Abstract
Esters are formed by the condensation of acids with alcohols. The esters isoamyl acetate and butyl butyrate are used for food and beverage flavorings. Alcohol acetyltransferase is one enzyme responsible for the production of esters from acetyl-CoA and different alcohol substrates. The genes ATF1 and ATF2, encoding alcohol acetyltransferases from the yeast Saccharomyces cerevisiae have been sequenced and characterized. The production of acids and alcohols in mass quantities by the industrially important Clostridium acetobutylicum makes it a potential organism for exploitation of alcohol acetyltransferase activity. This report focuses on the heterologous expression of the alcohol acetyltransferases in Escherichia coli and C. acetobutylicum. ATF1 and ATF2 were cloned and expressed in E. coli and ATF2 was expressed in C. acetobutylicum. Isoamyl acetate production from the substrate isoamyl alcohol in E. coli and C. acetobutylicum cultures was determined by head-space gas analysis. Alcohol acetyltransferase I produced more than twice as much isoamyl acetate as alcohol acetyltransferase II when expressed from a high-copy expression vector. The effect of substrate levels on ester production was explored in the two bacterial hosts to demonstrate the efficacy of utilizing ATF1and ATF2 in bacteria for ester production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The technology of producing "natural" flavorings from a microorganism may prove to be very useful to food and beverage industries as well as to the perfume industry. An advantage of producing flavor compounds such as esters via microbial fermentation is the elimination of chemical synthesis. The populace is becoming more concerned with having "natural" products, and since microbial metabolites are biologically derived chemical compounds, this is an attractive method for producing these esters.
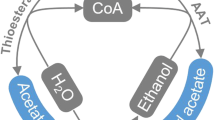
The food industry in particular is interested in the production of esters for use as flavoring compounds. Examples of such esters include butyl butyrate, ethyl acetate, and isoamyl acetate. Butyl butyrate, noted for its strong pineapple flavoring, has also been used as an additive for pear, butter, and butterscotch artificial flavoring. Ethyl acetate and isoamyl acetate are the two major products used for enhancing the flavor of beer and wine [3]. These esters were originally thought to be produced by the action of esterases and lipases [9, 15]. However, the enzyme alcohol acetyltransferase (AATase) is additionally responsible for the conversion of acetyl-CoA and alcohols to their corresponding acetate esters [4, 11, 13, 17].
The genes for two AATases, ATF1 and ATF2, have been cloned, sequenced, and characterized from Saccharomyces cerevisiae. AATases will bind to a variety of substrates; however, binding is preferential for acetyl-CoA and straight-chain alcohols. The ability to create large quantities of an ester, or to genetically alter a host for the production of a stronger or altered ester scent, would have many industrial applications. In an analysis of the formation of esters in sake wine, the yeast genes ATF1 and ATF2 were identified and analyzed for the purpose of producing more flavorful alcohols by increasing ester production during the yeast fermentation process [4, 13, 16, 17]. Fujii et al. [3] have used mutational analysis to develop altered yeast strains that produce a variable flavor of wine by increasing isoamyl acetate concentrations.
Clostridium acetobutylicum has been utilized in industry for acid and solvent production, and ethanol and butanol, which are produced in large amounts, have been used as fuel sources, precursors for explosives production, and various other solvent uses [6, 7, 14]. The ability of C. acetobutylicum to produce high concentrations of acids and solvents from simple starting materials without the need for aeration makes it an attractive alternative for production of these materials. Thus, expressing the AATases in C. acetobutylicum may lead to a new system for producing esters.
Materials and methods
Bacterial strains and plasmids
The host E. coli strain TOPO (Invitrogen) was used for cloning purposes and strain DH10β (mcrA, ΔmcrBC, rec A1) (Gibco BRL) for expression and methylation of plasmid DNA for transformation into C. acetobutylicum. C. acetobutylicum ATCC 824 (Rockville, Md., USA) and strain M5 [2] were used for expressing target DNA in solvent producing strains.
Construction of Plasmids. pPTB
The shuttle vector pSA12 [18], which has origins of replication for E. coli and C. acetobutylicum and confers erythromycin resistance, was used as a template to create pPTB. The constitutive promoter for phosphotransbutyrylase ( ptb) without its associated ribosomal binding site was inserted between the Sal I and Bam HI sites of pSA12 as confirmed by DNA sequencing analysis (Fig. 1) and was expressed in both E. coli and C. acetobutylicum.
pPTB. Shuttle vector used to express ATF2 in both Escherichia coli and Clostridium acetobutylicum. Origins of replication Ori II and ColEI are indicated. The ptb promoter was inserted in a multi-cloning region for ease of expressing numerous genes. The Bam HI site truncates the promoter region 10 bp before the putative ribosomal binding site as determined by sequence analysis
pD8/3
Alcohol acetyltransferase II (AATase II) was PCR-amplified from genomic DNA of S. cerevisiae strain W303. The forward primer, 5′-GCGGATCCAATAAAAATTGGGAGGGATAATTATGGAAGATATAGAAGG-3′ contains a Bam HI restriction site at its 5′ end (underlined), a ribosomal binding site (italics ) and is complementary to the region upstream of the gene. The reverse primer, 5′CATCTAGACGCTACGGCAGTATCGCATTAAAGCG-3′ contains an Xba I restriction site (underlined) and is complementary to the region containing and immediately following the stop codon for the gene. The resulting PCR product was ligated to the pBAD-TOPO cloning vector (Invitrogen, Carlsbad, Calif., USA) according to the manufacturer's protocol. After transformation, selection, and characterization of the constructed plasmid, it was digested with the appropriate enzymes to isolate the 1.7-kb gene fragment. This was ligated to the purified Bam HI-XbaI digested pPTB. Selection and characterization yielded pD8/3. ATF2 was sequenced from pD8/3 and found to be intact and in frame. The gene was positioned as expected under the control of the constitutively active PTB promoter.
pTrc-ATF1/pTrc-ATF2
Plasmid pTrcHIS (Invitrogen) was used for subcloning ATF1 and ATF2. The same primers used for PCR amplification of ATF2 for insertion in pPTB were used to amplify the gene. The forward primer for amplification of ATF1 was 5′-TATACCATCAAGGGATCAGCTCTCATGAATGAA-3′ and the reverse was 5′-TCAAGCATCATGTGAGATCTAAGGGCCTAAAAG-3′. PCR product was ligated to the cloning vector which was then used to transform the host strain according to the manufacturer's instructions. The supplied plasmid pTrc-LacZ was used as a control for all experiments with pTrcHIS derivatives.
DNA isolation, transformation, and manipulation
E. coli plasmid DNA was purified for sequencing and transformation using a Qiaprep Mini/Midi Kit according to the manufacturer's instructions (Qiagen, Chatsworth, Calif., USA). Restriction endonuclease enzymes and T4 ligase were purchased from New England Biolabs (Beverly, Mass., USA) and used according to the manufacturer's instructions. E. coli strains were transformed using a standard protocol (DH10β) or according to the manufacturer's instructions (TOPO). Prior to transformation with C. acetobutylicum, pD8/3 and pPTB were methylated in E. coli harboring the methylating plasmid pAN1 [12].
Media and growth conditions
E. coli strains were grown aerobically in 5 ml Luria-Bertani (LB) broth at 37 °C in a shaker or anaerobically in LB without shaking in airtight sealed vials. E. coli cultures were supplemented with erythromycin or ampicillin to a concentration of 400 μg and 100 μg per ml, respectively, and 10 mM isoamyl alcohol where appropriate. C. acetobutylicum cultures were grown anaerobically at 37 °C in clostridial growth media (CGM) [8]. Transformed strains were selected on Reinforced Clostridial Media (Difco, Detroit, Mich., USA) or CGM agar plates supplemented with 40 μg erythromycin/ml. Cultures for analysis were grown in the presence of 15 mM isoamyl alcohol unless otherwise indicated.
Preparation of cell-free extracts
E. coli (250 ml cultures) were grown as described above. The cultures were centrifuged at 5,000 g to pellet the cells which were resuspended in Tris-HCl buffer (pH 7.8), 1 mM dithiothreitol, and 10% glycerol, and lysed by incubation with lysozyme and subsequent sonication. Cell debris was separated from the lysate by 20-min centrifugation at 20,000 g. The lysate was filtered through 0.22-µm filters (TPP, Midwest Scientific, St. Louis, Mo., USA) before analysis.
Gas chromatography
Isoamyl acetate was quantified upon separation on an 80/100 mesh Poropak QS 6-foot glass column (2-mm I.D.; Alltech, Deerfield, Ill., USA) using a HP 5890 Series II gas chromatograph equipped with a flame ionization detector with nitrogen as the carrier gas. The injector and detector temperatures were 215 °C and 245 °C, respectively. One ml of head-space gas from each of the sealed cultures or lysates was analyzed at 220 °C for 15 min. The culture supernatant was analyzed for acids and alcohols to determine the stage of growth and to confirm C. acetobutylicum strain ATCC 824 vs. strain M5 as the host strain. The temperature profile for analysis of the supernatant was initially 125 °C for 5 min followed by a 30 °C/min ramp to 190 °C and held for 6 min before another 30 °C/min ramp to 220 °C and then held for 20 min. The supernatant samples were acidified before injection.
Results
Isoamyl acetate production by alcohol acetyltransferase in E. coli
The genes for S. cerevisiae AATase I and AATase II were PCR-cloned and inserted in the pTrcHIS cloning vector for rapid detection and to allow for a simple comparison of expression. Cultures of E. coli expressing ATF1 and ATF2 from pTrc-ATF1 and pTrc-ATF2, and the control pTrc-LacZ were grown for 18 or 40 h as described above and analyzed for ester content (Fig. 2a). Cultures expressing ATF1 consistently produced more ester than those expressing ATF2. The 5-ml cultures harboring pTrc-ATF1 produced 1.8 mM isoamyl acetate whereas the cultures with pTrc-ATF2 produced 0.52 mM. The OD600 of the cultures expressing ATF1 were consistently lower than those expressing ATF2. Cultures of E. coli expressing ATF2 from pD8/3 produced more isoamyl acetate than those harboring pTrc-ATF2 (Fig. 2).
Isoamyl acetate production in a E. coli transformed with pTrc-ATF1 or pTrc-ATF2 and b E. coli and C. acetobutylicum transformed with pD8/3. Cultures of E. coli and C. acetobutylicum expressing ATF1 or ATF2 were sampled after 18 (black) and 40 (gray) h. Head-space gas was analyzed by gas chromatography (GC) and standardized to determine ester concentration. Error bars One standard deviation from the mean, a n =6, b n =3
Isoamyl acetate production in cell-free extracts
E. coli lysates were standardized to 5 mg/ml total protein concentration. Ten mM isoamyl alcohol and 0.8 mM acetyl-CoA were added to a 2-ml reaction mixture in a 10-ml vial, sealed, and incubated at room temperature for 1 h. Each vial was placed in a 50 °C water bath for 30 min before analysis by gas chromatography. Lysates containing AATase I produced 0.39±0.14 mM isoamyl acetate whereas lysates containing AATase II produced 0.15±0.03 mM ester. No ester was produced in the control strain LacZ.
Isoamyl acetate production in C. acetobutylicum
The shuttle vector pPTB was used to express ATF2 in E. coli and C. acetobutylicum. Cultures of C. acetobutylicum expressing ATF2 from pD8/3 and the control vector pPTB were grown anaerobically for 18 and 40 h as described above. Isoamyl alcohol was added to a concentration of 15 mM for isoamyl acetate production. Isoamyl acetate was detected and compared to levels produced in E. coli (Fig. 2b). Cultures of C. acetobutylicum produced approximately half the isoamyl acetate that was produced in E. coli. Additionally, ester levels were lower in the 40-h cultures of C. acetobutylicum than in the 18-h cultures, suggesting hydrolysis of the ester. No ester was produced by the control strain.
Isoamyl acetate degradation in C. acetobutylicum
C. acetobutylicum has the potential to degrade isoamyl acetate based on its ability to convert acetate and butyrate to their derivatives. This was evidenced by the decrease in ester after 40 h growth vs. 18 h growth. Wild-type strain 824 and mutant strain M5 without vectors were grown in CGM with 2.5 mM isoamyl acetate and incubated for 30 h to determine whether the ester is actively degraded. The ester was reduced to 2.0 mM in the media control without C. acetobutylicum, but was actively reduced to almost zero in cultures of wild-type C. acetobutylicum ATCC 824 and to 0.5 mM in cultures of mutant strain M5 (Fig. 3). The decreased degradation of ester by mutant strain M5 may be due to slower growth of that strain.
Isoamyl acetate degradation by C. acetobutylicum ATCC 824 was greater than that by mutant strain M5. Cultures of C. acetobutylicum strains ATCC 824 and M5 and a control of clostridial growth media (CGM) were incubated with 2.5 mM isoamyl acetate for 18 or 40 h before head-space gas analysis by GC. Ester degradation was determined by comparison to a fresh standard of 2.5 mM isoamyl acetate. Error bars One standard deviation from the mean, n =3
Effect of alcohol substrate levels on isoamyl acetate production
Cultures of C. acetobutylicum strains ATCC 824 and M5 expressing ATF2 were grown with increasing levels of isoamyl alcohol (from 0 to 30 mM). Strain M5 lacks the extrachromosomal plasmid pSOL1, which contains the genes for acetone and butanol production ( adhE) and has a CoA transferase gene ( ctfA/ctfB). The level of isoamyl acetate increased with the corresponding increase in substrate concentration up to 25 mM (Fig. 4). Isoamyl acetate levels were also higher in the wild-type than in strain M5. Although C. acetobutylicum ATCC 824 degrades the ester more efficiently than strain M5, the accumulation of ester in C. acetobutylicum ATCC 824 cultures expressing ATF2 is five-fold that of the mutant M5 strain expressing ATF2 at the substrate level of 25 mM isoamyl alcohol (Fig. 4).
Isoamyl acetate levels in C. acetobutylicum increase with corresponding increase in concentrations of the substrate isoamyl alcohol. Cultures of C. acetobutylicum strains ATCC 824 and M5 harboring the ATF2 -bearing plasmid, pD8/3, were grown in 5 ml CGM with a range of substrate concentrations from 0 to 30 mM isoamyl alcohol for 30 h at 37 °C before analysis for ester production. One ml of head-space gas was used for analysis by GC, n =3
Availability of acetyl-CoA is a factor in isoamyl acetate concentrations
Acetyl-CoA levels vary among bacterial strains and can fluctuate during growth. To determine whether the AATases have a threshold for ester production based on acetyl-CoA levels, acetyl-CoA was added to crude lysates in increments from 0 to 3.2 mM with 10 mM isoamyl alcohol. Increasing the level of acetyl-coA available to the AATases in vitro resulted in a corresponding increase in ester concentration (Fig. 5). This effect was more pronounced in lysates from strains expressing ATF1 than from those expressing ATF2. For AATase I, ester continued to accumulate up to 2.8 mM acetyl-CoA (ester synthesized=1.3 mM) whereas ester concentrations from the AATase II extracts leveled off at a lower level.
Isoamyl acetate production (mM) in cell-free extracts with increasing levels of acetyl-CoA. Ester levels increase with the corresponding increase of acetyl-CoA. Cell-free extracts ( n =1) of E. coli harboring pTrc-ATF1 or pTrc-ATF2 were incubated for 1 h at 25 °C. One ml of head-space gas was analyzed by GC for the presence of isoamyl acetate
Discussion
The alcohol acetyltransferases from S. cerevisiae have been well characterized in yeast, but their activities have not been well studied in other expression systems. We found that ATF1 and ATF2 expression in E. coli was similar to that reported for S. cerevisiae. As reported for AATases in yeast, isoamyl acetate concentrations were greater in E. coli cultures expressing ATF1 than in those expressing ATF2. Expression of ATF1 and ATF2 from multi-copy plasmids in yeast results in 11- and six-fold increases in AATase activity, respectively [4]. Additionally in yeast, 80% of the isoamyl acetate produced during fermentation has been determined to come from AATase I using a null mutant, atf1 Δ [5]. A comparison of AATase activity in E. coli shows that twice as much ester was produced from cultures expressing ATF1 than from those expressing ATF2.
An increase in either isoamyl alcohol or in acetyl-CoA addition correlated directly to an increase in the quantity of ester produced by the enzymes. Ester accumulation was also greater in in-vitro assays of AATase I vs. AATase II from E. coli. This was the case when either acetyl-CoA or isoamyl alcohol substrate levels were increased, and is consistent with what has been reported previously in S. cerevisiae. The ester levels synthesized from E. coli cell lysates were 39 µmol·h−1·mg−1 total protein (AATase I) and 15 µmol·h−1·mg−1 total protein (AATase II) compared to 0.237 µmol·h−1·mg−1 total protein reported for yeast cell lysates [10].
C. acetobutylicum ATCC 824 expressing ATF2 produced levels of isoamyl acetate similar to those produced by E. coli only when the concentration of isoamyl alcohol was increased to 25 mM. To date, ATF1 has not been able to be inserted into an appropriate shuttle vector for expression in C. acetobutylicum. Evidence suggests that C. acetobutylicum expressing ATF genes will produce higher concentrations of ester during early growth periods than during later growth. This may be due to a number of factors. Firstly, acetyl-CoA pools in C. acetobutylicum vary during growth and among strains. Acetyl-CoA levels in C. acetobutylicum drop from approximately 436 µM during acidogenesis to 316 µM during solventogenesis, and acetyl-CoA levels in strain M5 are 70% that of C. acetobutylicum ATCC 824 [1]. Thus, acetyl-CoA may be more readily available to AATase II early in growth, before the substrate is fully utilized for acid production, and the ester may be able to accumulate faster during early growth when more acetyl-CoA is available. Secondly, the ester is not as effectively degraded after 18 h as compared to after 40 h of growth. Thirdly, the enzyme has very low activity at pH values below 5.0 [17]. In pH-uncontrolled batch culture of C. acetobutylicum, the external pH typically drops below 5.0 after 12 h of growth [1], which could lead to a decrease in AATase activity.
Isoamyl acetate was degraded by C. acetobutylicum. However, the degradation of the ester in strain M5 was less than in C. acetobutylicum ATCC 824, yet accumulation of the ester in C. acetobutylicum ATCC 824 was more than twice as much as that in M5. There are several enzymes which may be responsible for the hydrolysis of the ester. Potential coding sequences include regions for possible acetylxylan esterases, an acyl-CoA esterase, and a lipase-esterase related protein.
Expression of the yeast alcohol acetyltransferase genes in E. coli and C. acetobutylicum demonstrates the ability of these organisms to make use of a foreign gene and produce a new compound. Although ATF2 appears to be redundant in yeast, both genes have applicability for exploring metabolic flux in E. coli and C. acetobutylicum. However, controlled pH fermentation would be necessary for C. acetobutylicum to produce sufficient quantities of the ester for this to be a feasible option for mass ester production.
References
Boynton ZL, Bennett GN, Rudolph FB (1994) Intracellular concentrations of coenzyme A and its derivatives from Clostridium acetobutylicum ATCC 824 and their roles in enzyme regulation. Appl Environ Microbiol 60:39–44
Clark SW, Bennett GN, Rudolph FB (1989. Isolation and characterization of mutants of Clostridium acetobutylicum ATCC 824 deficient in acetoacetyl-coenzyme A:acetate/butyrate:coenzyme A-transferase (EC 2.8.3.9) and in other solvent pathway enzymes. Appl Environ Microbiol 55:970–976
Fujii T, Iwamatsu A, Yoshimoto H, Minetoki T, Bogaki, Nagasawa N (1998) USA patent 5,728,412
Fujii T, Nagasawa N, Iwamatsu A, Bogaki T, Tamai Y, Hamachi M (1994) Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl Environ Microbiol 60:2786–2792
Fujii T, Yoshimoto H, Nagasawa N, Bogaki T, Tamai Y, Hamachi M (1996) Nucleotide sequences of alcohol acetyltransferase genes from lager brewing yeast, Saccharomyces carlbergensis. Yeast 12:593–598
Girbal L, Croux C, Vasconselos I, Soucaille P (1995) Regulation of metabolic shifts in Clostridium acetobutylicum ATCC 824. FEMS Microbiol Rev 17:287–297
Grohmann K, Wymann CE, Himmel ME (1992) Potential for fuels from biomass and wastes. In: Rowell RM, Schults TP,Narayan R (ed)Emerging technologies for materials and chemicals from biomass. American Chemical Society, Washington, DC, pp. 354–392
Hartmanis MGN, Gatenbeck S (1984) Intermediary metabolism in C. acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47:1277–83
Howard D,Anderson RG (1976) Cell-free synthesis of ethyl acetate by extracts from Saccharomyces cerevisiae. J Inst Brew 82:70–71
Malcorps P, Dufour JP (1992) Short-chain and medium-chain aliphatic-ester synthesis in Saccharomyces cerevisiae. Eur J Biochem 210:1015–1022
Malcorps P, Dufour JP (1987) Ester synthesis by Saccharomyces cerevisiae: location of the acetyl-CoA:isoamyl alcohol acetyltransferase. European Brewery Convention 21:377–384
Mermelstein LD, Papoutsakis E (1993) In vivo methylation in Escherichia coli by the Bacillus subtilis phage ϕ3TI methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59:1077–1081
Nagasawa, N, Bogaki T, Iwamatsu A, Hamachi M, Kumagai C (1998) Cloning and nucleotide sequence of the alcohol acetyltransferase II gene ( ATF2) from Saccharomyces cerevisiae Kyokai No. 7. Biosci Biotechnol Biochem 62:1852–1857
Wayman M, Parekh SR (1990) Biotechnology of biomass conversion. Prentice Hall, Englewood Cliffs, New Jersey
Yamakawa Y, Goro S, Yokotsuka I (1978) Fractionation and some properties of acetic-ester synthesizing enzyme from Cladosporium cladosporioides. Agric Biol Chem 42:269–274
Yoshimoto H, Fujiwara D, Momma T, Tanaka K, Sone H, Nagasawa N, Tamai Y (1999) Isolation and characterization of the ATF2 gene encoding alcohol acetyltransferase II in the bottom fermenting yeast Saccharomyces pastorianus. Yeast 15:409–417
Yoshioka K,Hashimoto N (1981) Ester formation by alcohol acetyltransferase from brewers' yeast. Agric Biol Chem 45:2183–2190
Zhao Y, Nurman LA, Chuang A, Monroe-Augustus M, Lyristis M, Harrison ML, Rudolph FB, Bennett GN (2003) Expression of a cloned cyclopropane fatty acid synthase gene reduces solvent formation in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 69 (in press)
Acknowledgements
We thank Michael Gustin for supplying S. cerevisiae genomic DNA and Miles Scotcher for thoughtful discussions. This research was supported by the National Science Foundation grant BES-0001288, and the Robert A. Welch Foundation grants C-1268 and C-1372.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horton, C.E., Huang, KX., Bennett, G.N. et al. Heterologous expression of the Saccharomyces cerevisiae alcohol acetyltransferase genes in Clostridium acetobutylicum and Escherichia coli for the production of isoamyl acetate. J IND MICROBIOL BIOTECHNOL 30, 427–432 (2003). https://doi.org/10.1007/s10295-003-0070-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0070-0