Abstract
The mixed culture fermentation is an important environmental biotechnology that converts biodegradable organic wastes to valuable chemicals such as hydrogen, methane, acetate, ethanol, propionate, and so on. For the multistep process of hydrolysis, acidogenesis, acetogenesis/homoacetogensis, and methanogenesis, the typical metabolic reactions are firstly summarized. And then, since the final metabolites are always a mixture, the separation and purification processes are necessary to couple with anaerobic fermentation. Therefore, several typical coupling technologies including biogas upgrading, two-stage fermentation, gas stripping, membrane technology of pervaporation, membrane distillation, electrodialysis, bipolar membrane electrodialysis, and microbial fuel cells are summarized to separate the metabolites and recover energy. At last, the novel technologies such as the controlled metabolite production, medium chain carboxylic acid production, and high temperature ethanol recovery in thermophilic mixed culture fermentation are also reviewed. However, the novel concepts are still needed to meet the demands of better overall performances and lower total costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the aspirational theme to meet the needs of worldwide sustainability, governments and researchers pay much more attention to the green biofuels such as hydrogen and ethanol instead of the traditional fossil fuels (Angenent et al. 2004; Kleerebezem et al. 2015; Pawar and Niel 2013; Zhang et al. 2016a). Meanwhile, the sustainability goal also pushes human society to use and reuse the waste recourses. And, mixed culture fermentation (MCF) is well known to be the traditional environmental biotechnology that converts biodegradable organic wastes including activated sludge and household solid or cattle manure to biogas (Bastidas-Oyanedel et al. 2015; Batstone and Virdis 2014; Chen et al. 2007; Kleerebezem et al. 2015; Nielsen et al. 2007).
The processes of biodegradable organic wastes in MCF are the cascade bioreactions consisting of four main steps of hydrolysis, acidogenesis, acetogenesis/homoacetogensis, and methanogenesis, with distinct different groups of functional microorganisms including fermentative bacteria, acetogens, homoacetogens, and hydrogenotrophic and aceticlastic methanogens involved (Agler et al. 2011; Kleerebezem and van Loosdrecht 2007), as shown in Fig. 1. Once the methanogens are inhibited, the intermediates such as hydrogen, acetate, ethanol, and butyrate accumulate notably in the reactor, which are paid more and more attentions since the usage of chemical commodity, clean fuels, and building blocks (Bastidas-Oyanedel et al. 2015; Ghimire et al. 2015; Kleerebezem et al. 2015; Temudo et al. 2007; Zhang et al. 2012). In the present work, two main fields are analyzed and summarized. On one hand, the typical biochemical reactions are fundamental to understand the MCF; therefore, the main metabolites are summarized.
On the other hand, the metabolites both in headspace and in liquid solutions are always a mixture in MCF, which have to be concentrated and purified before the utilization. For example, the produced biogas generally consists of methane (40–75%); carbon dioxide (25–60%); and other trace gases, such as H2S, ammonia, siloxanes, and moisture (Chen et al. 2013; Ryckebosch et al. 2011). For hydrogen production in MCF, the theoretical maximum yield (4 mol/mol-glucose) represents only 25% conversion of substrate COD into BioH2 (Hallenbeck and Ghosh 2009). And, the practical yield is generally 2–3 mol/mol-glucose in MCF (Bastidas-Oyanedel et al. 2012; Temudo et al. 2007; Zhang et al. 2012). And, the metabolites in liquid solutions are also a mixture of acetate, butyrate, and ethanol. Thereby, several processes such as biogas upgrading, membrane-related technologies, microbial fuel cells (MFCs) are recently proposed to couple with MCF and are summarized below. At last, several novel technologies such as the controlled metabolite production, medium chain carboxylic acid production, and high temperature ethanol recovery in thermophilic mixed culture fermentation are also reviewed.
The basic bioreactions in MCF
Hydrolysis
Normally, only simple substrates such as glucose, sucrose, xylose, and glycerol can be directly utilized for volatile fatty acid (VFA, such as acetate, propionate, and butyrate) production. Recently, the worldwide interesting and promising substrates for MCF cover industrial and agricultural wastewater, biomass, household solids, algal, and even municipal sludge. But, these substrates could not be easily utilized and consequently, the pretreatment and hydrolysis are necessary in MCF. For example, the biomass is deemed to be the most abundant renewable organic material on earth; however, most of the degradable cellulose and hemicellulose in the biomass are packed with lignin that are resistant to microbial degradation (Abubackar et al. 2011). And, several methods including acid-based methods, hydrothermal processing, mild alkaline methods, oxidative methods, steam explosion, and ionic liquid solvents are proposed to remove lignin and hemicelluloses (Jönsson and Martín 2016). Interestingly, Yang et al. (2009) reported that one extreme thermophilic bacterium, Caldicellulosiruptor bescii sp. nov., can efficiently degrade the lignocellulosic biomass to hydrogen, acetate, and ethanol without any pretreatment. And, the related bacteria were recently also enriched in extreme thermophilic MCF, which meant the possibility of biomass bioconversion without pretreatments (Zhang et al. 2014a, 2016b). Recently, Fang et al. (2017) proposed to revive the effectiveness of cellulose digestibility by coupling hydrothermal pretreatment with deep eutectic solvent (DES) treatment and the results showed the significant increase of both xylan (25%) and lignin (22%) removals.
Acidogenesis
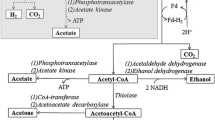
In the following acidogenesis step, the fermentative microorganisms utilize the aforementioned sugars into VFAs (such as acetate, propionate, and butyrate), alcohols such as ethanol and butanol, H2, and CO2. The main catabolic reactions from glucose in MCF are shown in Fig. 2 (Lengeler et al. 1999; Temudo et al. 2007; Zhang et al. 2013c). The energy conservation mechanism includes substrate level phosphorylation (SLP), ion motive force coupling with mainly H+ and Na+, and energy conversation electron bifurcation reaction (Lengeler et al. 1999; Thauer et al. 2008).
For example, once the extracellular glucose is transported into the cytoplasm by phosphotransferase system (PTS), glucose is mainly converted to pyruvate in the Embden-Meyerhof (EM) pathway (Eq.1); the percentages of other pathways such as the Entner-Doudoroff (ED) pathway and the pentose phosphate (PP) pathway are relative low; for example, de Vrije et al. found that in Caldicellulosiruptor saccharolyticus, the percentage of glycolysis via EM pathway reached to 99% (de Vrije et al. 2007).
-
Acetate: Acetate is formed directly from acetyl-CoA via phosphotransacetylase and acetate kinase with 1 ATP generated (Lengeler et al. 1999), as shown in Eq. 2. While, the formation of acetyl-CoA form pyruvate can flow through two pathways. The first pathway is the pyruvate formate-lyase (PFL) pathway that commonly occurs in facultative anaerobes and produces aceyl-CoA and formate, such as Escherichia coli (Sawers 2005). The second one is the pyruvate/ferredoxin oxidoreductase (PFOR) pathway that generates acetyl-CoA and reduced ferredoxin (Fdred) (Lengeler et al. 1999; Madigan et al. 2002) and is the typical pathway in strict anaerobes such as Clostridium sp. For the low redox potential and commonly pre-treated methods in MCF, the PFOR pathway is generally predominant (Lee et al. 2009).
-
Butyrate and butanol: Two main enzyme groups control the butyrate production: phosphotransbutyrylase and butyrate kinase and butyryl-CoA/acetate CoA-transferase. Louis et al. (2004) found that for the bacteria in the human colon, the first enzyme group was the main pathway for butyrate production (Eq. 3) under the low acetate concentration, while at the higher acetate concentration (30–80 mM), the pathway switched to use the second enzyme group (Eq. 4) to decrease the acetate toxicity.
Butanol can be produced from the reduction of butyryl-CoA with NADH by the bacteria of genus Clostridium in a typical biphasic process of the acetone-butanol-ethanol (ABE) fermentation (Moon et al. 2016). Generally, butanol is not the main metabolite in MCF and generally accumulated in pure culture fermentation (PCF) of C. acetobutylicum (Moon et al. 2016).
-
Ethanol: the formation of ethanol is reduced by NADH via acetyl-CoA from pyruvate, with acetaldehyde as an intermediate, as shown in Eq.5. Lactate heterofermentation from xylulose 5-phoshpate can generate ethanol too, but this type of fermentation is not common in glucose fermentation (Madigan et al. 2002). Ethanol can also be produced by decarboxylation of pyruvate via acetaldehyde in yeast and Zymomonas (Lengeler et al. 1999).
-
Lactate: the formation of lactate from glucose has mainly two modes (Price et al. 2004). The first one is homofermentation, that is, pyruvate produced from glucose via EMP pathway is reduced by NADH directly to lactate, as shown in Eq.6. The other one is heterofermentation, in which lactate and acetate (or ethanol) are produced from xylulose 5-phoshpate pathway, following with one CO2 produced. Normally, the first pathway is deemed to be the only pathway because of its higher energy yield and the most genera involved (Maris et al. 2004).
-
Propionate: the two main pathways of propionate formed from pyruvate are the methylmalonyl-CoA pathway and acryloyl-CoA pathway (Stams et al. 1998). Fumarate and succinate are the intermediates in the first pathway with 2/3 ATP generated (Lengeler et al. 1999) (Eq. 7), which is also the common pathway. The second pathway is directly from lactate with no ATP yielded, which is beneficial for the faster specific substrate turnover of C. homopropionicum (Seeliger et al. 2002).
-
Hydrogen formed form NADH and Fdred: generally, four pathways control the hydrogen production by the redox couple of NADH/NAD+, Fdred/Fdox, and formate (Schut and Adams 2009). Firstly, most of the researchers consider that NADH is the main electron donor for the reduction of H+ to H2 (Eq. 8) (Rodriguez et al. 2006). However, the potential of NADH/NAD+ is only −320 mV, which is higher than the potential of H2/H+ (−414 mv); consequently, the formation of hydrogen from NADH is not possible except under rather low hydrogen partial pressure (60 Pa) (Angenent et al. 2004). Secondly, Fdred/Fdox, of which the potential is below −400 mV (Thauer et al. 2008), is the more suitable electron donor for H+ reduced (Eq. 9) (Temudo et al. 2007). And, the hydrogen production from Fdred/Fdox is verified in Thermoanaerobacter tengcongensis (Soboh et al. 2004). Moreover, with temperature increasing, the potential of Fdred/Fdox becomes much lower (Smith et al. 1995). Thirdly, hydrogen can be also produced from formate (Eq. 10). Both Temudo et al. and our group found that the ratio of hydrogen verse (hydrogen + formate) was related to the thermodynamics of formate dehydrogenation to H2 both in mesophilic and thermophilic MCF (Temudo et al. 2007; Zhang et al. 2015a).
Schut and Adams (2009) found the fourth pathway named the electron bifurcation reaction that a new type of [FeFe] hydrogenase in a hyderthermophilic bacterium Thermotoga maritima could simultaneously utilize NADH and Fdred as the electron donors to produce hydrogen in an approximately 1:1 ratio (Eq. 11); thus, the hydrogen yield could reach the maximum 4 mol/mol-glucose even in normal hydrogen partial pressure. They also announced that lots of known H2 production bacteria might have this potential mechanistic function for hydrogen production (Schut and Adams 2009).
Acetogenesis and homoacetogenesis
In the third stage of anaerobic digestion, the produced VFAs and alcohols are converted to acetate, H2 and CO2 by acetogenic bacteria under rather low hydrogen partial pressure; for example, the hydrogen partial pressure in the conversion of propionate to acetate (Eq. 12) is lower than 10 Pa (Angenent et al. 2004). Therefore, propionate is rather hard to be consumed and easily accumulated in anaerobic digester when the reactor encounters fluctuation or shock (Hori et al. 2006).
Conversely, high hydrogen partial pressure favors homoacetogens such as C. ljungdahlii to produce acetate (Eq. 13) (Zhang et al. 2013b). For example, Poehlein et al. calculated that the minimal hydrogen concentration in the reactor should be above 250 Pa to allow homoacetogen growth on hydrogen and CO2 (Poehlein et al. 2012). Besides, other factors, for example temperature and hydraulic retention time (HRT), also affect the homoacetogen activities. For example, the Gibbs-free energy of homoacetogenesis (Eq. 13) at 70 °C (−83.2 kJ/mol) is higher than that of 25 °C (−104.5 kJ/mol) under standard conditions (Zhang et al. 2014b). Siriwongrungson et al. reported homoacetogenesis in a thermophilic continuously stirred tank reactor (CSTR) with 6-day HRT, while recently, our group did not detect homoacetogenesis in both thermophilic and extreme thermophilic CSTR with HRT below 1 day (Siriwongrungson et al. 2007; Zhang et al. 2016a, b).
Methanogenesis
Finally, methanogens utilize acetate or H2/CO2 to CH4, which is named as aceticlastic methanogenesis (Eq. 14) or hydrogenotrophic methanogenesis (Eq. 15), respectively (Thauer et al. 2008). The former reaction is carried out by Methanosarcinaceae and Methanosaetaceae, while the latter one is performed by Methanomicrobiales and Methanobacteriales (Karakashev et al. 2006). Normally, methanogenesis is the rate-limiting step in anaerobic digesters because methanogens grow slowly and are susceptible to toxins and operational conditions (for example pH, NH4 + and temperature) (Karakashev et al. 2005). On the other hand, hydrogenotrophic methanogens are considered to be more resistant to toxicity or operational factors (Demirel and Scherer 2008). For example, Ho et al. (2013) reported that the percentage of hydrogenotrophic methanogens was above 20% at 55 °C, and recently, Zhang et al. found that its percentage even approached to 100% of methanogens at 70 °C (Zhang et al. 2014b, 2015b).
Process coupling and perspective
Because of the stepwise bioreactions in MCF, the metabolites are always a mixture; coupling processes are necessary to utilize of the products in MCF. As shown in Fig. 3, several novel technologies such as biogas upgrading, two-stage fermentation, gas striping, membrane technology, and microbial fuel cells are reviewed and the details are discussed as follows.
Biogas upgrading
The produced H2 and CH4 in MCF have high calorific values. For example, the calorific value of typical methane biogas from 5.5 to 6.5 kWh m−3 is comparable to that of natural gas (5.8–7.8 kWh m−3); biogas can be used as a substitute for natural gas to generate heat and electricity (Yu et al. 2010). Recently, Sowunmi et al. (2016) showed that the energy of methane production from biomasses in Abu Dhabi Emirates were theoretically able to meet 6% of household electricity consumption. However, the impurities such as CO2, H2S, and siloxanes shall be removed firstly. Especially, CO2 are considered to be the main contributor for the greenhouse effect and gas corrosion. As demonstrated by Deng and Hägg (2010), upgrading the methane concentration to 90% could increase the heating value efficiently; furthermore, the upgraded biogas above 98% methane could be compressed and liquefied as vehicle fuel (Chen et al. 2013). As summarized by Petersson and Wellinger (2009), the number of worldwide biogas upgrading plants increased notably in the past 30 years and was around 100 in 2009.
Water washing, membrane separation, chemical absorption, and pressure swing adsorption (PSA) are the common four CO2 removal techniques (Deng and Hägg 2010). For example, the commercial membranes for CO2/CH4 separation are generally conventional polymeric dense membranes according to the solution-diffusion mechanism (Deng and Hägg 2010). CO2 is more readily absorbed in water than CH4; therefore, in the water absorption column, more CO2 is removed (Nock et al. 2014). While in chemical absorption technology, the alkali adsorption (such as NaOH) could reduce CO2 and H2S simultaneously and avoid the pre-cleaning of H2S, and the required operational pressure is also low (Petersson and Wellinger 2009). Chen et al. proposed a coupled system of bipolar membrane electrodialysis (BMED) and microbial fuel cell (MFC) for alkali production and CO2 adsorption with low operation cost. The final CH4 content increased notably in headspace and even reached 100% (Chen et al. 2013).
Recently, several biotechnologies are proposed to improve the biogas purity or convert the biogas (H2 and CO2) to other valuable biochemicals (Luo et al. 2012; Nie et al. 2007; van der Ha et al. 2012; Zhang et al. 2013a, b). For example, Luo et al. (2012) proposed to convert hydrogen to methane by hydrogenotrophic methanogenesis. While a second homoacetogenesis bioreactor was proposed by Nie et al. (2007) to couple with a MCF bioreactor and converted CO2 and H2 to improve the final acetate yield around 53%. In a hollow-fiber membrane bioreactor under acidic pH, the metabolite from CO2 and H2 was rather simple and the acetate fraction was higher than 99% in both batch and continuous modes (Zhang et al. 2013a). Recently, a methane oxidizing bacteria Methylocystis parvus was used to convert biogas to polyhydroxybutyrate (PHB) by van der Ha et al. (2012) and the final concentration of 295 ± 50 mg intracellular PHB g−1 cell dry weight was achieved.
Alkali adsorption can remove H2S in biogas; however, this method is not a selective process and the CO2 content is also decreased. Recently, activated carbon adsorption was suggested to be more suitable for H2S pretreatment; the surface acidity was found to be the key factor; however, the present of CH4 and CO2 notably reduced the H2S removal capacity (Yentekakis and Goula 2017). On the other hand, siloxanes were also the main problems for biogas utilization, because silica microparticulates formed at high temperatures can create fouling and abrasion effects to natural gas-fueled vehicles (Cabrera-Codony et al. 2014; Yentekakis and Goula 2017). Recently, Cabrera-Codony et al. proposed activated carbon for siloxane removal, but both the presence of CH4 and CO2 and biogas humidity can reduce the adsorbing ability and siloxane polymerization cannot be avoided under long-term operation. Therefore, developing suitable methods such as selective separating membrane technologies to remove H2S and/or siloxanes are the prerequisite for biogas utilization.
Two-stage fermentation for hydrogen and methane production
Because of the notable different cultivating conditions, such as pH and HRT, between fermentative bacteria and methanogens, commonly, the anaerobic digester is operated in a suboptimal condition that leads to low methanogen activity (Li and Yu 2011). Meanwhile, the fast-growing acidogens result in the notable acidification of bulk solution and consequently, it can inhibit the activities of methanogens. The two-stage fermentation for hydrogen and methane production is proposed by the separation of hydrolysis/acidogenesis and methanogenesis steps (Li and Yu 2011; Ueno et al. 2007). The separated hydrolysis/acidogenesis reactor can promote the complex substrate hydrolysis, too. For example, Kraemer et al. proposed that the first phase was operated at pH 5.5 for hydrogen production, and the second phase was utilized for methanogenesis at pH 6.8 (Kraemer and Bagley 2005). Meanwhile, the effluent of second reactor was recycled to the first one to reduce the required alkalinity for pH control (Kraemer and Bagley 2005).
Moreover, operating a thermophilic reactor in the first stage raises much more attention because high temperature benefits for the high substrate degradation rate, efficient heat utilization of some wastewater, and better pathogen destruction etc. (Nielsen et al. 2007; Zhang et al. 2014b). For example, Ueno et al. (2007) constructed a pilot-scale thermophilic two-stage plant for the production of hydrogen (at 60 °C) and methane (at 55 °C). Their results showed that this mode was rather stable even using the organic waste at a pilot-scale that the working volumes of hydrogen and methane production reactors were 200 and 500 L, respectively. Bolzonella et al. (2007) proposed an extreme-thermophilic MCF at 70 °C to accelerate the hydrolysis and acidogenesis of waste-activated sludge in the first hydrogen producing process. However, the overall operating costs after integrating thermophilic reactors still require further evaluation.
On the other hand, the extra alkali dosing increases the operational cost. Recently, Wu et al. proposed an acidic reactor at pH 4.0 to reduce the alkali addition in the two-phase anaerobic digestion for fruit and vegetable waste treatment and the system exhibited a low HRT (3.56 days) with a high methane yield (348.5 ml/g VS removed) (Wu et al. 2016). But, after long time operation, alkali will also need to be added periodically according to the variation of pH. Therefore, novel methods to reduce operational cost are still necessary.
Gas stripping
As the perspective substitutes for petroleum fuel, ethanol and butanol are paid more attentions for their higher energy density, less corrosiveness, and better compatibility with gasoline (Xue et al. 2013). However, the metabolites in MCF are always a mixture of ethanol, butanol, acetate, and butyrate. Therefore, the following processes are necessary to recover and concentrate ethanol and/or butanol from the fermentation broth. For the high volatility under high temperature, ethanol and butanol can be easily recovered by gas stripping technology after coupling with MCF (Xue et al. 2012, 2013). Moreover, reducing the accumulation of ethanol and butanol in the bulk solution also could increase the bacteria activities (Liu and Qureshi 2009). For example, Löser et al. (2005) found more than 30% of produced ethanol could be stripped off under practical conditions. Hashi et al. (2010) used CO2 to remove ethanol from the fermentation broth and reduced the level of ethanol toxicity, and then the following adsorption was used to recover residual ethanol from the CO2 vapor phase. Recently, butanol recovery is paid much more attention (Xue et al. 2013); for example, Xue et al. (2016) developed a two-stage gas stripping and pervaporation process integrated with ABE fermentation for butanol recovery. The results showed that much more ABE (27.5 g/L of acetone, 75.5 g/L of butanol, 7.0 g/L of ethanol) were produced in the fed-batch fermentation (Xue et al. 2016).
On the other hand, gas striping is also used to reduce the hydrogen content in reactor headspace and increase the hydrogen yield. For example, Kim et al. (2006) reported that the hydrogen yield increased from 0.9 to 1.2 mol/mol-glucose by gas sparging in mesophilic MCF, and Zhang et al. (2013d) also found that the hydrogen yield increased from 0.64 to 1.1 mol/mol-glucose in extreme-thermophilic MCF with N2 sparging. Excitingly, Bastidas-Oyanedel et al. (2012) reported the maximum H2 yield of 3.25 mol/mol-glucose at pH 4.5 and the N2 sparging rate of 58 L/day, which might be due to the inhibition of homoacetogenesis and methanogensis in MCF. However, using N2 will dilute the produced hydrogen content, and therefore, the following purifying process is also needed. Bastidas-Oyanedel et al. (2012) proposed one CH4 stripping process and the produced gas named biohythane consisting CH4 and H2, which is beneficial for increasing both the H2 yield and the calorific value. But, high gas stripping rates can bring some adverse effects, such as microbial activity inhibition and energy wasting, and coupling biomass immobilization with gas stripping may be a sound method but it still needs to be assessed in the future.
Membrane technologies
Membrane technologies have been proving their advance in the fields of separation and purification processes (Moon and Yun 2014). The applications of membrane separation technologies in MCF cover pervaporation, membrane distillation, electrodialysis (ED), BMED, and so on (Jones et al. 2015; Zacharof and Lovitt 2013). Pervaporation and membrane distillation could enable selective separation of volatile alcohols from the fermentation broth or purify H2 (Lewandowicz et al. 2011). For example, Lewandowicz et al. (2011) applied membrane distillation of a capillary polypropylene microfiltration unit for ethanol recovery. Bakonyi et al. (2013) proposed to use a commercial polyimide membrane module to purify hydrogen, and the highest H2/CO2 gas selectivity of 1.62 could be achieved.
On the other hand, ED is a traditional technology and can be used to separate and concentrate organic acids (Moresi and Sappino 2000; Zhang et al. 2011). For example, Meynial-Salles et al. (2008) proposed a novel three-stage continuous fermentation process, which combined an integrated membrane bioreactor-ED system to produce and concentrate succinic acid, and the maximum concentration reached 83 g/L. Redwood et al. (2012) proposed an integrated hydrogen refinery of food wastes in a synergic combination of photofermentation, extractive fermentation, and hydrothermal hydrolysis, in which ED provided the key link in the concept of waste to energy for the selective separation of organic acids. BMED is a technique with integrated bipolar membrane with traditional ED and can significantly reduce the cost of acid and alkali in traditional organic separation and purification processes (Zhang et al. 2009). Recently, Wang et al. constructed an equipment coupled lactic acid fermentation with BMED stack and achieved a lactic acid recovery ratio of 86% in the batch mode (Wang et al. 2012) and 69.5% in the continuous mode (Wang et al. 2013). Zhang et al. (2009) proposed a two-phase BMED that a mixture of water and ethanol could be used as the media to enhance the solubility of sebacic acid, which can also benefit for the recovery of the medium long-chain acids such as caproate and caprylate and needs the following research works.
Besides, other membrane technologies, such as microfiltration (MF) and nanofiltration (NF), and membrane extraction are also proposed to couple with MCF. For example, Zacharof and Lovitt (2013) reported that the waste effluent from an anaerobic digester of agricultural waste was treated by MF and NF to concentrate VFA. The results showed that MF produced the sterile, particle-free solution with a VFA concentration of 21.08 mM acetic acid and 15.81 mM butyric acid, while NF achieved retention ratios up to 75% and the final concentrations up to 53.94 mM acetate and 28.38 mM butyrate (Zacharof and Lovitt 2013). On the other hand, Bonk et al. (2015) carried out the economical assessment for the purification of VFAs and the results show that this process is economically feasible given that the separation technologies such as membrane technologies can be realized on a large scale. However, the pilot-scale experiments for VFA separation from real effluent of MCF also need to be proven in the future.
The membrane technologies are prevalent as reducing cost, but the overall cost is still high and membrane fouling cannot be avoided. For example, Zheng et al. (2010) reported that the membrane failed even after 24 h of operation. The addition of powered activated carbon (PAC) was proposed to reduce the membrane fouling, but methanogens may grow on the surface of PAC and consume metabolites such as acetate. Forward osmosis (FO), as a novel membrane technology, is notably characterized as having both low energy cost and low organic fouling (Mi and Elimelech 2010; Wang et al. 2016), which is also a promising coupled technology for MCF.
As is known, except the bacteria metabolites of organic acids and alcohols, the components of MCF broth normally also include various kinds of inorganic salts that affect the real separating factors. For example, Zhang et al. (2011) analyzed the ion competition between organic acids (such as formate, acetate, propionate, and butyrate) and inorganic salts (such as HPO4 2− and Cl−) and found that membrane selectivity was dependent on the size, charge, and functional groups of the organic ions; consequently, the concentrations of acetate, propionate, and butyrate decreased slower because there were still inorganic ions present. So, the developments of selective separating membranes for the specific metabolites are urgently needed. On the other hand, Bonk et al. (2015) reported that VFA concentrations were important factors to analyze the purification cost. Recently, the final concentration of acetate without any concentrated treatments reached 570 mM (Zhang et al. 2014b), which was higher than the above reported results. Therefore, future works are still needed to assess MF and NF for VFAs purification.
Microbial fuel cells
The produced metabolites in MCF also can be converted to electricity. Microbial fuel cells (MFCs) are a fast growing environmental biotechnology where the bio-convertible substrates are consumed in the anodic chamber with simultaneous electron generation (Logan and Regan 2006; Lovley 2008). This technology offers the benefits of convenient electricity recovery in ambient condition and has been proposed for energy recovery, wastewater treatment, bioremediation, and valuable chemical production, and so on (Li et al. 2014; Sun et al. 2016).
The MFC cost is a notable obstacle for its application, while coupling MFC with other biotechnologies can diminish this drawback somehow. For example, MFC could be coupled with MCF (Modin and Gustavsson 2014), and Koch et al. (2015) proposed the combination of anaerobic digestion and microbial electrochemical technology to efficiently produce methane and electrical energy from complex biomass. Ren et al. (2014) constructed a two-stage laboratory-scale combined treatment process, consisting of microbial fuel cells and an anaerobic fluidized bed membrane bioreactor, to produce high-quality effluent with energy recovery. Ki et al. (2015) recently investigated the combination of primary sludge pre-fermentation to produce volatile fatty acids (VFAs) as the electron donor for microbial electrolysis cells (MECs), and the results demonstrated successful performance including Coulombic efficiency (95%), Coulombic recovery (80%), and COD-removal efficiency (85%). Till now, researchers mainly focus on mesophilic MFC, while thermophilic microbial fuel cell (TMFC) is seldom reported (Dopson et al. 2016; Ha et al. 2012). As shown above, MCF under thermophilic conditions offers many advantages over mesophilic MCF; therefore, the development of TMFC could broaden its applications.
The voltage of MFC is too low (around 0.5–0.7 V) to be used directly for many practical applications (Kim et al. 2011). The development of energy collecting technologies such as capacitors would realize real energy recovery. For example, Kim et al. (2011) demonstrated that the parallel charging of the capacitors can avoid voltage reversal, while discharging the capacitors in series produced up to 2.5 V. Santoro et al. recently proposed a coupled supercapacitive MFC that produced the highest power value of 19 mW (Santoro et al. 2016). On the contrary, the feasibility of MFC as a biosensor with low voltage output was proposed for monitoring VFAs in anaerobic digestion and the results showed that the detection range was much broader than that of other biosensors (Jin et al. 2016). Moreover, the majority of present MFC works are carried out in lab scale, while pilot- or full-scale experiments are seldom reported; thus, the total costs are hard to evaluate.
Perspectives
Several novel concepts are proposed recently in MCF. Firstly, the production of sole metabolite in MCF can be separated under the lower cost. For example, after selective enrichment of hydrogenotrophic methanogens over aceticlastic methanogens, our group could solely produce acetate (the fraction in bulk solutions >90%) from glucose, glycerol, or Tofu wastewater in extreme-thermophilic MCF (Chen et al. 2016b; Zhang et al. 2014b, 2015b). And at acidic pH 4.5, the homoacetogens could produce acetate with fraction even above 99% from H2 and CO2 in a hollow fiber membrane bioreactor (Zhang et al. 2013a). The high-purity propionate production from glycerol or glucose in MCF induced by ammonium was recently investigated by Chen et al., and the final purity of propionate was 91–100% (Chen et al. 2016a, 2017). Recently, Bonk et al. (2017) proposed the selective lactic acid production from food waste by in-situ product extraction using activated carbon, and the results showed that the lactic acid concentration reached 32 gCOD/L with the selectivity of 93% in a semi-continuous mode.
Secondly, an alternative process for anaerobic wastewater treatment with methane or acetate recovery is to elongate the carbon chain of VFAs to the medium chain carboxylic acids such as n-caproic acid from ethanol, acetate, glycerol, and syngas (Leng et al. 2017; Zhang et al. 2013b). For example, Zhang et al. (2013b) realized in situ H2 utilization in a hollow-fiber membrane bioreactor under neutral pH and the final metabolic concentrations of acetate, butyrate, caproate, and caprylate were 7.4, 1.8, 0.98, and 0.42 g/L, respectively. For a longer carbon chain and lower O/C ratio, the mixture of produced medium chain fatty acids also could be upgraded to biofuels by hydrogen reduction (Steinbusch et al. 2011; Zhang et al. 2013b). For example, Xu et al. (2015) extracted n-caproate from the bioreactor broth by hollow fiber membrane and found the selective phase separation occurred due to the low maximum solubility of this acid, which allowed simple product separation into an oily liquid containing 90% n-caproic and n-caprylic acids. However, the biomass toxicities of medium chain carboxylic acids are still needed to be considered (Zhang et al. 2013b).
Finally, the volatility of ethanol is known to increase notably under high temperature. Thus, Frock et al. proposed the possibility that biofuels such as ethanol can be recovered directly through direct evaporation and distillation in high-temperature MCF (Frock and Kelly 2012). And, Fernández-Naveira et al. recently demonstrated the production of butanol and ethanol from CO and found low pH was more favorable to produce these solvents (Fernández-Naveira et al. 2016). The utilization of industrial waste hot water to heat thermophilic MCF could reduce the cost moreover.
Therefore, the typical metabolic pathways in acidogenesis, acetogenesis/homoacetogensis, and methanogenesis of mixed culture fermentation are summarized in this work. And then, several coupling technologies including biogas upgrading, two-stage fermentation, gas stripping, and membrane technologies are reviewed to recover and concentrate the metabolites. However, the novel concepts are still needed to meet the high demand including performances and total costs. Therefore, the process coupling is necessary in MCF to promote its worldwide application.
References
Abubackar HN, Veiga MC, Kennes C (2011) Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuels Bioprod Biorefin 5(1):93–114
Agler MT, Wrenn BA, Zinder SH, Angenent LT (2011) Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol 29(2):70–78
Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domíguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22(9):477–485
Bakonyi P, Kumar G, Nemestóthy N, Lin CY, Bélafi-Bakó K (2013) Biohydrogen purification using a commercial polyimide membrane module: studying the effects of some process variables. Int J Hydrog Energy 38(35):15092–15099
Bastidas-Oyanedel J-R, Mohd-Zaki Z, Zeng RJ, Bernet N, Pratt S, Steyer J-P, Batstone DJ (2012) Gas controlled hydrogen fermentation. Bioresour Technol 110:503–509
Bastidas-Oyanedel J-R, Bonk F, Thomsen M, Schmidt J (2015) Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Biotechnol 14(3):473–498
Batstone DJ, Virdis B (2014) The role of anaerobic digestion in the emerging energy economy. Curr Opin Biotechnol 27:142–149
Bolzonella D, Pavan P, Zanette M, Cecchi F (2007) Two-phase anaerobic digestion of waste activated sludge: effect of an extreme thermophilic prefermentation. Ind Eng Chem Res 46(21):6650–6655
Bonk F, Bastidas-Oyanedel J-R, Schmidt JE (2015) Converting the organic fraction of solid waste from the city of Abu Dhabi to valuable products via dark fermentation - economic and energy assessment. Waste Manag 40:82–91
Bonk F, Bastidas-Oyanedel J-R, Yousef AF, Schmidt JE (2017) Exploring the selective lactic acid production from food waste in uncontrolled pH mixed culture fermentations using different reactor configurations. Bioresour Technol 238:416–424
Cabrera-Codony A, Montes-Morán MA, Sánchez-Polo M, Martín MJ, Gonzalez-Olmos R (2014) Biogas upgrading: optimal activated carbon properties for siloxane removal. Environ Sci Technol 48(12):7187–7195
Chen M, Zhang F, Zhang Y, Zeng RJ (2013) Alkali production from bipolar membrane electrodialysis powered by microbial fuel cell and application for biogas upgrading. Appl Energy 103:428–434
Chen Y, Jiang S, Yuan H, Zhou Q, Gu G (2007) Hydrolysis and acidification of waste activated sludge at different pHs. Water Res 41(3):683–689
Chen Y, Wang T, Shen N, Zhang F, Zeng RJ (2016a) High-purity propionate production from glycerol in mixed culture fermentation. Bioresour Technol 219:659–667
Chen Y, Zhang F, Wang T, Shen N, Yu Z-W, Zeng RJ (2016b) Hydraulic retention time affects stable acetate production from tofu processing wastewater in extreme-thermophilic (70 °C) mixed culture fermentation. Bioresour Technol 216:722–728
Chen Y, Shen N, Wang T, Zhang F, Zeng RJ (2017) Ammonium level induces high purity propionate production in mixed culture glucose fermentation. RSC Adv 7(1):518–525
de Vrije T, Mars A, Budde M, Lai M, Dijkema C, de Waard P, Claassen P (2007) Glycolytic pathway and hydrogen yield studies of the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Microbiol Biotechnol 74(6):1358–1367
Demirel B, Scherer P (2008) The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev Environ Sci Biotechnol 7(2):173–190
Deng L, Hägg M-B (2010) Techno-economic evaluation of biogas upgrading process using CO2 facilitated transport membrane. Int J Greenhouse Gas Control 4(4):638–646
Dopson M, Ni G, Sleutels THJA (2016) Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiol Rev 40(2):164–181
Fang C, Thomsen MH, Frankær CG, Brudecki GP, Schmidt JE, AlNashef IM (2017) Reviving pretreatment effectiveness of deep eutectic solvents on lignocellulosic date palm residues by prior recalcitrance reduction. Ind Eng Chem Res 56(12):3167–3174
Fernández-Naveira Á, Abubackar HN, Veiga MC, Kennes C (2016) Efficient butanol-ethanol (B-E) production from carbon monoxide fermentation by Clostridium carboxidivorans. Appl Microbiol Biotechnol 100(7):3361–3370
Frock AD, Kelly RM (2012) Extreme thermophiles: moving beyond single-enzyme biocatalysis. Curr Opin Chem Eng 1(4):363–372
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95
Ha PT, Lee TK, Rittmann BE, Park J, Chang IS (2012) Treatment of alcohol distillery wastewater using a Bacteroidetes-dominant thermophilic microbial fuel cell. Environ Sci Technol 46(5):3022–3303
Hallenbeck PC, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol 27(5):287–297
Hashi M, Tezel FH, Thibault J (2010) Ethanol recovery from fermentation broth via carbon dioxide stripping and adsorption†. Energy Fuel 24(9):4628–4637
Ho DP, Jensen PD, Batstone DJ (2013) Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl Environ Microbiol 79(20):6491–6500
Hori T, Haruta S, Ueno Y, Ishii M, Igarashi Y (2006) Dynamic transition of a methanogenic population in response to the concentration of volatile fatty acids in a thermophilic anaerobic digester. Appl Environ Microbiol 72(2):1623–1630
Jin X, Angelidaki I, Zhang Y (2016) Microbial electrochemical monitoring of volatile fatty acids during anaerobic digestion. Environ Sci Technol 50(8):4422–4429
Jones RJ, Massanet-Nicolau J, Guwy A, Premier GC, Dinsdale RM, Reilly M (2015) Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour Technol 189:279–284
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Karakashev D, Batstone DJ, Angelidaki I (2005) Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl Environ Microbiol 71(1):331–338
Karakashev D, Batstone DJ, Trably E, Angelidaki I (2006) Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl Environ Microbiol 72(7):5138–5141
Ki D, Parameswaran P, Popat SC, Rittmann BE, Torres CI (2015) Effects of pre-fermentation and pulsed-electric-field treatment of primary sludge in microbial electrochemical cells. Bioresour Technol 195:83–88
Kim D-H, Han S-K, Kim S-H, Shin H-S (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrog Energy 31(15):2158–2169
Kim Y, Hatzell MC, Hutchinson AJ, Logan BE (2011) Capturing power at higher voltages from arrays of microbial fuel cells without voltage reversal. Energy Environ Sci 4(11):4662–4667
Kleerebezem R, van Loosdrecht MCM (2007) Mixed culture biotechnology for bioenergy production. Curr Opin Biotech 18(3):207–212
Kleerebezem R, Joosse B, Rozendal R, Loosdrecht MCM (2015) Anaerobic digestion without biogas? Rev Environ Sci Biotechnol 14(4):787–801
Koch C, Kuchenbuch A, Kretzschmar J, Wedwitschka H, Liebetrau J, Muller S, Harnisch F (2015) Coupling electric energy and biogas production in anaerobic digesters - impacts on the microbiome. RSC Adv 5(40):31329–31340
Kraemer JT, Bagley DM (2005) Continuous fermentative hydrogen production using a two-phase reactor system with recycle. Environ Sci Technol 39(10):3819–3825
Lee HS, Krajmalinik-Brown R, Zhang HS, Rittmann BE (2009) An electron-flow model can predict complex redox reactions in mixed-culture fermentative BioH2: microbial ecology evidence. Biotechnol Bioeng 104(4):687–697
Leng L, Yang P, Mao Y, Wu Z, Zhang T, Lee P-H (2017) Thermodynamic and physiological study of caproate and 1,3-propanediol co-production through glycerol fermentation and fatty acids chain elongation. Water Res 114:200–209
Lengeler JW, Drews G, Schlegel HG (1999) Biology of the prokaryotes. Georg Thieme Verlag, New York
Lewandowicz G, Białas W, Marczewski B, Szymanowska D (2011) Application of membrane distillation for ethanol recovery during fuel ethanol production. J Membr Sci 375(1–2):212–219
Li W-W, Yu H-Q (2011) From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: a future paradigm. Biotechnol Adv 29(6):972–982
Li W-W, Yu H-Q, He Z (2014) Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ Sci 7(3):911–924
Liu S, Qureshi N (2009) How microbes tolerate ethanol and butanol. New Biotechnol 26(3–4):117–121
Logan B, Regan J (2006) Microbial fuel cells-challenges and applications. Environ Sci Technol 40(17):5172–5180
Löser C, Schröder A, Deponte S, Bley T (2005) Balancing the ethanol formation in continuous bioreactors with ethanol stripping. Eng Life Sci 5(4):325–332
Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ (2004) Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol 186(7):2099–2106
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotech 19(6):564–571
Luo G, Johansson S, Boe K, Xie L, Zhou Q, Angelidaki I (2012) Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol Bioeng 109(4):1088–1094
Madigan M, Martinko J, Parker J (2010) Brock biology of microorganisms (13th ed.). Benjamin Cummings, San Francisco
Maris AJA, Konings WN, Dijken JP, Pronk JT (2004) Microbial export of lactic and 3-hydroxypropanoic acid: implications for industrial fermentation processes. Metab Eng 6(4):245–255
Meynial-Salles I, Dorotyn S, Soucaille P (2008) A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol Bioeng 99(1):129–135
Mi B, Elimelech M (2010) Organic fouling of forward osmosis membranes: fouling reversibility and cleaning without chemical reagents. J Membr Sci 348(1–2):337–345
Modin O, Gustavsson DJI (2014) Opportunities for microbial electrochemistry in municipal wastewater treatment—an overview. Water Sci Technol 69(7):1359–1372
Moon HG, Jang YS, Cho C, Lee J, Binkley R, Lee SY (2016) One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett 363(3):fnw001
Moon S-H, Yun S-H (2014) Process integration of electrodialysis for a cleaner environment. Curr Opin Chem Eng 4:25–31
Moresi M, Sappino F (2000) Electrodialytic recovery of some fermentation products from model solutions: techno-economic feasibility study. J Membr Sci 164(1–2):129–140
Nie Y, Liu H, Du G, Chen J (2007) Enhancement of acetate production by a novel coupled syntrophic acetogenesis with homoacetogenesis process. Process Biochem 42(4):599–605
Nielsen HB, Mladenovska Z, Ahring BK (2007) Bioaugmentation of a two-stage thermophilic (68°C/55°C) anaerobic digestion concept for improvement of the methane yield from cattle manure. Biotechnol Bioeng 97(6):1638–1643
Nock WJ, Walker M, Kapoor R, Heaven S (2014) Modeling the water scrubbing process and energy requirements for CO2 capture to upgrade biogas to biomethane. Ind Eng Chem Res 53(32):12783–12792
Pawar S, Niel EJ (2013) Thermophilic biohydrogen production: how far are we? Appl Microbiol Biotechnol 97(18):7999–8009
Petersson A, Wellinger A (2009) Biogas upgrading technologies—developments and innovations. IEA-Task 37:20
Poehlein A, Schmidt S, Kaster A-K, Goenrich M, Vollmers J, Thürmer A, Bertsch J, Schuchmann K, Voigt B, Hecker M, Daniel R, Thauer RK, Gottschalk G, Müller V (2012) An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7(3):e33439
Price ND, Reed JL, Palsson BO (2004) Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat Rev Microbiol 2(11):886–897
Redwood MD, Orozco RL, Majewski AJ, Macaskie LE (2012) An integrated biohydrogen refinery: synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Bioresour Technol 119:384–392
Ren L, Ahn Y, Logan BE (2014) A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ Sci Technol 48(7):4199–4206
Rodriguez J, Kleerebezem R, Lema JM, van Loosdrecht MCM (2006) Modeling product formation in anaerobic mixed culture fermentations. Biotechnol Bioeng 93(3):592–606
Ryckebosch E, Drouillon M, Vervaeren H (2011) Techniques for transformation of biogas to biomethane. Biomass Bioenergy 35(5):1633–1645
Santoro C, Soavi F, Serov A, Arbizzani C, Atanassov P (2016) Self-powered supercapacitive microbial fuel cell: the ultimate way of boosting and harvesting power. Biosens Bioelectron 78:229–235
Sawers RG (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33(Pt 1):42–46
Schut GJ, Adams MWW (2009) The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: a new perspective on anaerobic hydrogen production. J Bacteriol 191(13):4451–4457
Seeliger S, Janssen P, Schink B (2002) Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol Lett 211(1):65–70
Siriwongrungson V, Zeng RJ, Angelidaki I (2007) Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res 41(18):4204–4210
Smith ET, Blamey JM, Zhou ZH, Adams MWW (1995) A variable-temperature direct electrochemical study of metalloproteins from hyperthermophilic microorganisms involved in hydrogen production from pyruvate. Biochemist 34(21):7161–7169
Soboh B, Linder D, Hedderich R (2004) A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology 150(7):2451–2463
Sowunmi A, Mamone RM, Bastidas-Oyanedel J-R, Schmidt JE (2016) Biogas potential for electricity generation in the emirate of Abu Dhabi. Biomass Conv Bioref 6(1):39–47
Stams AJM, Dijkema C, Plugge CM, Lens P (1998) Contribution of 13C-NMR spectroscopy to the elucidation of pathways of propionate formation and degradation in methanogenic environments. Biodegradation 9(6):463–473
Steinbusch KJJ, Hamelers HVM, Plugge CM, Buisman CJN (2011) Biological formation of caproate and caprylate from acetate: fuel and chemical production from low grade biomass. Energy Environ Sci 4(1):216–224
Sun M, Zhai L-F, Li W-W, Yu H-Q (2016) Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem Soc Rev 45(10):2847–2870
Temudo MF, Kleerebezem R, van Loosdrecht M (2007) Influence of the pH on (open) mixed culture fermentation of glucose: a chemostat study. Biotechnol Bioeng 98(1):69–79
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6(8):579–591
Ueno Y, Fukui H, Goto M (2007) Operation of a two-stage fermentation process producing hydrogen and methane from organic waste. Environ Sci Technol 41(4):1413–1419
van der Ha D, Nachtergaele L, Kerckhof F-M, Rameiyanti D, Bossier P, Verstraete W, Boon N (2012) Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ Sci Technol 46(24):13425–13431
Wang L, Zhang W, Chu H, Dong B (2016) Forward osmosis filtration for removal of organic foulants: effects of combined tannic and alginic acids. Water Res 91:251–263
Wang X, Wang Y, Zhang X, Xu T (2012) In situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: operational compatibility and uniformity. Bioresour Technol 125:165–171
Wang X, Wang Y, Zhang X, Feng H, Xu T (2013) In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: continuous operation. Bioresour Technol 147:442–448
Wu Y, Wang C, Liu X, Ma H, Wu J, Zuo J, Wang K (2016) A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour Technol 211:16–23
Xu J, Guzman JJL, Andersen SJ, Rabaey K, Angenent LT (2015) In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem Commun 51(31):6847–6850
Xue C, Zhao J, Lu C, Yang S-T, Bai F, Tang IC (2012) High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol Bioeng 109(11):2746–2756
Xue C, Zhao JB, Liu FF, Lu CC, Yang ST, Bai FW (2013) Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresour Technol 135:396–402
Xue C, Liu F, Xu M, Zhao J, Chen L, Ren J, Bai F, Yang S-T (2016) A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production. Biotechnol Bioeng 113(1):120–129
Yang S-J, Kataeva I, Hamilton-Brehm SD, Engle NL, Tschaplinski TJ, Doeppke C, Davis M, Westpheling J, Adams MWW (2009) Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe “Anaerocellum thermophilum” DSM 6725. Appl Environ Microbiol 75(14):4762–4769
Yentekakis IV, Goula G (2017) Biogas management: advanced utilization for production of renewable energy and added-value chemicals. Front Environ Sci 5:7. doi:10.3389/fenvs.2017.00007
Yu Z, Morrison M, Schanbacher FL (2010) Biomass to biofuels: strategies for global industries. Blackwell Publishing Ltd., Hoboken
Zacharof MP, Lovitt RW (2013) Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci Technol 69(3):495–503
Zhang F, Huang C, Xu T (2009) Production of sebacic acid using two-phase bipolar membrane Electrodialysis. Ind Eng Chem Res 48(16):7482–7488
Zhang F, Zhang Y, Chen M, Zeng RJ (2012) Hydrogen supersaturation in thermophilic mixed culture fermentation. Int J Hydrog Energy 37(23):17809–17816
Zhang F, Ding J, Shen N, Zhang Y, Ding Z-W, Dai K, Zeng RJ (2013a) In situ hydrogen utilization for high fraction acetate production in mixed culture hollow-fiber membrane biofilm reactor. Appl Microbiol Biotechnol 97(23):10233–10240
Zhang F, Ding J, Zhang Y, Chen M, Ding Z-W, van Loosdrecht MCM, Zeng RJ (2013b) Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res 47(16):6122–6129
Zhang F, Zhang Y, Chen M, van Loosdrecht MCM, Zeng RJ (2013c) A modified metabolic model for mixed culture fermentation with energy conserving electron bifurcation reaction and metabolite transport energy. Biotechnol Bioeng 110(7):1884–1894
Zhang F, Chen Y, Dai K, Zeng R (2014a) The chemostat study of metabolic distribution in extreme-thermophilic (70 °C) mixed culture fermentation. Appl Microbiol Biotechnol 98(24):10267–10273
Zhang F, Zhang Y, Ding J, Dai K, van Loosdrecht MCM, Zeng RJ (2014b) Stable acetate production in extreme-thermophilic (70 °C) mixed culture fermentation by selective enrichment of hydrogenotrophic methanogens. Sci Rep 4:5268
Zhang F, Chen Y, Dai K, Shen N, Zeng RJ (2015a) The glucose metabolic distribution in thermophilic (55 °C) mixed culture fermentation: a chemostat study. Int J Hydrog Energy 40(2):919–926
Zhang F, Zhang Y, Chen Y, Dai K, van Loosdrecht MCM, Zeng RJ (2015b) Simultaneous production of acetate and methane from glycerol by selective enrichment of hydrogenotrophic methanogens in extreme-thermophilic (70 °C) mixed culture fermentation. Appl Energy 148:326–333
Zhang F, Yang J-H, Dai K, Chen Y, Li Q-R, Gao F-M, Zeng RJ (2016a) Characterization of microbial compositions in a thermophilic chemostat of mixed culture fermentation. Appl Microbiol Biotechnol 100(3):1511–1521
Zhang F, Yang J-H, Dai K, Ding Z-W, Wang L-G, Li Q-R, Gao F-M, Zeng RJ (2016b) Microbial dynamics of the extreme-thermophilic (70 °C) mixed culture for hydrogen production in a chemostat. Int J Hydrog Energy 41(26):11072–11080
Zhang Y, Pinoy L, Meesschaert B, Van der Bruggen B (2011) Separation of small organic ions from salts by ion-exchange membrane in electrodialysis. AICHE J 57(8):2070–2078
Zhang Y, Zhang F, Chen M, Chu P-N, Ding J, Zeng RJ (2013d) Hydrogen supersaturation in extreme-thermophilic (70°C) mixed culture fermentation. Appl Energy 109:213–219
Zheng H, O'Sullivan C, Mereddy R, Zeng RJ, Duke M, Clarke WP (2010) Experimental and theoretical investigation of diffusion processes in a membrane anaerobic reactor for bio-hydrogen production. Int J Hydrog Energy 35(11):5301–5311
Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (51408530, 50978244, and 51478447), the Natural Science Foundation of Hebei Province (E2015203306), the Foundation of Hebei Education Department (BJ2017014), and the Program for Changjiang Scholars and Innovative Research Team in University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Dai, K., Wen, JL., Zhang, F. et al. Valuable biochemical production in mixed culture fermentation: fundamentals and process coupling. Appl Microbiol Biotechnol 101, 6575–6586 (2017). https://doi.org/10.1007/s00253-017-8441-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8441-z