aureus 815 clinically isolated and S. epidermidis ATCC 35984. S. aureus and S. epidermidis strains have been characterised for many biofilm-related properties including viscoelastic properties, ica and agr typing as previously reported (Blanco et al. 2005; Di Stefano et al. 2009). We used S. epidermidis ATCC 35984 because it is the positive control for biofilm production and also it may represent a potential source of contamination with health hazards.
Cultures were grown overnight in 10 mL of tryptic soy broth (L. monocytogenes) and tryptic soy broth +1% glucose (S. aureus and S. epidermidis) and diluted in growth medium to 5 × 105 CFU/ml as previously described (Nostro et al. 2007). Aliquotes of 3 ml were dispensed into glass test tubes containing either the EVA14 or the EVA28/nisin film (1 cm2). After incubation at 37°C for 24 h, the planktonic bacterial growth was evaluated by measuring the optical density at 492 nm and the biofilm formed on the polymeric films was evaluated by biofilm biomass measurement and by a Live/Dead BacLight viability kit (Molecular Probes).
Biofilm biomass measurement
The polymeric films were washed twice with sterile PBS (pH 7.4), dried, stained for 1 min with 0.1% safranin and washed with water. The stained biofilms were resuspended in acetic acid 30% (v/v) and biofilm biomass was quantitated by measuring the optical density at 492 nm. Each assay was performed in triplicate and repeated at least three times. The relative inhibition of biofilm formation was calculated as: 100−[(OD492 EVA with nisin/OD492 EVA without nisin)×100].
Live/Dead BacLight viability kit
The polymeric films were rinsed once with PBS and stained by using a Live/Dead BacLight viability kit (Molecular Probes). The solution (1 ml) containing SYTO 9 and propidium iodide mixed in a ratio of 1:1 was added to the film. The films were incubated at room temperature for 15 min in the dark. After incubation, residual stain was removed. The images were observed using a fluorescent microscope (Reichert) equipped with a halogen lamp, Neoplan 100/1.25 (oil) objective, and 1713 filter cube (Fluorescein; 490/510/520).
Statistical analysis
The percentage values of biofilm inhibition were analysed by gerarchic ANOVA tests following angular transformation. The differences between groups (different nisin concentrations) as well as within groups (different nisin) were considered significant at p < 0.05.
Results
In order to evaluate whether the nisin incorporation could modify films surface properties, contact angle measurements and scanning electron microscopy were performed. For EVA14/nisin films, both the presence of nisin 1000 and nisin 8000 caused a slight decrease of contact angle values when compared with the contact angle of the neat matrice (Table 1). This phenomenon was more evident at higher amounts of nisin.
As expected, a lower contact angle was observed with neat EVA 28 compared with EVA14, reasonably due to the higher amount of vinyl-acetate contained. Actually, incorporation of nisin 8000 caused a very slight decrease of contact angles in comparison with the value of neat EVA28. The morphologies of nisin 1000 and nisin 8000 are shown in Fig. 1a, b, respectively. Both samples presented an evident heterogeneity. Nisin was derived from milk and the commercial powder used in this work contained milk proteins, carbohydrates and sodium chloride. Indeed, it was possible to individuate aggregates in which nisin was dispersed together with other components. SEM micrographs of neat film showed the surface of these samples was smooth and homogeneous (Fig. 1c–d).
Regarding the film morphology, SEM micrographs showed a different surface of films containing nisin compared with that of the neat polymer (Fig. 2a–d). In particular, the EVA14 film containing the highest content of nisin 1000 (1%) (Fig. 2c), showed a surface uneven and rough whereas the film containing only the 0.1% of nisin 1000 (Fig. 2a) showed a surface very similar to that of the polymer without nisin. EVA14 containing 0.5% of nisin 1000 (Fig. 2b) exhibited a surface morphology with an intermediate roughness between that shown by the two previous samples.
The SEM micrographs of EVA14/nisin 8000 systems, not reported here for sake of brevity, showed a surface morphology very similar to EVA14/nisin 1000 systems.
The EVA28/nisin 8000 (1%) (Fig. 2d) showed again a very rough and uneven surface. Actually it was even greater than that shown by the EVA14/nisin 1000 (1%) sample.The results can be extended to all the samples of EVA28/nisin 8000 (0.1% and 0.5%) not reported here for sake of brevity.
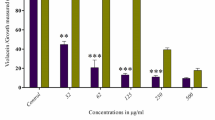
The data of growth and biofilm formation on EVA films are shown in Tables 2, 3, 4 and 5. A slight reduction (7–26%) of bacterial planktonic growth was observed in the presence of all EVA/nisin films, except for EVA/nisin 8000 (1%) films which caused a strong inhibition (87–92%) of L. monocytogenes planktonic growth. Results of biofilm biomass measurements showed that the biofilm was formed differently according to surfaces type and strain. Although a generally lower biofilm biomass was documented on the EVA28 films compared with EVA14 films, L. monocytogenes was proved to express minor ability to biofilm formation when compared with S. aureus and S. epidermidis, but produced a biofilm that was in according to the literature data (Harvey et al. 2007; Stepanović et al. 2004).
Regarding biofilm inhibition, the data revealed the efficacy of EVA14/nisin films in reducing biofilm production on their surfaces. Significant differences (p < 0.05) in biofilm formation values were observed between EVA14/nisin films and EVA14 films without nisin but not between nisin 1000 and 8000. Moreover, EVA14/nisin (1% and 0.5%) films produced a greater biofilm inhibition than EVA14/nisin (0.1%) films, with more evident effect for S. aureus and S. epidermidis compared with L. monocytogenes. However, a lower staphylococcal biofilm inhibition was observed in the presence of EVA14/nisin (1%) compared with EVA14/nisin (0.5%).
The situation was different for EVA28 films, with a slight biofilm inhibition observed in the presence of EVA28/nisin only for S. aureus and L. monocytogenes. The reduction (83%) of L. monocytogenes biofilm biomass on EVA28/nisin (1%) was probably related to the strong growth inhibition. Instead, a different behavior was found for S. epidermidis, the biofilm amount formed on EVA 28/nisin being more compared to the value obtained on the EVA28 films without nisin.
These findings were confirmed by fluorescence microscopy images which revealed poor biofilm formation on EVA14/nisin films, also characterised by the presence of dead cells. In contrast, biofilm formed on EVA14 films without nisin was dense with marked cell viability (Fig. 3).
Fluorescence microscopy images. L. monocytogenes ATCC 7644, S. aureus 815 and S. epidermidis ATCC 35984 biofilms formed on EVA14/nisin 1000 films were stained by using a Live/Dead BacLight viability kit. a Biofilm formed on EVA14 film; b biofilm formed on EVA14/nisin 1000 (0.5%) film; c biofilm formed on EVA14/nisin 1000 (1%) film. The green fluorescence labelled live cells, the red fluorescence labelled cells with damaged membranes
Discussion
During the past decades, the bacterial ability to adhere to surfaces (polymers, metals, glass) and form biofilm has represented a serious health problem. The development of polymeric films with different chemical compositions and surface properties activated by nisin could be a promising way of overcoming this problem. While previous studies have documented the efficacy of nisin when coated or incorporated into different polymers (Coma et al. 2001; Cutter et al. 2001; Guerra et al. 2005; Mauriello et al. 2005; Neetoo et al. 2007), limited information is available on the antibiofilm activity of nisin when incorporated into a blend polymeric film. To date, Bower et al. (1995) investigated the activity of the antimicrobial peptide after adsorption to silica surfaces and stated that nisin maintained activity and killed the adhered cells. The present manuscript shows for the first time the antibiofilm-activity of nisin when incorporated into polymeric matrix in the melt. Here we reported the effects of EVA blend films incorporating nisin on biofilm formation. In particular we demonstrated the efficacy antibiofilm of EVA14/nisin films and the unsatisfactory activity of EVA28/nisin.
In contrast, a poor activity of EVA/nisin films on planktonic growth was observed except for L. monocytogenes planktonic growth which showed a strong inhibition in the presence of EVA/nisin 8000 (1%) films. The possible cause of weak antibacterial activity of the films may be related to the effect of dilution (3 ml medium ) of nisin released (data of kinetics nisin release not shown) while the strong effect of EVA/nisin 8000 (1%) on L. monocytogenes may be due to both the nisin (8000) as well as the susceptibility of the strain. We used L. monocytogenes sensitive to nisin because it may show resistance in the sessile form (Norwood and Gilmour 2000).
Although the mechanism of biofilm inhibition is still unclear, the reason for the observed effects could be due to factors acting alone or in concert. The antimicrobial activity of nisin was largely attributed to the nisin-lipid II complex that interferes with the peptidoglycan synthesis and causes cytoplasmic membrane pore formation thus allowing the efflux of cellular material such as ions, ATP and nucleic acid (Abee et al. 1994; Chan et al. 1996; Wiedemann et al. 2001). Nisin incorporated into polymeric film could cause bacterial cell surface modification and compromise the initial attachment phase to EVA film.
In this context, it is also possible that the initial attachment phase was in part influenced by the hydrophobicity slight reduction of EVA/nisin films (documented by contact angle values) thereby leading to a less attractive strength and consequently a lower adhesion of hydrophobic staphylococci. The hydrophobicity appears to be the main factor influencing bacterial adhesion, particularly when films were challenged with S. aureus and S. epidermidis. On the other hand, even if there have been inconsistent reports (Cerca et al. 2005), it is known that generally bacteria with hydrophobic properties such as the staphylococci prefer hydrophobic material surfaces (Doyle 2000). Higashi et al. (1998) reported that hydrophobic interactions enhanced bacterial adhesion on polyethylene while diminished with hydrophilic protein adsorption. In according with our results, Bower et al. (1995) demonstrated that protein-free surfaces evoked greater adhesion than nisin-covered surfaces. In addition, the presence of milk proteins in the commercial powder could also contribute to the reduced biofilm-forming ability (Barnes et al. 1999).
Also the different biofilm amount of S. epidermidis formed on EVA 28 compared with the value obtained on the EVA14 film without nisin can be explained by the different intrinsic hydrophobicity of EVA14 and EVA28. As expected, a lower contact angle was observed with neat EVA 28 compared with EVA14, reasonably due to the higher amount of vinyl-acetate contained.
However, it should be noted that the surface homogenity changes occurred after nisin incorporation may be responsible for the lower staphylococcal biofilm inhibition on EVA14/nisin (1%) compared with EVA14/nisin (0.5%), as well as EVA28/nisin and EVA28 without nisin. From SEM images, the EVA14/nisin (1%) film and EVA28/ nisin 8000 (1%) displayed a slightly uneven surface with capes and roughness. It is known from literature (Scaffaro et al. 2009b; Tang et al. 2009) that surface roughness encourages bacterial adhesion. In fact, when bacteria grow on roughened surfaces are more numerous than the bacteria grown on smooth surfaces as the irregular surfaces provide a larger contact area of the cells with the substrate and the bacteria colonise the hills and valleys on the surface. Moreover, a high amount of nisin can cause a negative side effect, developing resistant strains that, as consequence, are non-sensitive to the nisin action (Chi-Zhang et al. 2004; Scaffaro et al. 2009c).
It should be noted also that nisin was incorporated into two grades of EVA (EVA14 and EVA28) in the melt during a common film blowing operation. The two polymers used in this work have the same chemical nature but different rheological properties and consequently different processability. The SEM micrographs revealed that the EVA28 based films have a rougher and more uneven surface if compared with the EVA14 based films. In the manuscript, for sake of brevity, it is reported only the sample with the 1% of nisin (Fig. 2d) but the results can be extended to all the samples. The higher adherent bacteria formed on EVA28/nisin 8000 0.1% with respect to EVA14/nisin 8000 0.1% was probably due to surface roughness which encourages bacterial adhesion.
Taken together these considerations suggest that it is important to take into account that the incorporated molecule retains its activity for the development of effective antimicrobial/antibiofilm polymeric films but it is equally essential to obtain a polymeric film with appropriate morphological and structural properties.
To the best of our knowledge, no study reporting to nisin antibiofilm-activity when incorporated into polymeric matrix in the melt is present in literature. So the results of this study contribute to enrich the literature data and could have important implications for the development and implementations of novel strategy and control measures either in medical, industrial and environmental area.
References
Abee T, Rombouts FM, Hugenholtz J, Guihard G, Letellier L (1994) Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol 60:1962–1968
Arnold RR, Wei HH, Simmons E, Tallury P, Barrow DA, Kalachandra S (2008) Antimicrobial activity and local release characteristics of chlorhexidine diacetate loaded within the dental copolymer matrix, ethylene vinyl acetate. J Biomed Mat Res Part B: Appl Biomat 86B:506–513
Barnes LM, Lo MF, Adams MR, Chamberlain AHL (1999) Effect of milk proteins on adhesion of bacteria to stainless steel surfaces. Appl Environ Microbiol 65:4543–4548
Blanco AR, Sudano-Roccaro A, Spoto GC, Nostro A, Rusciano D (2005) Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Ch 49:4339–4343
Bower CK, McGuire J, Daeschel MA (1995) Suppression of Listeria monocytogenes colonization following adsorption of nisin onto silica surfaces. Appl Environ Microbiol 61:992–997
Brumfitt W, Salton MRJ, Hamilton-Miller JMT (2002) Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Antimicrob Chemother 50:731–734
Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J (2005) Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res Microbiol 156:506–517
Chan WC, Leyland M, Clark J, Dodd HM, Lian LY, Gasson MJ, Bycroft BW, Roberts GCK (1996) Structure-activity relationships in the peptide antibiotic nisin: antibacterial activity of fragments of nisin. FEBS Lett 390:129–132
Chi-Zhang Y, Yam KL, Chikindas ML (2004) Effective control of Listeria monocytogenes by combination of nisin formulated and slow released into a broth system. Int J Food Microbiol 90:15–22
Coma V, Sebti I, Pardon P, Deschamps A, Pichavant FH (2001) Antimicrobial edile packaging based on cellulosic ethers, fatty acids and nisin incorporation to inhibit Listeria innocua and Staphylococcus aureus. J Food Prot 64:470–475
Cutter CN, Willett JL, Siragusa GR (2001) Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett Appl Microbiol 33:325–328
Delves-Broughton J, Gasson MJ (1994) Nisin. Natural antimicrobial system in food preservation. CAB International, Wallingford, UK
Di Stefano A, D’Aurizio E, Trubiani O, Grande R, Di Campli E, Di Giulio M, Di Bartolomeo S, Sozio P, Iannitelli A, Nostro A, Cellini L (2009) Viscoelastic properties of Staphylococcus aureus and Staphylococcus epidermidis mono-microbial biofilms. Microbial Biotechnology 2:634–641
Doyle R (2000) Contribution of the hydrophobic effect to microbial infection. Microb Infect 2:391–400
Ercolini D, La Storia A, Villani F, Mauriello G (2006) Effect of a bacteriocin-activated polythene film on Listeria monocytogenes as evaluated by viable staining and epifluorescence microscopy. J Appl Microbiol 100:765–772
Guerra NP, Araujo AB, Barrera AM, Agrasar AT, Macias CI, Carbacllo J, Pastrana L (2005) Antimicrobial activity of nisin adsorbed to surfaces commonly used in food industry. J Food Prot 68:1012–1019
Guiga W, Galland S, Peyrol E, Degraeve P, Carnet-Pantiez A, Sebti I (2009) Antimicrobial plastic film: physico-chemical characterization and nisin desorption modelling. Inn Food Sci Emerg Technol 10:203–207
Harvey J, Keenan KP, Gilmour A (2007) Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol 24:380–392
Higashi JM, Wang IW, Shlaes DM, Anderson JM, Marchamt RE (1998) Adhesion of Staphylococcus epidermidis and transposon mutant strains to hydrophobic polyethylene. J Biomed Mater Res 39:341–350
Hogt AH, Dankert J, Feijen J (1986) Adhesion of coagulase-negative staphylococci to methacrylate polymers and copolymers. J Biomed Mater Res 20:533–545
Ji J, Zhang W (2009) Bacterial behaviors on polymer surfaces with organic and inorganic antimicrobial compounds. J Biomed Mater Res 88:448–453
Jin T, Zhang H (2008) Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. J Food Sci 73:127–134
Jin T, Liu L, Zhang H, Hicks K (2009) Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int J Food Sci Technol 44:322–329
Joerger RD (2007) Antimicrobial films for food applications: an analysis of quantitative results. Packag Technol Sci 20:231–273
Kim YM, An DS, Park HJ, Park JM, Lee DS (2002) Properties of nisin-incorporated polymer coatings as antimicrobial packaging materials. Packag Technol Sci 15:247–254
Kirwan MJ, Strawbridge JW (2003) Plastics in food packaging. In: Coles R, McDowell D, Kirwan MJ (eds) Food packaging technology. Blackwell Publishing Ltd, London, pp 174–240
La Storia A, Ercolini D, Marinello F, Mauriello G (2008) Characterization of bacteriocin-coated antimicrobial polyethylene films by atomic force microscopy. J Food Sci 73:48–54
Le Magrex-Debar E, Lemoine J, Gellé MP, Jacquelin LF, Choisy C (2000) Evaluation of biohazards in dehydrated biofilms on foodstuff packaging. Int J Food Microbial 55:239–243
Lee CH, An DS, Park HJ, Lee DS (2003) Wide-spectrum antimicrobial packaging materials incorporating nisin and chitosan in the coating. Packag Technol Sci 16:99–106
Lee CH, An DS, Lee SC, Park HJ, Lee DS (2004) A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and α-tocopherol. J Food Engin 62:323–329
Liu LS, Finkenstadt VL, Liu CK, Jin T, Fishman ML, Hicks KB (2007) Preparation of poly(lactic acid) and pectin composite films intended for applications in antimicrobial packaging. J Appl Polym Sci 106:801–810
Liu L, Jin T, Coffin DR, Hicks KB (2009) Preparation of antimicrobial membranes: coextrusion of poly(lactic acid) and Nisaplin in the presence of Plasticizers. J Agric Food Chem 57:8392–8398
Mafu AA, Roy D, Goulet J, Magny P (1990) Attachment of Listeria monocytogenes to stainless steel, glass, polypropylene, and rubber surfaces after short contact times. J Food Prot 53:742–746
Mah TC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Mauriello G, Ercolini D, La Storia A, Casaburi A, Villani F (2004) Development of polyethene films for food packaging activated with an antilisterial bacteriocin from Lactobacillus curvatus 32Y. J Appl Microbiol 97:314–322
Mauriello G, De Luca E, La Storia A, Villani F, Ercolini D (2005) Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett Appl Microbiol 41:464–469
Neetoo H, Ye M, Chen H (2007) Effectiveness and stability of plastic films coated with nisin for inhibition of Listeria monocytogenes. J Food Prot 70:1267–1271
Norwood DE, Gilmour A (1999) Adherence of Listeria monocytogenes strains to stainless steel coupons. J Appl Microbiol 86:576–582
Norwood DE, Gilmour A (2000) The growth and resistance to sodium ipochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J Appl Microbiol 88:512–520
Nostro A, Sudano Roccaro A, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523
Rieu A, Lemaître JP, Guzzo J, Pivetau P (2008) Interactions in dual species biofilms between Listeria monocytogenes EGD-e and several strains of Staphylococcus aureus. Int J Food Microbiol 126:76–82
Scaffaro R, Botta L, La Mantia FP (2009a) Preparation and characterization of polyolefin-based nanocomposite blown films for agricultural applications. Macromol Mater Engin 294:445–454
Scaffaro R, Morreale M, Lo Re G, La Mantia FP (2009b) Degradation of mMater-Bi®/wood flour biocomposites in active sewage sludge. Polym Degrad Stab 94:1220–1229
Scaffaro R, Botta L, Marineo S, Puglia AM (2009c) Incorporation of nisin in poly (ethylene-co-vinyl acetate) films by melt processing: a study on the antimicrobial properties and the bacteriocin release. LWT (in press)
Schlegelová J, Babák V, Holasová M, Dendis M (2008) The biofilm-positive Staphylococcus epidermidis isolates in raw materials, foodstuffs and on contact surfaces in processing plants. Folia Microbiol 53:500–504
Siragusa GR, Cutter CN, Willett JL (1999) Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol 16:229–235
Smith K, Hunter IS (2008) Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol 57:966–973
Stepanović S, Cirković I, Ranin L, Svabić-Vlahović M (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol 38:428–432
Tai YC, McGuire J, Neff JA (2008) Nisin antimicrobial activity and structural characteristics at hydrophobic surfaces coated with the PEO–PPO–PEO triblock surfactant Pluronic® F108. J Colloid Interface Sci 322:104–111
Tang H, Cao T, Liang X, Wang A, Salley SO, McAllister J, Ng KY (2009) Influence of silicone surface roughness and hydrophobicity on adhesion and colonization of Staphylococcus epidermidis. J Biomed Mater Res A 88:454–463
Wiedemann I, Breukink I, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, Sahl HG (2001) Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem 276:1772–1779
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nostro, A., Scaffaro, R., Ginestra, G. et al. Control of biofilm formation by poly-ethylene-co-vinyl acetate films incorporating nisin. Appl Microbiol Biotechnol 87, 729–737 (2010). https://doi.org/10.1007/s00253-010-2598-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2598-z