Abstract
Butyl butyrate (BB) has been widely used as a flavor and fragrance compound in the beverage, food, perfume, and cosmetic industries. Currently, BB is produced through two-step processes; butanol and butyrate are first produced and are used as precursors for the esterification reactions to yield BB in the next step. Recently, an alternative process to the current process has been developed by using microorganisms for the one-pot BB production. In the one-pot BB process, alcohol acyl transferases (AATs) and lipases play roles in the esterification of butanol together with their co-substrates butyryl-CoA and butyrate, respectively. In this paper, we review the characteristics of two enzymes including AAT and lipase in the esterification reaction. Also, we review the one-pot processes for BB production by employing the wild-type and engineered Clostridium species and the engineered Escherichia coli strains, with the combination of AATs and lipases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Butyl butyrate (BB) is one of the short-chain esters known as a flavor and fragrance compound (Matte et al. 2016; Varma and Madras 2008; Zabetakis and Holden 1997). In nature, BB has routinely been found in flowers, fruits, and fermented beverages. The sweet and sour flavor found in nature is often due to the presence of BB together with other short-chain esters. Thus, BB has been used as a flavoring agent in the beverages, foods, perfumes, and cosmetic industries (Jenkins et al. 2013; Santos et al. 2007).
For the industrial-scale production of BB, the catalytic and enzymatic processes have been developed and used. In the catalytic BB process, the esterification reaction has been performed under the high temperature and pressure conditions by supplying precursors butanol and butyrate and using hydrofluoric acid and sulfuric acid as catalysts (Han and Zhou 2011). By using such acid catalysts, the process has caused some problems in terms of corrosiveness, formation of environmentally hazardous byproducts, and difficulty in catalyst recovery (Han and Zhou 2011; Liu and Zhang 2018; Park et al. 2017a). To overcome such problems in BB production, an enzymatic process has been developed by employing immobilized lipases, which catalyze esterification reaction under atmospheric condition (Kirdi et al. 2017; Yeom and Go 2018). It has been demonstrated that the lipase-catalyzed esterification reaction allowed production of various esters including BB with high conversion yields (Chowdary and Prapulla 2002; Dhake et al. 2012; Gim and Kim 2018; Lozano et al. 2002; Park et al. 2005).
In both current catalytic and enzymatic processes for BB production, the precursors butanol and butyrate should be externally supplemented. Thus, the current BB processes typically comprise two independent steps including the precursor production step and the esterification reaction step. As an alternative to the current processes, one-pot processes have recently been developed for BB production by employing microorganisms (Horton and Bennett 2006; Rodriguez et al. 2014). In these alternative processes, the precursors are produced as metabolic intermediates (or end-products) in microorganisms including Clostridium species and engineered Escherichia coli strains (Layton and Trinh 2014; Noh et al. 2018; Rodriguez et al. 2014).
In this paper, the characteristics of two key enzyme alcohol acyltransferase (AAT) and lipase involved in the esterification reaction for BB production in the one-pot processes are reviewed. The metabolic engineering strategies employed for BB production by microorganisms including C. acetobutylicum and E. coli are also reviewed. Furthermore, BB production by employing Clostridium species together with the extracellular lipases is reviewed. Finally, perspectives and future research directions are suggested.
AAT and lipase
AAT and lipase are used as key enzymes in the recent studies on the development of the one-pot processes for BB production (Horton et al. 2003; Langrand et al. 1990; Layton and Trinh 2016b). Thus, this section begins with a brief overview of their characteristics, which play important roles in the esterification reaction.
AAT
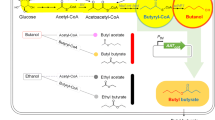
AAT is an enzyme catalyzing the condensation reaction of alcohols together with acyl-CoA to produce esters including BB (Fig. 1) (Günther et al. 2011; Nancolas et al. 2017; Olias et al. 1995; Salas 2004). Various AATs are found in yeasts as well as fruits including strawberry, banana, melon, and apple (Balbontin et al. 2010; Defilippi et al. 2005; Kruis et al. 2017). AATs are distinguished from wax synthase/diacylglycerol acyltransferases (WS/DGATs) by the substrate preference towards relatively shorter carbon length (Menendez-Bravo et al. 2017). For this reason, AATs have been employed for the production of short carbon chain esters like BB, while WS/DGATs have been used for the production of long carbon chain esters like biodiesel (d’Espaux et al. 2015; Kalscheuer et al. 2006; Kumar et al. 2018; Li et al. 2008; Park et al. 2017b; Sudheer et al. 2017).
In wild-type Saccharomyces cerevisiae, the AATs, ATF1 and ATF2, are encoded by the genes ATF1 and ATF2, respectively (Saerens et al. 2010). ATF1 and ATF2 are mainly involved in the formation of acetate esters in S. cerevisiae (Kruis et al. 2017; Li et al. 2018; Nancolas et al. 2017; Zhang et al. 2013). While a recent report indicated that ATF1 had a substrate preference for C4 to C6 alcohols and acetyl-CoA in an engineered E. coli strain (Layton and Trinh 2016a), the substrate preference of ATF2 has not yet been defined in detail. The homologs of ATF1 and ATF2 have been identified in other yeast strains, including Saccharomyces carlsbergensis, Candida glabrata, and Kluyveromyces lactis (Fujii et al. 1996; Fujiwara et al. 1999; Kim et al. 2017; Schneiderbanger et al. 2016; Van Laere et al. 2008; Zhang et al. 2014).
AATs have also been isolated from fruits, such as strawberry (SAAT and FaAAT2 from Fragaria × ananassa, VAAT from Fragaria vesca, and FcAAT1 from Fragaria chiloensis), banana (BanAAT from Musa sapientum), melon (MAAT from Cucumis melo), and apple (AAAT from Malus sp.) (Beekwilder et al. 2004; Defilippi et al. 2005). The AATs from such fruits have wide substrate specificities that range from C1 alcohols to C10 or higher alcohols (Beekwilder et al. 2004). Beekwilder et al. (2004) reported that SAAT exhibited its highest activity in a reaction supplemented with geraniol and acetyl-CoA, but this activity was reduced to 5% and 11% of the highest activity when geraniol was replaced by methanol and butanol, respectively. When butyryl-CoA was supplied as a co-substrate in the SAAT-mediated esterification reaction, the highest activity was reported when octanol was used as the second substrate, and 81% of this activity level was retained when butanol was used as the second substrate for BB production (Beekwilder et al. 2004). However, in the reaction of butanol together with butyryl-CoA, the exact kinetics were limited by FaAAT2, which has a Kcat/KM value of 0.04/s/μM (Cumplido-Laso et al. 2012).
Lipase
In contrast to AATs, which catalyze the esterification of alcohols with acyl-CoA, lipases catalyze the esterification of alcohols with acids to yield esters in the organic phase (Kim 2017). Thus, during the lipase-mediated production of BB, butanol and butyrate are used as precursors (Fig. 1). Lipase reacts with butyrate to yield a lipase-butyrate complex, followed by isomerization of the complex to form acyl-lipase intermediate. In the next step, acyl-lipase catches butanol to yield another complex, followed by isomerization of the complex to form butyl butyrate–lipase complex. In final, BB was released from the complex. Lipases can also catalyze the hydrolysis of fatty acid esters, including triacylglycerol, via their α/β-hydrolase activity (Haque et al. 2018; Jung et al. 2010; Langrand et al. 1990; Mancheno et al. 2003; Yu et al. 2012).
Lipases have been identified from various microorganisms, including Achromobacter sp., Bacillus sp., Burkholderia sp., Thermomyces lanuginosus, Candida antarctica, and Candida rugosa (Gupta et al. 2004; Martins et al. 2013; Selvam et al. 2013). In particular, Candida antarctica lipase B (CALB) is a well-known enzyme that robustly catalyzes diverse reactions, including the syntheses of flavor and fragrance esters (short-chain esters), biodiesels (long-chain esters), and modified glycerides. For commercial applications, CALB is typically used in an immobilized form under biphasic conditions because the free enzyme is unstable in the aqueous condition (Dhake et al. 2012; Hasan et al. 2006; Kim and Suh 2016). In a study for BB synthesis, kinetic parameters of a CALB lipase immobilized on acrylic resin, Novozym 435, were determined in the absence of the product: Vmax of 2.22 mol/g/h, KM with butanol of 530 mM, and KM with butyrate of 350 mM (Varma and Madras 2008). The immobilized CALB used in such work is routinely produced from recombinant yeast and fungi (Emond et al. 2010; Han et al. 2009; Tamalampudi et al. 2007).
Metabolic engineering of microorganisms for AAT-mediated BB production
In recent studies, AATs have been used to construct synthetic pathways for BB production in C. acetobutylicum and E. coli (Layton and Trinh 2014, 2016a, b; Noh et al. 2018). In the engineered microorganisms, BB was formed via butanol and butyryl-CoA, which were obtained from glucose catabolism or external supplementation. To generate these two precursors for BB production, the native pathway was used in C. acetobutylicum, whereas a synthetic pathway was constructed in E. coli (Layton and Trinh 2014; Noh et al. 2018; Rodriguez et al. 2014).
Wild-type C. acetobutylicum forms butanol via butyryl-CoA from glucose (Fig. 2), making the strain a promising host for BB production (Desai et al. 1999; Horton et al. 2003; Noh et al. 2018; Woo et al. 2018). In this organism, butyryl-CoA and butanol are formed from the sequential transformation of two acetyl-CoA molecules by four enzymes: thiolase, 3-hydroxybutyryl-CoA dehydrogenase, crotonase, and butyryl-CoA dehydrogenase (Jang et al. 2012; Wiesenborn et al. 1988; Yeon et al. 2016). Butyryl-CoA can also be formed by the reassimilation of butyric acid by CoA transferase (Desai et al. 1999; Lee et al. 2012; Wiesenborn et al. 1988). In a recent study, SAAT and AAAT from F. ananassa and Malus sp., respectively, were introduced into C. acetobutylicum (Fig. 2). The codon-optimized SAAT and AAAT genes were expressed under the control of the thl promoter (Noh et al. 2018). In anaerobic cultures of the engineered C. acetobutylicum strains, BB productions of 50.07 mg/L (SAAT) and 40.60 mg/L (AAAT) were achieved without precursor feeding (Table 1). The BB selectivity of this ester production was 84.8% and 87.4% for C. acetobutylicum strains harboring the SAAT and AAAT genes, respectively (Noh et al. 2018).
Metabolic pathway of the engineered C. acetobutylicum strain harboring the AAT genes for BB production (Noh et al. 2018). The SAAT and AAAT genes were introduced from Fragaria × ananassa and Malus sp., respectively, into C. acetobutylicum. Gene abbreviations encoding enzymes: ctfAB, CoA transferase; adhE1, aldehyde/alcohol dehydrogenase; buk, butyrate kinase; ptb, phosphotransbutyrylase; thl, thiolase; hbd, 3-hydroxybutyrate dehydrogenase; crt, crotonase; bcd, butyryl-CoA dehydrogenase
Unlike C. acetobutylicum, wild-type E. coli does not possess a native pathway for forming butanol and butyryl-CoA. Thus, genetic modules must be heterologously introduced to enable the generation of precursors if the goal is to produce BB in E. coli. In one study, to supply butyryl-CoA for BB production, Rodriguez et al. (2014) engineered an E. coli mutant, that had low levels of aldehyde and alcohol dehydrogenase activity, by introducing the branched-chain keto acid dehydrogenase complex (KDHC) operon from Pseudomonas putida. 2-Ketovalerate was externally supplied to yield butyryl-CoA via KDHC, as was butanol, which was not produced by the mutant E. coli (Fig. 3a). For BB production, the engineered E. coli strain was further transformed by constructs encoding one of two AATs: EHT1 from S. cerevisiae or the chloramphenicol acetyltransferase-encoding cat gene (Rodriguez et al. 2014). When the final strains were cultured in medium containing 3 g/L of 2-ketovalerate and 3 g/L butanol, the BB productions were 14.9 mg/L (ETH1 strain) and 10.6 mg/L (cat strain) (Table 1).
Metabolic pathway of the engineered E. coli strains harboring the AAT genes for BB production. a Butyryl-CoA was formed from the externally added 2-ketovalerate through the branched-chain keto acid dehydrogenase complex (KDHC) encoded from the P. putida bkdA1, bkdA2, bkdB, and lpdV genes (Rodriguez et al. 2014). The other precursor butanol was also externally supplied for BB production. The S. cerevisiae EHT1 and the chloramphenicol resistant cat genes were used for the esterification reaction. b Butyryl-CoA was formed from glucose through the chimeric butanol pathway involving the enzymes encoded from the atoB, hbd, crt, and ter genes (Layton and Trinh 2014). Butanol could form through enzymes encoded from the adh and adhB genes. The Fragaria × ananassa SAAT gene was used for the esterification reaction. c Butyryl-CoA was formed from the externally added butyrate through the acyl-CoA transferase encoded from the C. propionicum pct gene (Layton and Trinh 2016a). The other precursor butanol was formed from butyryl-CoA via the enzymes encoded from the adhB and endogenous adhE genes. The Fragaria × ananassa SAAT and Fragaria vesca VAAT genes were used for the esterification reaction. Abbreviations for microorganisms: Pp, P. putida; Zm, Z. mobilis; Ca, C. acetobutylicum; Td, Treponema denticola; Ec, E. coli; Cp, C. propionicum. Gene abbreviations encoding enzymes: bkdA1, 2-oxoisovalerate dehydrogenase α subunit; bkdA2, 2-oxoisovalerate dehydrogenase β subunit; bkdB, dihydrolipoyl transacylase; lpdV, dihydrolipoamide dehydrogenase; atoB, acetyl-CoA acetyltransferase; hbd, 3-hydroxybutyrate dehydrogenase; crt, crotonase; ter, trans-2-enoyl-CoA reductase; adhE, alcohol dehydrogenase; adhB, alcohol dehydrogenase II; pdc, pyruvate decarboxylase, pct, propionyl-CoA transferase
In the same year, another group used two different modules to generate butyryl-CoA and alcohol in an engineered E. coli (Layton and Trinh 2014). The butyryl-CoA-producing module was constructed on the basis of the butanol pathway known in C. acetobutylicum and previous work demonstrating butanol production in E. coli (Inui et al. 2008). An alcohol production module was constructed by cloning the Zymomonas mobilis adhB and pdc genes, which encode alcohol dehydrogenase II and pyruvate decarboxylase, respectively (Fig. 3b). To enable the esterification reaction, the SAAT gene was incorporated downstream of the T7 promoter in the butyryl-CoA production module. Culture of the mutant E. coli strain harboring these modules without feeding of any precursor yielded production of 0.75 mg/L for BB and 37.16 mg/L for ethyl butyrate (Layton and Trinh 2014). In a fermentation with in situ recovery using the same strain, Layton and Trinh (2014) obtained production of 36.83 mg/L for BB and 134.00 mg/L for ethyl butyrate (Table 1).
In subsequent studies, the same research group replaced the butyryl-CoA production module with the acyl-CoA transferase encoded by the Clostridium propionicum pct gene (Layton and Trinh 2016a, b). Butyrate was externally fed to form butyryl-CoA through acyl-CoA transferase in the engineered E. coli (Fig. 3c). Layton and Trinh (2016a) used the alcohol-forming enzymes encoded from the adhB and pdc genes and tested five different AATs for the esterification reaction in the mutant E. coli. Among the engineered E. coli strains, those harboring the SAAT and VAAT genes yielded BB productions of 47.63 mg/L and 2.76 mg/L, respectively, and ethyl butyrate productions of 134.43 mg/L and 141.60 mg/L, respectively (Layton and Trinh 2016a). Conversely, strains expressing ATF1, ATF2, and AeAT9 (Actinidia eriantha) exhibited negligible BB productions of 0.17–0.28 mg/L (Layton and Trinh 2016a).
BB production by employing Clostridium species and lipase supplementation
As Clostridium species can generate precursors for BB production, some studies have employed C. acetobutylicum, Clostridium beijerinckii, and Clostridium tyrobutyricum, together with lipases, to produce BB (Fig. 4 and Table 2). For example, hexadecane-extractive fed-batch fermentation of C. acetobutylicum in the presence of beads harboring immobilized Candida antarctica lipase B (CALB) yielded a BB production of 4.9 g/L from glucose (van den Berg et al. 2013).
Production of BB using Clostridium species together with lipases. a BB production using the solventogenic C. acetobutylicum, Clostridium sp. BOH3, and C. beijerinckii spo0A mutant (Seo et al. 2017; van den Berg et al. 2013; Xin et al. 2016). b BB production using the acidogenic C. tyrobutyricum (Zhang et al. 2017). Abbreviations: LCREx, externally added Candida rugosa lipase; CALBEx, externally added C. antarctica lipase B; LipaseBOH3, native lipase secreted from Clostridium sp. BOH3; butanolEx, externally added butanol; and butyrateEx, externally added butyrate. Gene abbreviations encoding enzymes: thl, thiolase; hbd, 3-hydroxybutyrate dehydrogenase; crt, crotonase; bcd, butyryl-CoA dehydrogenase; adhE1, aldehyde/alcohol dehydrogenase; ptb, phosphotransbutyrylase; buk, butyrate kinase; and pct, propionate:acetate CoA transferase
Clostridium sp. strain BOH3, which harbors native lipase activity, was recently used for BB production (Xin et al. 2016). This strain yielded 1.7 g/L BB from xylose under olive oil–based lipase induction and 6.3 g/L BB from xylose using an oil sludge remover for lipase induction and extraction (Xin et al. 2016). In the same study, BB production of 22.4 g/L was achieved from 70 g/L xylose and 7.9 g/L exogenous butyrate in kerosene-extractive fed-batch fermentation with externally supplemented Candida rugosa lipase (Table 2).
The C. beijerinckii spo0A mutant has also been tested for BB production, as it has a high capability for producing butanol and butyrate (Seo et al. 2017). In hexadecane-extractive batch-fermentation using the spo0A mutant, 3.32 g/L BB was produced from 60 g/L glucose and 5 g/L exogenous butanol (Table 2).
In a more recent study, the hyper butyrate producer, C. tyrobutyricum, was tested for BB production in medium containing exogenous butanol and CALB (Zhang et al. 2017). In hexadecane-extractive fermentation using C. tyrobutyricum, 34.7 g/L BB production was achieved by CALB from 80 g/L glucose and 10 g/L butanol (Table 2).
Conclusions
One-pot processes for producing BB have been developed by employing wild-type and engineered Clostridium species as well as engineered E. coli strains. In these processes, AATs and lipases contribute to esterifying butanol together with butyryl-CoA and butyrate, respectively, to yield BB. Butanol, butyryl-CoA, and butyrate are formed as metabolites in wild-type Clostridium species, and a number of studies have shown that these strains are promising hosts for BB production (Noh et al. 2018; Seo et al. 2017; van den Berg et al. 2013; Xin et al. 2016; Zhang et al. 2017). On the other hand, as wild-type E. coli does not produce butanol and butyryl-CoA, synthetic modules were constructed to form precursors for BB production in the engineered strains (Layton and Trinh 2014, 2016a, b; Rodriguez et al. 2014). Although the engineered and/or externally supplemented AATs and lipases function properly in these one-pot processes, the BB yields obtained to date are not sufficient to allow these strategies to replace the current catalytic and enzymatic processes. To overcome this hurdle, it will be necessary to improve the affinity (KM) of AATs for butanol and butyryl-CoA by evolutionary enzyme engineering. Moreover, for processes involving lipases, system metabolic engineering could be used to optimize the metabolic pathways of Clostridium to produce butanol and butyrate at a proper precursor ratio for BB production.
References
Balbontin C, Gaete-Eastman C, Fuentes L, Figueroa CR, Herrera R, Manriquez D, Latche A, Pech J-C, Moya-León MaA (2010) VpAAT1, a gene encoding an alcohol acyltransferase, is involved in ester biosynthesis during ripening of mountain papaya fruit. J Agr Food Chem 58:5114–5121
Beekwilder J, Alvarez-Huerta M, Neef E, Verstappen FW, Bouwmeester HJ, Aharoni A (2004) Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol 135:1865–1878
Chowdary GV, Prapulla SG (2002) The influence of water activity on the lipase catalyzed synthesis of butyl butyrate by transesterification. Process Biochem 38:393–397
Cumplido-Laso G, Medina-Puche L, Moyano E, Hoffmann T, Sinz Q, Ring L (2012) The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J Exp Bot 63:4275–4290
d’Espaux L, Mendez-Perez D, Li R, Keasling JD (2015) Synthetic biology for microbial production of lipid-based biofuels. Curr Opin Chem Biol 29:58–65
Defilippi BG, Kader AA, Dandekar AM (2005) Apple aroma: alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci 168:1199–1210
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Dhake KP, Thakare DD, Bhanage BM (2012) Lipase: a potential biocatalyst for the synthesis of valuable flavor and fragrance ester compounds. Flavour Frag J 28:71–83
Emond S, Montanier C, Nicaud J-M, Marty A, Monsan P, André I, Remaud-Siméon M (2010) New efficient recombinant expression system to engineer Candida antarctica lipase B. Appl Environ Microbiol 76:2684–2687
Fujii T, Yoshimoto H, Nagasawa N, Bogaki T, Tamai Y, Hamachi M (1996) Nucleotide sequences of alcohol acetyltransferase genes from lager brewing yeast, Saccharomyces carlsbergensis. Yeast 12:593–598
Fujiwara D, Kobayashi O, Yoshimoto H, Harashima S, Tamai Y (1999) Molecular mechanism of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast 15:1183–1197
Gim GH, Kim SW (2018) Optimization of cell disruption and transesterification of lipids from Botryococcus braunii LB572. Biotechnol Bioprocess Eng 23:550–556
Günther CS, Chervin C, Marsh KB, Newcomb RD, Souleyre EJ (2011) Characterisation of two alcohol acyltransferases from kiwifruit (Actinidia spp.) reveals distinct substrate preferences. Phytochem 72:700–710
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Han X, Zhou L (2011) Optimization of process variables in the synthesis of butyl butyrate using acid ionic liquid as catalyst. Chem Eng 172:459–466
Han S-Y, Pan Z-Y, Huang D-F, Ueda M, Wang X-N, Lin Y (2009) Highly efficient synthesis of ethyl hexanoate catalyzed by CALB-displaying Saccharomyces cerevisiae whole-cells in non-aqueous phase. J Mol Catal B Enzym 59:168–172
Haque MA, Hong SY, Hwang CE, Kim SC, Cho KM (2018) Cloning of an organophosphorus hydrolase (opdD) gene of Lactobacillus sakei WCP904 isolated from chlorpyrifos-impregnated kimchi and hydrolysis activities of its gene product for organophosphorus pesticides. Appl Biol Chem 61:643–651
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzyme Microb Tech 39:235–251
Horton CE, Bennett GN (2006) Ester production in E. coli and C. acetobutylicum. Enzym Microb Technol 38:937–943
Horton CE, Huang K-X, Bennett GN, Rudolph FB (2003) Heterologous expression of the Saccharomyces cerevisiae alcohol acetyltransferase genes in Clostridium acetobutylicum and Escherichia coli for the production of isoamyl acetate. J Ind Microbiol Biotechnol 30:427–432
Inui M, Suda M, Kimura S, Yasuda K, Suzuki H, Toda H, Yamamoto S, Okino S, Suzuki N, Yukawa H (2008) Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl Microbiol Biotechnol 77:1305–1316
Jang Y-S, Lee JY, Lee J, Park JH, Im JA, Eom M-H, Lee J, Lee S-H, Song H, Cho J-H (2012) Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. mBio 3:e00314–12
Jenkins RW, Munro M, Nash S, Chuck CJ (2013) Potential renewable oxygenated biofuels for the aviation and road transport sectors. Fuel 103:593–599
Jung S-M, Park Y-C, Park K (2010) Effects of environmental conditions and methanol feeding strategy on lipase-mediated biodiesel production using soybean oil. Biotechnol Bioprocess Eng 15:614–619
Kalscheuer R, Stöveken T, Luftmann H, Malkus U, Reichelt R, Steinbüchel A (2006) Neutral lipid biosynthesis in engineered Escherichia coli: jojoba oil-like wax esters and fatty acid butyl esters. Appl Environ Microbiol 72:1373–1379
Kim J (2017) Surface display of lipolytic enzyme, lipase A and lipase B of Bacillus subtilis on the Bacillus subtilis spore. Biotechnol Bioprocess Eng 22:462–468
Kim RJ, Suh MC (2016) The GxSxG motif of Arabidopsis monoacylglycerol lipase (MAGL6 and MAGL8) is essential for their enzyme activities. Appl Biol Chem 59:833–840
Kim D-H, Jeon Y-J, Chung M-J, Seo J-G, Ro Y-T (2017) Complete sequence and gene analysis of a cryptic plasmid pLU4 in Lactobacillus reuteri strain LU4 (KCTC 12397BP). Appl Biol Chem 60:145–153
Kirdi R, Ben Akacha N, Messaoudi Y, Gargouri M (2017) Enhanced synthesis of isoamyl acetate using liquid-gas biphasic system by the transesterification reaction of isoamyl alcohol obtained from fusel oil. Biotechnol Bioprocess Eng 22:413–422
Kruis AJ, Levisson M, Mars AE, van der Ploeg M, Daza FG, Ellena V, Kengen SW, van der Oost J, Weusthuis RA (2017) Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab Eng 41:92–101
Kumar V, Kumar R, Rawat D, Nanda M (2018) Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl Biol Chem 61:7–13
Langrand G, Rondot N, Triantaphylides C, Baratti J (1990) Short chain flavour esters synthesis by microbial lipases. Biotechnol Lett 12:581–586
Layton DS, Trinh CT (2014) Engineering modular ester fermentative pathways in Escherichia coli. Metab Eng 26:77–88
Layton DS, Trinh CT (2016a) Expanding the modular ester fermentative pathways for combinatorial biosynthesis of esters from volatile organic acids. Biotechnol Bioeng 113:1764–1776
Layton DS, Trinh CT (2016b) Microbial synthesis of a branched-chain ester platform from organic waste carboxylates. Metab Eng Commun 3:245–251
Lee J, Jang Y-S, Choi SJ, Im JA, Song H, Cho JH, Papoutsakis ET, Bennett GN, Lee SY (2012) Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl Environ Microbiol 78:1416–1423
Li F, Wu X, Lam P, Bird D, Zheng H, Samuels L, Jetter R, Kunst L (2008) Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol 148:97–107
Li W, Cui D-Y, Wang J-H, Liu X-E, Xu J, Zhou Z, Zhang C-Y, Chen Y-F, Xiao D-G (2018) Overexpression of different alcohol acetyltransferase genes with BAT2 deletion in Saccharomyces cerevisiae affects acetate esters and higher alcohols. Eur Food Res Technol 244:555–564
Liu T, Zhang J (2018) High-level expression and characterization of Aspergillus niger ATCC 1015 xylanase B in Komagataella phaffii. Appl Biol Chem 61:373–381
Lozano P, Pérez-Marın A, De Diego T, Gomez D, Paolucci-Jeanjean D, Belleville M, Rios G, Iborra J (2002) Active membranes coated with immobilized Candida antarctica lipase B: preparation and application for continuous butyl butyrate synthesis in organic media. J Membr Biol 201:55–64
Mancheno JM, Pernas MA, Martınez MJ, Ochoa B, Rúa ML, Hermoso JA (2003) Structural insights into the lipase/esterase behavior in the Candida rugosa lipases family: crystal structure of the lipase 2 isoenzyme at 1.97 Å resolution. J Mol Biol 332:1059–1069
Martins AB, Friedrich JL, Cavalheiro JC, Garcia-Galan C, Barbosa O, Ayub MA, Fernandez-Lafuente R, Rodrigues RC (2013) Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene–divinylbenzene beads. Bioresour Technol 134:417–422
Matte CR, Bordinhão C, Poppe JK, Rodrigues RC, Hertz PF, Ayub MA (2016) Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. J Mol Catal B Enzym 127:67–75
Menendez-Bravo S, Comba S, Gramajo H, Arabolaza A (2017) Metabolic engineering of microorganisms for the production of structurally diverse esters. Appl Microbiol Biotechnol 101:3043–3053
Nancolas B, Bull ID, Stenner R, Dufour V, Curnow P (2017) Saccharomyces cerevisiae Atf1p is an alcohol acetyltransferase and a thioesterase in vitro. Yeast 34:239–251
Noh HJ, Woo JE, Lee SY, Jang Y-S (2018) Metabolic engineering of Clostridium acetobutylicum for the production of butyl butyrate. Appl Microbiol Biotechnol 102:8319–8327
Olias JM, Sanz C, Rios J, Perez AG (1995) Substrate specificity of alcohol acyltransferase from strawberry and banana fruits. ACS Symp Ser (USA) 596:134–141
Park S-C, Chang W-J, Lee S-M, Kim Y-J, Koo Y-M (2005) Lipase-catalyzed transesterification in several reaction systems: an application of room temperature lonic liquids for bi-phasic production of n-butyl acetate. Biotechnol Bioprocess Eng 10:99–102
Park S-J, Kim D-H, Yoo J, Hwang EY, Shin M-S, Lee N-T, Cho I-R, Kang H-G, Kim Y-J, Park S, Kim Y-W (2017a) Detection of organophosphate bound butyrylcholinesterase using a monoclonal antibody. Appl Biol Chem 60:233–240
Park S, Kim K, Han S-I, Kim EJ, Choi Y-E (2017b) Organic solvent-free lipid extraction from wet Aurantiochytrium sp. biomass for co-production of biodiesel and value-added products. Appl Biol Chem 60:101–108
Rodriguez GM, Tashiro Y, Atsumi S (2014) Expanding ester biosynthesis in Escherichia coli. Nat Chem Biol 10:259–265
Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol 3:165–177
Salas JJ (2004) Characterization of alcohol acyltransferase from olive fruit. J Agr Food Chem 52:3155–3158
Santos JC, Nunes GF, Moreira AB, Perez VH, de Castro HF (2007) Characterization of Candida rugosa lipase immobilized on poly (N-methylolacrylamide) and its application in butyl butyrate synthesis. Chem Eng Technol 30:1255–1261
Schneiderbanger H, Koob J, Poltinger S, Jacob F, Hutzler M (2016) Gene expression in wheat beer yeast strains and the synthesis of acetate esters. J I Brewing 122:403–411
Selvam K, Vishnupriya B, Maanvizhi M (2013) Enzymatic synthesis of fragrance ester by lipase from marine Actinomycetes for textile industry. Int J Eng Adv Technol 3:91–96
Seo S-O, Wang Y, Lu T, Jin Y-S, Blaschek HP (2017) Characterization of a Clostridium beijerinckii spo0A mutant and its application for butyl butyrate production. Biotechnol Bioeng 114:106–112
Sudheer PDVN, Yun J, Chauhan S, Kang TJ, Choi K-Y (2017) Screening, expression, and characterization of Baeyer-Villiger monooxygenases for the production of 9-(nonanoyloxy)nonanoic acid from oleic acid. Biotechnol Bioprocess Eng 22:717–724
Tamalampudi S, Talukder MMR, Hama S, Tanino T, Suzuki Y, Kondo A, Fukuda H (2007) Development of recombinant Aspergillus oryzae whole-cell biocatalyst expressing lipase-encoding gene from Candida antarctica. Appl Microbiol Biotechnol 75:387–395
van den Berg C, Heeres AS, van der Wielen LAM, Straathof AJJ (2013) Simultaneous clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate. Biotechnol Bioeng 110:137–142
Van Laere SD, Saerens SM, Verstrepen KJ, Van Dijck P, Thevelein JM, Delvaux FR (2008) Flavour formation in fungi: characterisation of KlAtf, the Kluyveromyces lactis orthologue of the Saccharomyces cerevisiae alcohol acetyltransferases Atf1 and Atf2. Appl Microbiol Biotechnol 78:783–792
Varma MN, Madras G (2008) Kinetics of synthesis of butyl butyrate by esterification and transesterification in supercritical carbon dioxide. J Chem Technol Biotechnol 83:1135–1144
Wiesenborn DP, Rudolph FB, Papoutsakis ET (1988) Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl Environ Microbiol 54:2717–2722
Woo JE, Lee SY, Jang Y-S (2018) Effects of nutritional enrichment on acid production from degenerated (non-solventogenic) Clostridium acetobutylicum strain M5. Appl Biol Chem 61:469–472
Xin F, Basu A, Yang K-L, He J (2016) Strategies for production of butanol and butyl-butyrate through lipase-catalyzed esterification. Bioresour Technol 202:214–219
Yeom SH, Go YW (2018) Optimization of a novel two-step process comprising re-esterification and transesterification in a single reactor for biodiesel production using waste cooking oil. Biotechnol Bioprocess Eng 23:432–441
Yeon YJ, Park H-Y, Park K, Park HJ, Yoo YJ (2016) Structural basis for the substrate specificity of 3-hydroxybutyrate dehydrogenase. Biotechnol Bioprocess Eng 21:364–372
Yu Y, Wu D, Liu C, Zhao Z, Yang Y, Li Q (2012) Lipase/esterase-catalyzed synthesis of aliphatic polyesters via polycondensation: a review. Process Biochem 47:1027–1036
Zabetakis I, Holden MA (1997) Strawberry flavour: analysis and biosynthesis. J Sci Food Agric 74:421–434
Zhang C-Y, Liu Y-L, Qi Y-N, Zhang J-W, Dai L-H, Lin X, Xiao D-G (2013) Increased esters and decreased higher alcohols production by engineered brewer’s yeast strains. Eur Food Res Technol 236:1009–1014
Zhang J, Zhang C, Qi Y, Dai L, Ma H, Guo X, Xiao D (2014) Acetate ester production by Chinese yellow rice wine yeast overexpressing the alcohol acetyltransferase-encoding gene ATF2. Genet Mol Res 13:9735–9746
Zhang ZT, Taylor S, Wang Y (2017) In situ esterification and extractive fermentation for butyl butyrate production with Clostridium tyrobutyricum. Biotechnol Bioeng 114:1428–1437
Funding
This work was supported by a grant from the Ministry of Science and ICT (MSIT) through the National Research Foundation (NRF) of Korea (NRF-2016R1D1A3B04933184). SYL was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the MSIT through the NRF of Korea (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noh, H.J., Lee, S.Y. & Jang, YS. Microbial production of butyl butyrate, a flavor and fragrance compound. Appl Microbiol Biotechnol 103, 2079–2086 (2019). https://doi.org/10.1007/s00253-018-09603-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09603-z