Abstract
Anaerobic fermentation of biodegradable organic materials is usually carried out to obtain the final product, methane, a valuable energy source. However, it is also well known that various intermediates are produced in this process, e.g. ethanol, volatile organic acids and hydrogen. All these species have applications and value as fuels or chemicals. This paper shows a critical analysis of the potential of using anaerobic fermentation by mixed cultures to produce intermediates, e.g. ethanol, acetic, lactic and butyric acid and hydrogen, rather than methane. This paper discusses the current processes to produce these chemicals and compares them with the alternative approach of using open mixed cultures to produce them simultaneously via fermentation from renewable resources. None of these chemicals is currently produced via mixed culture fermentation: ethanol and lactic acid are usually produced in pure culture fermentation using food crops, e.g. corn or sugar cane, as starting materials; hydrogen, acetic and butyric acids are mainly produced via chemical synthesis from fossil fuel derived starting materials. A possible flow-sheet for the production of these chemicals from organic waste using mixed culture fermentation is proposed and the advantages and disadvantages of this process compared to current processes are critically discussed. The paper also discusses the research challenges which need to be addressed to make this process feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mixed culture biotechnology can be defined as the study of those processes catalysed by undefined mixed microbial cultures (Kleerebezem and van Loosdrecht 2007; Temudo et al. 2007). At industrial scale, processes based on mixed culture biotechnology are currently used for biological wastewater or waste treatment, under aerobic (e.g. the activated sludge process) or anaerobic conditions. In particular, anaerobic digestion combines the treatment of organic waste with energy generation, since the process converts a fraction of the organic matter into methane, which can be burnt to generate electrical energy and heat (Mata-Alvarez et al. 2000). In spite of the considerable interest in the literature for the use of mixed cultures to produce valuable chemicals, e.g. biohydrogen and biopolymers (Cavinato et al. 2011; Dias et al. 2006; Guo et al. 2010; Villano et al. 2010), looking at industrial commercial-scale processes we observe that currently no chemicals other than methane are produced using mixed cultures. On the other hand, pure cultures of naturally occurring or genetically engineered microorganisms are currently used in many industrial processes, e.g. production of bioethanol (Cardona et al. 2010), lactic acid (Datta and Henry 2006) or poly-hydroxyalkanotaes (Ojumu et al. 2004). Compared with pure cultures, processes based on mixed cultures can potentially have various advantages (Dionisi et al. 2006; Kleerebezem and van Loosdrecht 2007): no need for sterilisation of the process lines and vessels, possibility of using mixed substrates like municipal solid waste, no inoculum cost and no need for sterile conditions for precultivation of the pure cultures. The possibility of using mixed substrates in mixed culture processes is particularly important and derives from the fact that in mixed cultures different microbial species, that coexist in the same vessel, can metabolise different substrates and can perform the various steps required for the conversion of the substrate into the desired product, i.e. hydrolysis of organic polymers and metabolisation of the monomers. This process happens in mixed culture processes for aerobic wastewater treatment and in anaerobic digestion.
A recent paper by this research group (Dionisi et al. 2015) has reviewed the potential of microbial processes to produce ethanol from lignocellulosic biomass. The aim of this paper is to extend the analysis to the potential and the limitations of using mixed cultures to produce a wider range of chemicals at commercial scale, which is a process initially hypothesised by Levy et al. (1981). In particular the focus of this paper are the intermediate products of anaerobic digestion, for which there is an increasing interest in the literature (Agler et al. 2011; Angenent et al. 2004; Kleerebezem et al. 2015; Weimer 2015). It is well known that anaerobic fermentation of biodegradable organic matter generates a variety of chemicals, alcohols, volatile fatty acids (VFA’s) and hydrogen, which in conventional anaerobic digestion are further converted to methane (Batstone et al. 2002; Demirel and Yenigün 2002). However, the intermediates products of anaerobic digestion have a value and a large market per se as energy vectors (e.g. ethanol) or chemicals (volatile fatty acids) or both (hydrogen).
The paper is organised as follows:
-
Section 2 reviews the manufacturing processes which are currently used to produce ethanol, acetic, lactic and butyric acid and hydrogen. The review of current manufacturing processes will provide a basis for a critical analysis of the advantages and disadvantages of mixed culture processes. Acetic, lactic and butyric acid and hydrogen have been chosen since they are among the most frequently observed intermediate products of anaerobic fermentation of organic matter, however the discussion and conclusions drawn in this paper can be extended to the production of other intermediates of anaerobic digestion;
-
Section 3 reviews the microbial reactions that lead to the formation of ethanol and organic acids from biomass while Sect. 4 gives examples of possible biomass substrates for the fermentation process;
-
Section 5 presents a possible process scheme to produce these chemicals from anaerobic fermentation of organic waste. This process is then critically discussed and compared with the current processes described in Sect. 2;
-
Section 6 reviews the state of the art regarding anaerobic fermentation by mixed cultures to produce ethanol, organic acids and hydrogen and Sect. 7 analyses the feasibility of the process at local and global scale;
-
Section 8 highlights and discusses the research needs in this area and Sect. 9 (conclusions) summarises the work.
2 Current manufacturing processes for ethanol, hydrogen and organic acids
Ethanol, hydrogen, acetic acid, lactic acid and butyric acid are important chemicals with a wide range of uses. The industrial applications, production rates and market price of these chemicals are summarised in Table 1.
Ethanol, which has the largest annual production, has its main application as fuel as a substitute for oil-derived petrol, however it also find applications in the chemical industry for the production of esters. Hydrogen is mainly used in the production of fertilisers (ammonia synthesis), methanol, oil refinery, e.g. hydrocraking and in the chemical industry for hydrogenation reactions. Interest in the use of hydrogen for fuel cells or vehicles has increased over the last decades (European Commission 2014), even though its use as fuel is currently only marginal. Acetic acid is widely used as an intermediate in the chemical industry, e.g. in the plastics, coating, painting and photographic industries (Yoneda et al. 2001). Production of lactic and butyric acids is lower than for the other chemicals. Lactic acid is used for the production of plastics (poly-lactic acid) and as food ingredient, while butyric acid has a variety of uses in the food, cosmetic and chemical industries.
In this section the main processes currently used for the manufacture of ethanol, hydrogen, acetic, lactic and butyric acids are described.
2.1 Ethanol
Ethanol is mainly produced via fermentation of organic materials by pure cultures of selected microorganisms. Different types of agricultural feedstock can be converted into ethanol such as corn, sugarcane, sugar beets, sorghum, molasses (Carioca and Leal 2011). The type of feedstock to be used depends, in general, on the geographical availability. Brazil has developed the technology to produce bioethanol from sugar cane, while the US has focused on the production via corn starch fermentation. Both countries are the largest bioethanol producers, comprising together about 88 % of the production in the world (Gupta and Verma 2015).
Figure 1 shows simplified flow sheets for bioethanol production from corn or sugar cane. When corn is used as feedstock, the typical sequence of operations is (adapted from Cardona et al. 2010 and Kwiatkowski et al. 2006): grinding of the feedstock, liquefaction, starch hydrolysis, fermentation and ethanol purification. The aim of the liquefaction stage, which is typically carried out at a temperature of 80–90 °C, is to solubilise all the starch components (amylose and amylopectin) in order to make them available for the next stage of starch hydrolysis. During liquefaction, thermoresistant α-amylase enzymes are also added in order to start starch hydrolysis which will be completed in the next step. Starch hydrolysis is carried out with hydrolytic enzymes under neutral or acidic pH. At the end of starch hydrolysis a concentrated glucose solution is obtained, which is fermented in the next stage using pure cultures of microorganisms giving high ethanol yield. Saccharomyces cerevisiae is one of the microbial species used more frequently. The fermentation reaction producing ethanol is the following, where biomass production is neglected:
Ethanol concentration at the end of the fermentation process is typically in the range 8–10 % by weight. Pure ethanol (>99 % by weight) is then obtained using a sequence of distillation columns followed by final purification using various technologies such as extractive distillation, azeotropic distillation or molecular sieves.
When sugar cane is used as feedstock, ethanol is usually produced as a co-product of the production of sugar. The process description below is adapted from (Amorim et al. 2011) and (Cardona et al. 2010).
The process begins with the sugarcane juice extraction in the mills, separating the sugarcane juice from the bagasse. Sugarcane is cleaned to remove sand, dirt and metals in the mills and fed to the juice treatment operations: clarification and concentration. The bagasse, a fibrous leftover of the extraction stage, is burnt in the boilers to supply the industrial plant energy demands, further exporting the surplus of electricity to the grid.
In the clarification stage, the sugarcane juice receives physical and chemical treatments to remove its impurities. Fibres and dirt particles are removed by screens and hydrocyclones and phosphoric acid is used to enhance the impurities removal during settlement. The sugar is then pre-heated up to 70 °C and receives lime. After being heated up again to 105 °C, the hot juice is flashed to remove air bubbles and then added in a settler with a flocculant polymer. The mud obtained in the settler can be filtered to enhance sugar recovery and to recycle it to the process. The filter cake can be used as fertirrigation (a fertilizer) in the fields. The clarified juice is concentrated and can be mixed with molasses to achieve a desired feed concentration. The juice is sterilised to avoid microbial contamination before being fed in the fermenter. During the fermentation stage, in about 6–12 h the alcohol concentration reaches 7–11 % in volume and the fermented broth is centrifuged to separate the yeast from the wine. The yeast receives an acid treatment at pH 2.0–2.5 to reduce bacterial contamination and, then, to be recycled in the next batch operation. Ethanol is then purified according to similar processes as the ones described above for the ethanol production from corn.
Table 2 shows the costs components in the production of ethanol from corn and from sugar cane. In both cases the main contributor to the total production cost is the cost of the feedstock. Corn represents almost 60 % of the total production cost and other raw materials, which include the chemicals and the microorganisms, add a further 7 %. Similarly, the cost of the raw material represents approximately 68 % of the total production cost of ethanol from sugar cane. The cost of the distillation process is given by the cost of the steam and by the fraction of the capital cost due to the distillation equipment. Overall, using corn as feedstock the distillation process accounts for little more than 10 % of the total production costs. Using sugar cane as feedstock the cost of the distillation is even lower, because all the required steam is generated by burning bagasse, and therefore the steam required for distillation becomes available at no cost. Other contributions to the total costs are utilities and labour. As far as the capacity of the plants is concerned, the average capacity of ethanol production plants from corn in the US is approximately 200,000 tonnes/year, but it varies in a wide range, from a few thousands to over 1,000,000 tonnes/year (Ethanol Producer Magazine 2015).
2.2 Lactic acid
Currently the main production process for lactic acid (Fig. 2) is based on the fermentation of variety of feedstocks using pure cultures of lactic acid bacteria (Litchfield 1996; Martinez et al. 2013; Miller et al. 2011; Randhawa et al. 2012). Often organic wastes such as cheese whey, molasses or starch-based wastes are used. Various microorganisms in pure cultures are currently used for lactic acid production, e.g. Lactobacillus casei. An advantage of lactic acid production by fermentation over its chemical synthesis is that only the desired optical isomer can be produced by microbial fermentation. The fermentation reaction for lactic acid production is shown below, ignoring the production of biomass
The fermentation usually occurs in batch or fed-batch operation mode in sterilised fermenters to prevent unwanted microbial growth. The temperature and pH are set in the fermenter depending on the microorganism, varying from 30 to 60 °C and pH 5–7. The values are specified to obtain the optimal lactic acid formation rate for the specific microbial strain. Calcium carbonate or calcium hydroxide is added in the fermenter to neutralise the acid production, since low pH can retard the fermentation process. Adding calcium hydroxide, the calcium lactate salt is formed. The length of fermentation also varies for different carbohydrate sources—1–2 days for a 5 % sugar feedstock or 2–6 days for feedstock with 15 % of sugar or sucrose. After fermentation, temperature and pH are increased to 80–100 and 10–11 °C respectively, in order to kill the microorganisms, solubilise the calcium lactate and degrade the residual sugar. Then, the broth is filtered to remove the biomass and sulphuric acid is added to obtain lactic acid from calcium lactate according to the stoichiometry below.
Insoluble waste gypsum (calcium sulphate) is consequently formed and removed by filtration. Crude lactic acid is concentrated by evaporation and taken to other purification steps. One of the main problems in the current production of lactic acid is the large production of waste gypsum, which makes the process economically and ecologically unattractive. This typical process produces approximately one metric tonne of waste gypsum for each tonne of lactic acid produced. Other technologies have been developed to avoid this waste production such as eletrodialysis, but still have high capital and operating costs (Miller et al. 2011).
Lactic acid production. Adapted from Inamdar (2012)
2.3 Acetic acid
Acetic acid is mainly produced by methanol carbonylation (around 60 % of the world manufacturing capacity), followed by acetaldehyde, ethanol and light hydrocarbon oxidations (Kent 2010; Sunley and Watson 2000). In general, the feedstock for acetic acid production derives entirely from fossil fuels. Both in the traditional Monsanto process and in the more recent Cativa process (Fig. 3) methanol carboxylation is catalysed by rhodium or iridium catalysts promoted with methyl (or lithium) iodide. The overall reaction can be written as follows:
The process operate at a pressure of 20–60 atm and at temperatures of 150–200 °C. After the reaction, purification of acetic acid from the rest of the reaction medium is required. The reaction medium includes methyl iodide, present up to 25 % by weight in the reactor, water, which is present up to 8 % in the Cativa and up to 15 % in the Monsanto process in order to activate and stabilise the catalyst, methyl acetate and heavier by-products, mainly propionic acid. Purification is typically achieved in a sequence of distillation columns. Also, non-condensable by products, mainly methane, hydrogen, carbon dioxide, are formed in the reactor, and these need to be scrubbed before release to the atmosphere. Scrubbing is obtained in an absorption process using a sequence of methanol, acetic acid and water as absorbing liquids, in order to ensure that no iodine-containing species are released to the atmosphere.
Acetic acid production. Adapted from Yoneda et al. (2001)
The costs components for the production of acetic acid are shown in Table 3. The cost of the raw materials, methanol and carbon monoxide, is an important contribution (30 %) to the total production cost. Investment cost is also an important cost factor, accounting for approximately 30 % of the total production cost. The catalysts, rhodium or iridium, is also very expensive (e.g. 1 mol of RhCl3·3H2O costs around $30,000). The high capital costs are in part due to the fact that the process requires expensive materials (Hastelloy in many parts) and appropriate systems to handle volatile iodide species (Kent 2010). Capacities of full scale acetic acid plants are usually of 100,000 tonnes/year or higher.
2.4 Butyric acid
Currently, n-butyric acid is mainly produced via petrochemical routes through the oxidation of n-butyraldehyde which is synthesised from propylene (Fig. 4) (Dwidar et al. 2012; Frohning et al. 2002; Kubitschke et al. 1986; Xu and Jiang 2011). The n-butyraldehyde synthesis reaction requires propylene, hydrogen and carbon monoxide according to the stoichiometry below:
Similarly to acetic acid, the starting materials for butyric acid production are in essence crude oil and natural gas. The n-butyraldehyde synthesis reaction occurs usually over rhodium catalyst with temperature at 70–150 °C and pressure between 15 and 50 atm. Depending on the operating conditions, process design, catalyst-ligand system, etc., different ratios of n/iso-butyraldehyde can be obtained. Then, n-butyraldehyde is oxidised by pure oxygen or air in a liquid phase to produce butyric acid, according to the stoichiometry below:
n-butyric acid production. Adapted from Lee et al. (2007)
2.5 Hydrogen
The main industrial process currently used for hydrogen production is steam methane reforming (SMR), and the overall chemical reaction is given below:
The particular SMR process described here is based on Balasubramanian et al. 1999. Figure 5 depicts a simplified SMR flow-diagram and divides the process into three different stages: reforming, shifting and carbon dioxide removal. In the reforming stage, desulphurised methane is mixed with superheated steam and the endothermic reforming reaction occurs at 900 °C over a nickel-based catalyst, with the reaction stoichiometry reported below:
The next step is the catalytic reaction (shifting) where carbon monoxide is reacted in two different stages: high-temperature (HTS) and low-temperature shift (LTS). In the HTS converter, the exothermic shift reaction occurs at 310–450 °C and 100–8375 kPa, converting around 94 % of carbon monoxide over an iron oxide catalyst (Fe 2 O 3). Owing to the exothermic reaction, the flow temperature increases along the length and the inlet temperature is controlled to prevent the exit temperature from exceeding 550 °C (Newsome 1980). Then, part of the remaining CO reacts in the LTS at a range of 200–250 °C over cooper oxide (CuO). The stoichiometry of the shifting reaction is:
In the carbon dioxide removal stage, the gas is compressed to approximately 35 atm and carbon dioxide is scrubbed with monoethanolamine. Steam is supplied to the stripper and the carbon dioxide content in the hydrogen stream is 0.1 % by weight.
Hydrogen production. Adapted from Balasubramanian et al. (1999)
According to Rostrup-Nielsen (2005) typical capacities of hydrogen production plants range from 10,000 to 100,000 Nm3/h, but smaller capacities, between 5 and 1000 Nm3/h, are typical when hydrogen is used for fuel cells applications. Hydrogen production cost is mainly determined by the operating costs (hydrocarbon and steam costs).
3 Fermentation of organic biomass
As an alternative to the conventional processes described above, ethanol, hydrogen, acetic, lactic and butyric acid could also, in principle, be produced by anaerobic fermentation of organic biomass by mixed microbial cultures. The routes leading to these products from the various components of organic biomass are summarised in Fig. 6. Other intermediates can also be produced from anaerobic fermentation of organic biomass, e.g. formic acid, propionic acid, succinic acid and others. Also, it is important to observe that all these species are intermediates and they all can be further metabolised to the end product, methane, if process conditions allow for the growth of methanogenic microorganisms.
Examples of products produced by anaerobic fermentation of organic matter. Main products shown only, other fermentation products are also possible. Hydrogen is reported in brackets because it is produced simultaneously to organic acids from sugars or long chain fatty acids but not necessarily from aminoacids
One of the factors that determines which product is obtained is the chemical nature of the substrate, which can be divided into carbohydrates, proteins and lipids.
Carbohydrates are defined as (poly)hydroxyaldeides or (poly)hydroxychetones made of carbon, hydrogen and oxygen. They can be present as monosaccharides (sugars) or as polysaccharides (cellulose, hemicellulose and starch). Only monomeric species, sugars, can be directly metabolised by microorganisms, while polysaccacharides need to be hydrolysed to sugars in order to be metabolised.
A few examples of fermentation reactions of carbohydrates in a mixed culture environment are reported below, using glucose as an example (Antonopoulou et al. 2008):
Other stoichiometries for lactic acid production are also possible. As far as the species considered in this paper are concerned, it is important to note that hydrogen is produced when acetic and butyric acids are produced, but not during ethanol or lactic acid production. The ability to ferment carbohydrates to organic acids, ethanol and hydrogen is widespread among many bacterial species, including Escherichia coli (Rosales-Colunga and de León 2015) and species recently isolated from Antarctica (Alvarado-Cuevas et al. 2015).
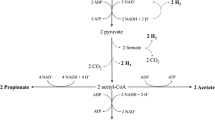
Proteins are nitrogen-containing organic substances of high molecular weight. Before being metabolised, proteins need to be hydrolysed to their monomeric building blocks, the aminoacids. Fermentation of aminoacids can yield various organic acids, most frequently acetic and butyric acids. Examples of reactions converting amino acids to acetic and butyric acids are the following (Ramsay and Pullammanappallil 2001):
As the reaction stoichiometry indicate, aminoacid metabolisation may produce hydrogen or consume it, depending on the particular amino acid being metabolised.
Lipids are high molecular weight substances made from the combination of long chain fatty acids and glycerol. Like the other polymeric organic substances, lipids metabolism requires hydrolysis to the monomeric building blocks, in this case fatty acids and glycerol. Under anaerobic conditions, long-chain fatty acids are usually converted to acetic acid and hydrogen in a sequence of reactions called beta-oxidation. Examples of anaerobic conversion of fatty acids are reported below (Sousa et al. 2007):
As indicated by the reaction stoichiometries above, lipid metabolism under anaerobic conditions does not generate CO2, which is a positive aspect since it indicates that all the carbon in the starting material is converted to useful products. From these reactions it can be calculated that anaerobic digestion of fatty acids generates the highest yield of hydrogen, e.g. 10 % of the mass of palmitic acid is transformed into hydrogen, while for glucose fermentation only approximately 4 % of the mass of the starting material is converted to hydrogen (in case of the fermentation of glucose to acetic acid). The stoichiometry of the various reactions showed in this section indicate that the nature of the starting material is an important factor in determining the fermentation products and their yields.
In addition to the reactions shown above many other reactions occur in an anaerobic environment with mixed cultures, and they are important because they can alter the final products obtained in the fermentation of organic substrates. One of these reactions is the conversion of VFA’s to acetic acid, e.g. butyric acid can be converted to acetic acid via the following reaction:
Also ethanol can be converted to acetic acid:
Very important reactions are the ones that remove acetic acid and hydrogen to generate methane:
Microorganisms that produce methane from hydrogen are called hydrogenotrophic methanogens, while microorganisms that use acetic acid as substrate are called acetoclastic methanogens. These two last reactions are the desired ones if anaerobic digestion is carried out to produce methane, while they are undesired if the aim of the process is to produce ethanol, VFA’s and hydrogen, which is the process discussed in this paper.
4 Sources of organic biomass for fermentation
The most obvious and environmentally friendly choice as a feedstock for anaerobic conversion to ethanol, organic acids and hydrogen by mixed cultures is biodegradable organic waste. Examples of biodegradable organic wastes and their composition are shown in Table 4. The figures about the annual production of organic wastes reported in Table 4 have to be taken as broad estimates rather than accurate figures, however they indicate that the production of biodegradable organic waste is in the order of many billions of tonnes per year. The composition of the organic waste is important since it is one of the main factors that determines the obtainable products. Agricultural waste such as rice straw, corn straw, etc. is typically mainly composed of lignin and cellulose/hemicellulose. Anaerobic fermentation of lignocellulosic substrates, although has been proven feasible even in absence of pretreatments, is usually considered to be very slow (Dionisi et al. 2015), but these substrates are potentially very interesting due to the large volumes produced and to the fact that carbohydrates fermentation can lead to the production of ethanol, which is the chemical required in the largest amount among the species considered here. Another very large source of biodegradable organic matter is municipal solid waste. This waste has a wide chemical composition and therefore it is likely to give a variety of species as intermediates of anaerobic digestion. Other important sources of organic waste is livestock manure, which is typically richer in proteins than other types of waste.
It is important to observe that many of these wastes may already have uses. E.g. many biodegradable organic wastes are used in anaerobic digesters for conversion to methane (De Bere 2000; Zhang et al. 2012a, b). Fruit and vegetable wastes can be used as livestock feed and a wide range of potential other uses has been suggested (Arvanitoyannis and Varzakas 2008; Wadhwa and Bakshi 2013). The organic fraction of municipal solid waste can be converted to compost and this process is carried out by many municipalities (Kumar 2011). However, in spite of the various potential uses and technologies for organic waste, a large fraction of it still is discharged in landfills, e.g. most of the global production of fruit and vegetable waste (Wadhwa and Bakshi 2013). It is estimated that over 2 million tonnes per year of municipal solid waste are landfilled every year in Scotland, of which 63 % is considered biodegradable (Zero Waste Scotland 2010).
It is not possible to make a comprehensive comparison of the various options and technologies for the conversion/recycle of organic waste and the most appropriate method/technology will be determined in the end by market conditions and local circumstances. However it is possible to make a simple, although certainly not exhaustive, comparison of the two process options for anaerobic fermentation: conversion to a mixture of ethanol, organic acids and hydrogen or conversion to methane, the final product. This comparison is based exclusively on the revenues obtained from the products and does not consider the process costs, in particular it does not consider the separation costs of the liquid products. A full economic assessment of the process, which includes the separation costs, will be needed in order to establish which process is more economically viable. In the following calculations market prices reported in Table 1 are assumed for ethanol, acetic acid and hydrogen, and for methane a market price of 0.17 US$/kg is assumed (NASDAQ 2015). Assuming glucose as model substrate for this example, the stoichiometry of glucose conversion to methane can be written as, ignoring the fraction of the substrate which is used for microorganisms’ growth:
From this stoichiometry we can calculate that anaerobic digestion to methane generates a product value of approximately 45 US$/1000 kg glucose.
Similarly, the stoichiometry of glucose conversion to ethanol, acetic acid and hydrogen can be written as, assuming that 50 % of the glucose is used for ethanol production and 50 % for acetic acid (and therefore hydrogen) production:
This reaction corresponds to a total product value of approximately 600 US$/1000 kg glucose, i.e. a much larger revenue than glucose conversion to methane. The larger revenue that can be obtained from conversion of glucose to ethanol, acetic acid and hydrogen can be explained considering that these chemicals are more versatile than methane. Indeed, methane is mainly used for energy generation, while ethanol, acetic acid and hydrogen have uses as a whole both as chemicals and as energy vectors. However this market analysis can only be considered very approximate. Besides, if large amounts of acetic acid and hydrogen are put on the market as a result of anaerobic fermentation of organic substrates, it is likely that the market price of these chemicals will decrease. However, it is also possible that the possibility of generating large volumes of these chemicals will contribute to develop new applications for them, with a consequent increase in demand.
5 Possible mixed culture process for production of ethanol, hydrogen and organic acids from renewable resources and critical comparison with current processes
Figure 7 shows a possible process scheme for the simultaneous production of ethanol, hydrogen and organic acids from biodegradable organic waste. The organic waste is digested in an anaerobic reactor where the fermentation is controlled to the production of intermediates, rather than driven to the final conversion of the organic matter to methane. Control of the fermentation conditions to produce intermediates and not methane is reviewed and discussed in the next section. Under these conditions it is expected that the gas phase from the reactor contains mainly hydrogen and carbon dioxide. Carbon dioxide can be separated using conventional absorption processes and purified hydrogen can be recovered and utilised as chemical or as fuel. The liquid–solid effluent is first subject to solid–liquid separation. The solid phase is made of the produced microorganisms and of any solids that have not been hydrolysed in the fermenter. The solids can be sent to further conversion to methane in a conventional anaerobic digester operated at a much longer residence time. The products in the liquid phase can be separated from water and purified. For illustrative purposes in Fig. 7 the liquid phase is assumed to be composed of water, ethanol, acetic, butyric and lactic acids and the components are separated in a sequence of distillation columns. This is in theory possible since these species have different boiling points, at atmospheric pressure the boiling temperatures are 78, 100, 118, 122 and 163 °C for ethanol, water, acetic, lactic and butyric acid respectively. However, separation by distillation may be uneconomical and various alternative separation processes are possible, as discussed in the next sections.
It is worth observing that two of the products from the process scheme in Fig. 7, hydrogen and acetic acid, are used in many reactions by the chemical industry as discussed in Sect. 2. This therefore indicates the possibility of integrating a biorefinery (Fig. 7) with a conventional chemical plant for the production of, for example, fertilisers (from hydrogen) and plastics (from acetic acid), with the significant advantage that some of the feedstock required for the chemical plant derives from organic waste rather than from fossil fuels.
Table 5 reports selected literature examples of lab-scale studies where mixed cultures have been investigated for the production of ethanol, VFA’s and hydrogen. On the basis of the data in Table 5 and of the process scheme in Fig. 7, the main advantages and limitations that a mixed-culture process for the production of ethanol, organic acids and hydrogen would have versus the current processes, shown in Figs. 1, 2, 3, 4 and 5, are discussed below.
-
Feedstock: current processes for the production of hydrogen, acetic acid and butyric acid use non-renewable feedstock derived from oil and gas. On the other hand, a process based on mixed-cultures can use organic waste, a renewable and cheap feedstock. In Table 5 various examples of chemicals production from wastes using mixed cultures are reported. This is clearly an advantage for the biological process because it would not impact on fossil fuel reserves, which are limited, and it would not contribute to increasing the carbon dioxide in the atmosphere, since the carbon dioxide generated from the fermentation is removed when the crops which originate the biodegradable waste grow. Compared to the current production process for ethanol from corn or sugarcane, production of ethanol using wastes and mixed cultures would avoid the undesirable food versus fuel competition. For the sugarcane process this is particularly true if sugarcane cultivation is exclusively dedicated to the production of ethanol without any production of sugar;
-
Process conditions: current processes for the production of hydrogen, acetic acid and butyric acid use high temperature (up to 900 °C for hydrogen production) and high pressure conditions (up to 60 atm for acetic acid synthesis). On the other hand, fermentation processes, such as the mixed-culture process described here, usually work at mild conditions (atmospheric pressure and temperatures typically not exceeding 50–60 °C) even though anaerobic fermentation studies at up to 80 °C have been reported (Wiegel 1980). Processes operating at high-temperature and pressure require more expensive materials for the reactors and may require additional consumption of non-renewable fuel to heat-up the reactants;
-
Reaction rates: comparing pure culture processes for ethanol production with mixed culture processes, in general higher reaction rates have been reported for pure cultures. Typical productivities reached by pure culture fermentation for ethanol production are in the range 25–75 kg/m3/day, with values of up to 300 kg/m3/day reported with special lab-scale reactor configurations (Cardona et al. 2010). On the other hand, reported values (Table 5) for ethanol productivities with mixed cultures are typically below 30 kg/m3/day, even though many literature studies were not aimed at maximising the productivity of any particular product and therefore the maximum productivity with mixed culture has certainly not been reached yet;
-
Use of heavy metals: in the production of hydrogen, acetic acid and butyric acid heavy metals, e.g. nickel and rhodium, are used as catalysts in the reaction stage. Heavy metals are non-renewable and expensive, e.g. as discussed in Sect. 2.3, in the acetic acid process one of the main cost factors is the rhodium or iridium catalyst. Fermentation processes do not require heavy metals as catalysts and this is clearly an economic and environmental advantage for the mixed-culture processes;
-
Purity of the reaction products: a major drawback of mixed-culture processes is that the fermentation products are likely to contain a mixture of substances which need to be separated into the pure components. Looking at Table 3, concentrations of each of the chemicals produced by mixed-culture anaerobic fermentation are typically no more than a few % each. On the other hand, both pure-culture fermentation processes and conventional non-fermentation processes produce a lower range of products from the reaction stage and the main product is usually more concentrated. For example, in industrial processes for ethanol production from mixed cultures, ethanol concentration at the end of the fermentation process is usually in the order of 10 % (Cardona et al. 2010). Therefore, the separation processes which follow the reaction stage are likely to be more difficult and more expensive in a mixed-culture process than in pure-culture fermentations and in current chemical processes. However, it is fair to say that even in current processes often separation processes are quite complex, e.g. in the manufacture of acetic acid at least two distillation columns are required to separate acetic acid from methyl iodide, water and other organic acids by-products;
-
Feasibility of integrated bioprocessing: the use of mixed cultures would allow a one-pot conversion from the feedstock to the products, due to the coexistence of different microbial populations which would be responsible for the hydrolysis and fermentation stages. Indeed, in Table 5 many lab-scale examples of one-stage conversion of organic wastes are reported. In general, pure cultures cannot carry out one-stage conversion of complex substrates into products because a given microbial species is usually active only on a limited number of substrates. As an alternative to mixed cultures, genetically modified microorganisms are being considered for integrated (or consolidated) bioprocessing, especially for the conversion of lignocellulosic materials into ethanol (Dionisi et al. 2015; Olson et al. 2012). However, in the industrial processes currently in use for ethanol production, integrated bioprocessing is not used, due to the limited capability of the pure cultures used. As an example, in current processes (Fig. 1) for ethanol production from corn using S. cerevisiae, several pre-treatment stages are required in order to hydrolyse starch to glucose, because S. cerevisiae is not able to hydrolyse starch.
5.1 Summary of economic and environmental considerations
At this stage it is not possible to make a complete cost comparison between the production of chemicals using the conventional processes and using mixed culture biotechnology. However, based on the considerations above and on the previous sections some important points can be highlighted.
In Sect. 2 it has been shown that a major cost component for the production of both ethanol and acetic acid using the conventional processes is the cost of the feedstock, which accounts for almost 60 % of the production costs for ethanol and for approximately 30 % in the case of acetic acid. On the other hand, if organic waste is used in a mixed culture biotechnology process, it is likely that the feedstock will come at very little or even negative cost (due to the offset of waste treatment costs). This will make the economics of a mixed culture process look favourable compared to conventional processes. Also, as discussed above, a biological process does not need heavy metals as catalysts, while many conventional processes for organic acids and hydrogen production do, and this is likely to give a further cost advantage to the mixed culture process. Another potential economic advantage of biological processes is the absence of sterilisation costs. Indeed, sterilisation requires high temperatures and increases the complexity of the process and it has been observed (Cardona et al. 2010) that one of the main issues in the pretreatment of starchy feedstock for ethanol production is the maintenance of sterile conditions during the starch pretreatment stages until the fermentation vessel. On the other hand, separation costs are likely to be lower for the conventional processes than for mixed culture processes. However, separation costs are typically a relatively minor fraction of the total production costs (Tables 2, 3) and may be counterbalanced by the savings on feedstock costs mentioned above.
These economic considerations are strictly linked with environmental considerations. Producing chemicals from waste is a way to treat the waste and reduces the amount of waste disposed into landfills. Also, producing ethanol from waste, instead than from food crops, would avoid any undesirable food versus fuel competition. In general, a biological process can use a renewable resource as feedstock, rather than resources derived from finite amounts of fossil fuels, and therefore would be, at least in this respect, more sustainable than conventional processes. Another important aspect is the use of heavy metals as catalysts, which are required in conventional processes based on fossil fuels but not in biological processes using organic waste as feedstock. Also, the use of milder conditions of temperature and pressure makes biological processes more environmentally acceptable than conventional chemical processes. On the other hand, biological processes have environmental issues which need to be considered and addressed: the use of the final digestate, the environmental impact of waste transportation and the energy consumption due to the separation processes.
6 State of the art of mixed-culture biorefinery
In this section we will critically analyse the mixed culture studies aimed at the production of intermediates, ethanol, organic acids and hydrogen, rather than methane. Here we don’t aim to provide a comprehensive coverage of the literature in this topic, but rather to address the main aspects which are critical for the development of the considered process at industrial scale. Comprehensive literature reviews on anaerobic fermentation for the production of intermediates, mainly focussed on hydrogen as desired product, have been published recently (Guo et al. 2010; Lin et al. 2012).
In general the evidence from literature studies, summarised in Table 5, is that it is certainly possible to control the anaerobic fermentation of organic substrates to intermediate species rather than to the final product, methane. The process has been demonstrated at lab scale for a wide range of substrates, from model substrates (e.g. Temudo et al. 2007), to real wastes or wastewaters (e.g. Jung et al. 2010; Traverso et al. 2000; Yang et al. 2007; Yu et al. 2002). In terms of the composition of the products in the liquid phase, a few general conclusions can be listed:
-
Acetic acid is usually one of the main products in the liquid phase. This is clearly justified considering that acetic acid is a central metabolite in anaerobic fermentation and can be produced from carbohydrates, proteins and fats (Fig. 6);
-
Apart from acetic acid, the presence of other species in the liquid phase is dependent on the nature of the substrate treated. Ethanol is often present in the product stream when the feed is mainly composed of carbohydrates (Temudo et al. 2007, 2009; Yu et al. 2002; Zoetemeyer et al. 1982a, b) while it is not present for substrates of different chemical composition. This is again in agreement with what is known about the metabolism of organic substrates (Fig. 6). In addition to acetic acid and ethanol, other species such as lactic acid, butyric acid and other VFA’s are often present in the effluent. Their relative concentration is to some extent dependent on the operating parameters of the fermentation reactor, such as pH and this will be discussed in the next subsections.
In the next subsections focus will be given to those aspects which are more important for full scale implementation of mixed culture processes, i.e. hydrolysis of lignocellulosic substrates, control of process conditions and substrate concentration in the feed.
6.1 Hydrolysis of lignocellulosic materials
As shown in Table 4, many organic wastes are lignocellulosic in nature. This means that, if a single-stage fermentation process without pretreatments is used, as shown in Fig. 7, then the mixed culture which develops in the fermenter needs to be able to hydrolyse lignin and cellulose. Microbial hydrolysis of lignocellulosic materials has been reviewed recently by this research group (Dionisi et al. 2015) and readers are addressed to this paper for details. So far, the very few attempts reported in the literature to use non-pretreated materials with high lignin and cellulose content as substrates for microbial conversion to ethanol, VFA’s or hydrogen production have been essentially unsuccessful. E.g. non-pretreated corn straw (Li and Fang 2007), corn stalk (Zhang et al. 2007) and grass silage (Karlsson et al. 2008) have been used as substrate for microbial hydrogen production, but the hydrogen yields have been very low. However, there is evidence in the literature that lignin and cellulose can be hydrolysed under anaerobic conditions by both pure and mixed cultures. Particularly interesting are, to this regard, the studies by Sharma et al. (1988) and by Turick et al. (1991). They reported methane production, which requires lignin and cellulose hydrolysis, from biomass with high lignin content, such as leaves and wood. However, the reported degradation rates are low.
While microbial hydrolysis has been so far unsuccessful, considerable interest is being given to chemical, physical or enzymatic pretreatments, which include steam explosion, acid hydrolysis, addition of solvents, of oxidising agents (e.g. ozone) and enzymes. These pretreatments have been reviewed recently (Galbe and Zacchi 2012; Rajendran and Taherzadeh 2014) and the reader is directed to these papers for a detailed description. Pretreatment of lignocellulosic materials has been shown to greatly enhance anaerobic digestibility. E.g. wheat straw showed almost no anaerobic biodegradation in the absence of pretreatments, but it gave high yields of hydrogen and VFA’s when anaerobic digestion followed acid and microwave pretreatment(Fan et al. 2006). The general evidence is that chemical physical technologies are usually effective in obtaining lignin and cellulose hydrolysis at high rates, however they typically require high temperatures and pressure and the addition of chemicals. For these reasons these pretreatments are usually expensive and not particularly environmentally friendly and this is limiting so far their application to full scale processes.
6.2 Control of process conditions
In a mixed culture environment the products obtained and their respective yields depend not only on the chemical nature of the feedstock but also on the applied process conditions. The most important operating conditions that may affect the product distribution are: microorganisms residence time, pH, temperature. Also, the partial pressure of hydrogen is another important variable that can affect the process. In this section we will review what is known about the effect of operating conditions on the mixed culture process considered here.
6.2.1 Effect of solids residence time
In order for the process described in Fig. 7 to be successful, the process conditions need to be controlled in order to favour the growth of the ethanol- and/or VFA-producing microorganisms, while washing out methanogenic microorganisms. One way of achieving this is by control of the residence time of the microorganisms (solids residence time, SRT) in the reactor. From the literature examples shown in Table 5, it is evident that if the solids residence time is controlled to a value which is low enough, methane formation can be suppressed. Indeed, all the studies reported in Table 5 have a SRT in the order of maximum a few days, while anaerobic digestion for methane production is usually carried out at SRT values of 10 days or more (De la Rubia et al. 2006; Nges and Liu 2010). To this regard, if we want to avoid any losses of ethanol, acids and hydrogen, it is important to wash out both acetoclastic methanogens (the microorganisms that produce methane from acetic acid) and hydrogenotrophic methanogens (the microorganisms that produce methane from hydrogen). Literature indicates that hydrogenotrophic methanogens can grow at lower residence times than acetoclastic methanogens (Eastman and Ferguson 1981). For example, under mesophilic conditions (30–40 °C) hydrogenotrophic methanogens have been reported to require a minimum residence time in the order of 1–2 days (Shea et al. 1968), while acetoclastic methanogens require a minimum residence time of approximately 2.5–4 days (Lawrence and McCarty 1969). Even though the exact values of residence times for wash-out of methanogenic microorganisms vary from study to study, there seems to be general agreement in the literature that it is easier to wash out acetoclastic methanogens than hydrogenotrophic methanogens. To illustrate, in the interesting study by Liu et al. 2008, the authors operated chemostats fed with household waste in a range of residence times from 1 to 6 days (at pH 7 and 70 °C). They observed at all these residence times high yields of VFA’s, which accumulated in the liquid phase. However, they also observed high methane production and very little hydrogen production at all residence times expect the lowest one (1 day). This indicates that, while acetoclastic methanogens were washed out in the whole range of residence times, hydrogenotrophic methanogens were only washed out at the shortest residence time. This finding confirms that, if hydrogen is one of the desired products, the residence time needs to be very short.
Another interesting question is whether, staying inside the range of residence times that prevent methanogenesis, it is possible to manipulate the residence time in order to control the products distribution, e.g. producing more ethanol and less VFA’s or vice versa. However, literature investigation on this aspect is very limited. Bengtsson et al. (2008) studied the effect of the residence time on anaerobic fermentation of whey and paper mill wastewaters. They observed that acetic acid yield was relatively unaffected by the residence time, while butyrate yield decreased at residence times higher than 10 h. In correspondence to the decrease in butyrate yield, propionate yield increased with increasing residence time. In the study mentioned above using household organic waste as substrate, Liu et al. (2008) observed that acetate was the main product in the liquid phase, accounting for more than 80 % of the total volatile fatty acids produced, at any residence times in the range 1–6 days.
So, the following conclusions can be drawn so far on the effect of solids residence time on the production of ethanol, organic acids and hydrogen by mixed cultures:
-
the process has to be operated at relatively short residence times, in the order of no more than a few days, in order to wash out methanogenic microorganisms;
-
it is easier to wash out acetoclastic than hydrogenotrophic methanogens, this means that production of VFA’s can be obtained in a wider range of residence times than hydrogen production. If hydrogen is the desired product, the residence time needs to be very short.
More study is needed to address the effect of residence time on the spectrum of product distribution.
6.2.2 Effect of pH
pH is also an important parameter in determining the performance of anaerobic fermentation. In general, fermentation of organic matter can occur in the wide range of pH values from 4 to 9 (Temudo et al. 2007). However, methanogenic microorganisms have a narrower pH range, and in general they have considered to have an optimum pH in the range 6.8–8 (Lay et al. 1997; Visser et al. 1993). Therefore, it is expected that pH control outside this range can be used to wash out methanogenic microorganisms and to control the process to the production of ethanol, VFA’s and hydrogen. Indeed, various studies seem to indicate that the optimum pH for hydrogen production is in the range 5.0–7.0 (Guo et al. 2010), i.e. in a range which is mostly outside the optimum pH of methanogenic microorganisms.
In addition to its effect on control of methanogenic microorganisms, pH may also have an effect on the product distribution between ethanol and acids and between the various acids and this has been investigated by several researchers. With waste activated sludge supplemented by carbohydrates as substrate (Feng et al. 2009), acetate and propionate were observed to be the main products of acidogenic fermentation, with propionate being the main product at pH 6–9 and acetate being the main one at pH 4,5,10 and 11. A similar evidence was reported for the fermentation of primary sludge (Wu et al. 2009). Using cattle wastewater as substrate Tang et al. (2008) observed that acetate and butyrate were the main products. Butyrate was observed to be more dependent on pH than acetate and a maximum in butyrate concentration was observed at pH 5.5. Interestingly, ethanol concentration, although lower than acetate and butyrate, was at its highest values at the extreme pH values reported in this study, i.e. 4.5 and 7.5. Liu et al. (2008) observed that pH had a strong effect on the acetate/lactate ratio. In studies with household waste at residence time of 3 days, at pH 6–7 acetate was the main fermentation product, while at lower pH, 5–5.5, the main product was lactate. Using glucose as model substrate, it was shown that at pH 4–5.5 acetate and butyrate were the main products, while at pH 6.25–8.5 the main products were acetate and ethanol.
It is important to observe that, in a system without external pH control, the pH of the digester will be affected by the applied organic load rate (OLR). Under conditions where methanogens are washed out, high values of the OLR will give higher concentrations of acids, causing the pH to drop. So, in the absence of methanogenesys, the pH of the process will naturally settle to acidic values, without external pH control.
Overall, even though more studies are need to clarify the effect of pH on the considered process, the following general conclusions can be drawn:
-
If pH is used to avoid methane formation and to maximise hydrogen production, pH values of 7.0 or lower should be used;
-
Acetic acid is often the main fermentation product in a wide range of pH values;
-
Butyrate and lactate production is favoured by acidic pH values, in the approximate range 4.5–6.0, while ethanol production is usually favoured by pH values higher than 6.0.
6.2.3 Effect of temperature
The effect of temperature can also be particularly important. Anaerobic digestion can be carried out in a wide temperature range, approximately in the range 25–75 °C. Within this range, the question is whether temperature has an effect on the rate of the process and on the distribution of products. In terms of reaction rates, in general the temperature range 50–60 °C gives the highest rates (Buhr and Andrews 1977), however this is clearly dependent on the nature of the substrate. Gilroyed et al. (2008) studied the fermentation of cattle manure in a range of temperatures from 36 to 60 °C and observed the highest hydrogen production rate at 52 °C. In the liquid phase, acetate was found to be the main product at temperatures higher than 52 °C, while at lower temperatures butyrate was the main product. In their analysis of the literature on hydrogen production from agricultural waste, Guo et al. 2010 observed that most literature studies were carried out under mesophilic conditions (30–45 °C), but that no optimal temperature could be determined due to the variability of the wastes and of the operating conditions used. Using cow waste as substrate, Yokoyama et al. (2007) investigated the effect of temperature on hydrogen production in the range 37–85 °C. They found 60 °C as the optimum temperature for hydrogen production and no hydrogen was produced at the highest temperature tested, 85 °C. The products in the liquid phase were measured at the temperature of 60 and 75 °C and acetate was found to be the main product at both temperatures, with very low concentrations of ethanol and other species. Interestingly, in a study with glucose as a model substrate Zoetemeyer et al. (1982a, b) observed the highest fermentation rates in the range 50–55 °C, and observed that butyrate was the main product at temperatures up to 50 °C, while ethanol and lactate were the main products at 55 and 60 °C (the study was carried out at pH 5.8).
Overall, it can be concluded that the effect of temperature is highly dependent on the nature of the substrate and certainly further study is needed, however the following general observations can be made:
-
temperatures in the range 50–60 °C seem the most interesting for anaerobic fermentation to ethanol, VFA’s and hydrogen;
-
in the liquid phase butyrate seems to be produced preferentially at temperatures not higher than 50 °C (however, butyrate production is also very dependent on pH as discussed in a previous section).
6.2.4 Effect of hydrogen partial pressure
Hydrogen partial pressure, which is linked to hydrogen concentration in the liquid phase, may potentially have multiple and complex effects on the performance of the process described here. Hydrogen pressure can be controlled by sparging with an inert gas, even though this is likely to be expensive in full scale plants. One effect is that high hydrogen partial pressure may inhibit carbohydrates uptake, as observed, e.g. by Chung (1976) and by Wiegel et al. (1979). However, other studies have reported no inhibition of carbohydrates fermentation even in a medium saturated with hydrogen (Denac et al. 1988; Roychowdhury et al. 1988). This apparent discrepancy in literature observations has been explained by Ruzicka (1996) and linked to the different glucose uptake rates in the various literature studies. Another effect of hydrogen partial pressure is the inhibition of the conversion of other VFA’s to acetic acid (Thauer et al. 1977). This effect is due to thermodynamic considerations, which indicate that the oxidation of VFA’s to acetate is only possible for very low hydrogen partial pressures. Similarly, conversion of alcohols, e.g. ethanol, to acetate is also inhibited by hydrogen partial pressure for the same thermodynamic reasons, even though alcohol’s conversion can occur at higher hydrogen pressures than VFA conversion (Majone et al. 2010). Since other VFA’s or ethanol are more reduced than acetate, conversion of VFA’s or ethanol to acetate occurs with generation of hydrogen (see also the stoichiometry of these reactions in Sect. 3). Therefore, if conversion of VFA’s or ethanol is inhibited by high hydrogen partial pressure, hydrogen production will be lower.
In the context of anaerobic fermentation for the production of ethanol, organic acids and hydrogen, relatively little systematic investigation has been carried out on the effect of hydrogen partial pressure. It is important to observe that in many of the studies on anaerobic digestion for hydrogen production the reactors are not sparged, and this indicates that conversion of the organic materials to hydrogen and VFA’s can occur even at high hydrogen partial pressures. A study by Mizuno et al. (2000) compared hydrogen production from glucose with and without nitrogen sparging and they observed a 70 % increase in the hydrogen production rate when the hydrogen partial pressure in the reactor was decreased from 0.5 to 0.05 atm. However, the reason for the increase in hydrogen production rate was not clear, since gas sparging did not have any effect on the glucose consumption rate or on the product distribution. A similar increase in hydrogen production rate with gas sparging was also observed by Liu et al. (2006) who reported a 90 % increase in hydrogen production rate from household solid waste. Overall, it seems that if the target products of the process are hydrogen and acetate, then a benefit may be gained by maintaining a low hydrogen pressure, while if the process is targeted to ethanol and other VFA production, then high hydrogen pressure should be beneficial. However, further investigation is needed on this topic.
6.3 Substrate concentration
Which is the maximum concentration of substrate that is possible to feed to the fermentation reactor? For a given residence time, higher substrate concentration corresponds to a higher concentration of products in the liquid phase in the effluent stream. As for the effluent gas phase, its composition is mainly determined by the nature of the substrate fed to the reactor rather than by its concentration. There are two reasons why it is important to maximise the concentration of products in the liquid phase leaving the fermentation reactor, i.e. to maximise the substrate concentration in the feed. The first reason is that higher concentration of products in the liquid phase corresponds to a higher volumetric productivity, for a given hydraulic residence time. This gives clearly an economic advantage. The second reason is that higher concentration of products makes their separation after the fermentation stage easier and cheaper. E.g. considering separation by distillation, a higher concentration of the desired species would correspond to a lower number of trays (lower capital cost) and a lower required reflux ratio (lower operating costs).
However, most literature studies which report anaerobic fermentation by mixed cultures to volatile fatty acids, ethanol and hydrogen are carried out at relatively low substrate concentrations. E.g. looking at Table 5, substrate concentration in the feed rarely exceeds 10 % and it is in many cases below 1 or 2 %. One of the reasons for this is that in most of these studies hydrogen is the desired product and the studies are focused on hydrogen yield, which is independent of substrate concentration. Since hydrogen is in the gas phase, it does not have to be separated from the other products in the liquid phase, and therefore increasing the substrate concentration in the feed does not give any advantage for hydrogen separation. Also, if the gas phase is only composed of hydrogen and carbon dioxide, which are produced simultaneously by fermentation of the organic matter (Sect. 3), increasing the substrate concentration in the feed will not have a large effect on the concentration of hydrogen in the gas phase. If hydrogen is the desired product the only benefit of higher substrate concentration in the feed is the higher volumetric productivity, which is however certainly a not negligible aspect.
There are several constraints on the maximum possible substrate and product concentration in the process under consideration here. The main constrain is related to the inhibiting effects of high concentrations of ethanol and volatile fatty acids. Pure culture studies have shown that the maximum ethanol concentration that can be tolerated by microorganisms is in the range 30–150 g/l. E.g. the bacterium Zymomonas mobilis has been reported to tolerate ethanol concentrations up to 100 g/l (Rogers et al. 1982), and ethanol concentrations up to 150 g/l have been reached with the yeast Saccharomyces cerevisiae (Alfenore et al. 2002). The bacterium Clostridium thermocellum is usually considered not able to tolerate ethanol concentrations higher than 30 g/l, although it has been shown that ethanol tolerance can be increased through adaptation (Brown et al. 2011; Williams et al. 2007). VFA inhibition on anaerobic digestion with mixed cultures has been studied by several researchers (Azman et al. 2015). Veeken et al. (2000) observed no inhibition by VFA on hydrolysis of organic solid waste up to the highest VFA concentration tested, 30 gCOD/l. However Siegert and Banks (2005) reported inhibition of cellulolytic activity at VFA concentration as low as 2 g/l and inhibition of glucose metabolism at VFA concentration of 8 g/l. Other possible inhibitors of anaerobic digestion are long chain fatty acids, which are generated from the hydrolysis of fats, present in many types of organic waste or wastewaters. Although many investigators have reported the inhibiting effect of LCFA’s (Chen et al. (2008), it has been shown (Beccari et al. 1996) that even lipid-rich wastewaters such as olive oil mill wastewaters can be converted to methane with high yield and that inhibition by LCFA’s can be overcome through acclimation of the microorganisms (Cirne et al. 2007).
7 Local and global potential of mixed culture biotechnology
In Table 4 we have reported estimated amounts of organic waste produced per year. However, in order for a biorefinery of organic waste to be feasible, the waste has to be available within a relatively small area, otherwise the high transportation costs will make the process prohibitively expensive. While a full analysis of which is the economic size of a mixed culture biorefinery would require a full life cycle assessment of the process and cannot be done here, in this section we aim to show a few examples of the potential product output obtainable by processing organic wastes available in a relatively small area. It is important to observe that the examples considered in this section have been made exclusively to illustrate the potential production of chemicals from anaerobic fermentation and do not mean that the calculated production of chemicals can actually be obtained with the current status of the technology. Further technological and scientific developments in the area of mixed culture fermentation are required to achieve the full potential of this technology, as discussed in the rest of this paper.
Four examples have been considered here, with organic waste originating from: a very large urban area; a relatively small urban area; a large biological wastewater treatment plant; a large dairy farm. In all these cases, the organic waste has been assumed to be composed entirely of carbohydrates and the yields of the various products have been assumed to be the ones reported by Temudo et al. (2007), using glucose as substrate, and at pH 6.25. The reasons for this choice are the following: the paper by Temudo et al. (2007) is one of the most comprehensive studies on the products distribution of mixed culture fermentation without methanogenesis; carbohydrates are often the main components present in a wide variety of organic wastes (Table 4); the choice of a single set of product yields for all the four considered examples allows to compare them on a uniform basis. With these considerations in mind, it is clear that the data in Table 6 are useful for an estimation of the amounts and composition spread of the products obtainable from fermentation of the organic waste for the considered examples, but cannot be considered accurate estimations of the actual products obtained by a mixed culture biorefinery. In particular, a fraction of the considered organic wastes will not be composed by carbohydrates, e.g. it will also include proteins and fats, and so it is likely that the actual fermentation products will contain less ethanol and more acetic acid and hydrogen than the figures reported in Table 6. Also, the data from Temudo et al. (2007) include a significant yield of formic acid among the fermentation products. Although not explicitly reported in this paper, formic acid is an important organic acid currently produced at 30,000 tonnes per year and with a market price of approximately 1000 $/tonne (Zacharof and Lovitt 2013).
The first example is about one very densely populated urban area and is based on the data reported by Satchatippavarn et al. (2015) for Bangkok, Thailand. Bangkok municipality has over 8 million inhabitants and collects about 2.7 million tonnes of organic waste per year (80 % of which are disposed of in landfills). Excluding plastics, from the data in the cited paper the biodegradable organic matter can be estimated to be approximately 2.0 million tonnes. Assuming a dry matter content of 35 %, within the reported range for municipal solid waste (Table 4), it can be estimated that over 100,000 tonnes per year of ethanol and acetic acid can be produced by fermentation of the organic waste collected in Bangkok. Compared to Sect. 2, these capacities are within the range of typical plants for bioethanol production from corn, and are comparable, although lower, to the typical capacities of acetic acid production plants from fossil fuels. The estimated production of butyric acid (17,000 tonnes per year) would corresponds to about 20 % of the total global production of this substance. The estimated production of hydrogen is in the region of 10,000 tonnes per year, which corresponds to approximately 12,800 Nm3/h (assuming continuous operation throughout the year), and is within the range of typical capacities of hydrogen plants reported in Sect. 2. Overall, this analysis shows that the organic waste generated in a single, very densely populated urban area, can sustain a large-scale biorefinery producing an amount of chemicals comparable to current full scale plants using conventional technologies.
The second example (small urban area) is based on the data of the household municipal solid waste collected by the Aberdeen City Council (SEPA 2014). Aberdeen, Scotland, UK, has a population of approximately 230,000 inhabitants and collects about 96,000 tonnes of household waste per year. Assuming a biodegradable fraction of 63 % (SEPA 2014) and a dry matter content of 35 %, it can be estimated that approximately 3500 and 4400 tonnes per year of ethanol and acetic acid, respectively, can be produced from fermentation of this waste. Also, lower but significant amounts, in the order of hundreds of tonnes per year, of butyric and formic acids and hydrogen can be obtained. The amounts of chemicals which can be obtained for the third and fourth example are similar to the ones of the second example. The third example, large wastewater treatment plant, is based on a typical 1 million population equivalent wastewater treatment plant, which is estimated to produce about 29,000 tonnes of waste sludge (as dry solids) per year. These figures have been calculated based on the data reported by DEFRA (2012) and by Corbitt (1998). The values reported in Table 6 have been obtained assuming that 60 % of the waste sludge solids are biodegradable (Appels et al. 2011). The data for the fourth example (large dairy farm) have been obtained for a large dairy farm (80,000 cows) in India (Global Methane Initiative 2013), which produce an estimated 350,000 tonnes per year of manure which is currently converted to biogas in an anaerobic digestion plant. The dry solids content of the manure has been taken as 7.5 % (Gerin et al. 2008).
Overall, the second, third and fourth examples give similar production of chemicals, which are much lower than the ones obtainable from the municipal solid waste generated by a big city, but still of industrial relevance. E.g. the hydrogen produced per year in these examples would still be more than enough for fuel cells applications, which have an estimated hydrogen requirements from 5 to 100 Nm3/h (Rostrup-Nielsen 2005), or for the fine chemical or pharmaceutical industry, which typically have plants requiring hydrogen at a rate of dozens to hundreds of tonnes per year (Bonrath et al. 2012). Similarly, the amount of ethanol produced would still be in the range of small scale bioethanol production plants currently in operation in the US (Ethanol Producer Magazine 2015).
Overall, depending on the amount of waste which is available locally, a biorefinery with open mixed cultures has the potential to produce chemicals in amounts comparable to large or small conventional chemical plants. In general, in order to reduce the transportation costs, it may make sense to convert the waste and use the fermentation products locally, as close as possible to where the waste is generated.
Looking at the global scale of waste production, the amount of chemicals that could be produced using mixed culture fermentation is huge. E.g. the global production of municipal solid waste has been estimated to be in the region of 1300 million tonnes (Table 4). With the same assumptions used in the paragraph above, i.e. 35 % dry matter, 63 % biodegradable matter content, products yields as per Temudo et al. (2007) at pH 6.25, we would be able to produce about 50 million tonnes of ethanol, 60 million tonnes of acetic acid and over 4 million tonnes of hydrogen. Also we would produce about 7 million tonnes of butyric acid and 10 million tonnes of formic acid. For ethanol, this approximately corresponds to 60–70 % of the current global production, and for hydrogen to about 8 % of the current global production. The amount of organic acids would largely exceed current production rate and market requirements, which could trigger their use for additional applications, as suggested by several researchers (Agler et al. 2011). The same approach can be used to estimate the amount of chemicals which could be produced by fermentation of sewage sludge, which, assuming a global annual production of 150 million tonnes (as dry weight), would give over 25 million tonnes of ethanol, 30 million tonnes of acetic acid and 2 million tonnes of hydrogen per year. A similar approach can be used to estimate the amount of chemicals which could be potentially produced from other types of waste.
In summary, the analysis carried out in this section shows that anaerobic fermentation of organic waste has the potential to produce a large amount of chemicals and fuels, which in many cases would satisfy or exceed current global requirements. However, due to the local nature of waste generation and to the cost associated with waste transportation, chemicals production via anaerobic fermentation would probably require the shift towards a more decentralised production of chemicals, with a larger number of smaller chemical plants, compared to today’s picture of a relatively small number of large plants. Overall, the sustainability of this approach will require a detailed life cycle analysis and cost-benefit evaluation.
8 Research needs for a mixed culture biorefinery
What are the factors that are currently preventing the use of mixed cultures for the industrial production of ethanol, hydrogen and volatile fatty acids? In which directions should the research efforts in this area go? Based on the analysis carried out in the previous sections we can highlight the following points:
-
Enhancing the rate of hydrolysis of lignocellulosic materials. One main limitation of mixed culture processes using organic waste as feedstock is the very low rate of microbial cellulose hydrolysis. As discussed in a recent paper by this group (Dionisi et al. 2015), possible ways to increase the rate of microbial hydrolysis are: enrichment studies, exploring the effect of microbial acclimation to lignocellulosic substrates, investigation of the effect of cellulose particle size and of the effect of process parameters. As an alternative to naturally-occurring microorganisms, genetically modified microorganisms, which could simultaneously hydrolyse cellulose and ferment the products to ethanol, are an interesting possibility and more research into this area is also needed. Studies on genetically modified microorganisms have also been reviewed in our previous paper (Dionisi et al. 2015). In parallel to biological processes, research is also needed on cheaper and more environmentally friendly chemical-physical pretreatments, but, even though many improvements to existing pretreatments are continuously published, e.g. F. Yang et al. (2015), there do not seem to be any game-changers in this area at the moment;
-
Investigating process performance with high concentration of waste in the feed and, consequently, high products concentration. Focus should be given to understanding which is the maximum concentration of substrate that can be fed to mixed culture bioreactors and consequently which is the maximum product concentration that can be obtained. To this regard, it is necessary to improve microbial tolerance to high concentration of ethanol and VFA’s, e.g. by enrichment and acclimation studies where microorganisms are subject to high products concentration for sufficiently long time;
-
Substrate selection. In order to reduce the number of species produced in anaerobic fermentation of mixed cultures, the types of wastes used as feedstock could be selected in order to maximise the yield of certain products. E.g. if the main desired product is ethanol, it would make sense to use mainly carbohydrates-based feedstock rather than mixed waste as starting material. On the other hand, if the main desired product is hydrogen it would be preferable to have fat-rich substrates in the feed, since anaerobic fermentation of fats can potentially give the highest hydrogen yields (as shown in Sect. 3);
-
Improved separation processes: even though there is interest in trying to direct the fermentation to single species, mixed culture fermentation is likely to always produce a mixture of products. So, how can we economically separate ethanol and mixtures of volatile organic acids in water into the single components? Various innovative separation processes have been and are currently being investigated, e.g. membrane processes (Timmer et al. 1994), azeotropic distillation (Helsel 1977), liquid–liquid extraction (Wardell and King 1978), adsorption (Kawabata et al. 1982), reactive extraction (Kumar and Babu 2008). While research in the area of innovative separation processes is certainly needed, in our opinion there is also the need to revisit the application of conventional distillation to the separation of fermentation products. Indeed, it is usually considered that distillation is not suitable to separate fermentation products because they are typically diluted and therefore the energy requirements for distillation would make the process too expensive. E.g. for bioethanol production, it has been reported (Weimer 2015) that an ethanol concentration of at least 52 g/l is required at the end of the fermentation to make the separation process economically viable. However, these considerations are valid for conventional processes, where the feedstock cost is very high and therefore the separation costs have to be as low as possible to make the process viable. On the other hand, a fermentation process based on organic waste would have a much lower feedstock costs and therefore it is likely to be economically sustainable even with higher separation costs. Considering the distillation of acetic acid and water, this is feasible and there are no azeotropes (Sebastiani and Lacquaniti 1967) and various schemes have been developed over the years (e.g. addition of third components) to increase the relative volatility and reduce the process costs. In our opinion, research and optimisation studies on conventional or advanced distillation of water mixtures of alcohols and acids is required, in the general framework of a mixed culture biomass biorefinery. Distillation would look even more attractive if its energy requirements were provided by renewable energy, e.g. sun or wind, and this is an area where considerable innovation is expected in the near future, similarly to what is currently happening for desalination processes powered by renewable energy (Ma and Lu 2011). It is also important to observe that separation of the organic acids may not be needed at all, if they are to be used as a concentrated mixture to be converted to higher chain length fatty acids or as a feedstock for biodegradable plastics production (Kleerebezem et al. 2015; Villano et al. 2010).
9 Conclusions
A biorefinery process based on undefined mixed microbial cultures has, in principle, many potential environmental and economic advantages over current processes for the production of ethanol, organic acids and hydrogen: possibility of using organic waste rather than food crops or fossil fuel derived materials, mild conditions of temperature and pressure, no need of heavy metals as catalysts, no requirement for sterilisation and possibility of integrated bioprocessing. However, the mixed culture process has also important drawbacks: higher separation costs due to the lower products concentration and lower productivities. The main challenges that research on mixed culture biotechnology needs to address have been discussed: fermentation of lignocellulosic substrates, achieving higher concentration of products, effect of process conditions on products yields and rates and improved/new separation processes. In parallel to the investigation on the technology, a full economic and environmental analysis of a mixed culture process for the conversion of biomass to chemicals, which compares this process to the current conventional processes, is also required.
References
Agler MT, Wrenn BA, Zinder SH, Angenent LT (2011) Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol 29:70–78
Alfenore S, Molina-Jouve C, Guillouet S et al (2002) Improving ethanol production and viability of saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl Microbiol Biotechnol 60:67–72
Alvarado-Cuevas Z, López-Hidalgo A, Ordoñeza LG (2015) Biohydrogen production using psychrophilic bacteria isolated from Antarctica. Int J Hydrog Energy 40:7586–7592
Amorim HV, Lopes ML, de Castro Oliveira JV, Buckeridge MS, Goldman GH (2011) Scientific challenges of bioethanol production in Brazil. Appl Microbiol Biotechnol 91:1267–1275
Angenent LT, Karim K, Al-Dahhan MH et al (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22:477–485
Antonopoulou G, Gavala HN, Skiadas IV et al (2008) Biofuels generation from sweet sorghum: fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour Technol 99:110–119
Appels L, Lauwers J, Degreve J et al (2011) Anaerobic digestion in global bio-energy production: potential and research challenges. Renew Sustain Energy Rev 15:4295–4301
Arvanitoyannis IS, Varzakas TH (2008) Vegetable waste treatment: comparison and critical presentation of methodologies. Crit Rev Food Sci Nutr 48:205–247
Aybeke M, Sidal U (2011) Effects of olive oil mill wastewater used as irrigation water on in vitro pollen germination. Pak J Biol Sci 14:703–708
Azman S, Khadem AF, Van Lier JB, Zeeman G, Plugge CM (2015) Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Crit Rev Environ Sci Technol 45(23):2523–2564
Balasubramanian B, Ortiz AL, Kaytakoglu S, Harrison D (1999) Hydrogen from methane in a single-step process. Chem Eng Sci 54:3543–3552
Batstone DJ, Keller J, Angelidaki I et al (2002) The IWA anaerobic digestion model no 1 (ADM 1). Water Sci Technol 45(10):65–73
Beccari M, Bonemazzi F, Majone M, Riccardi C (1996) Interaction between acidogenesis and methanogenesis in the anaerobic treatment of olive oil mill effluents. Water Res 30:183–189
Ben Sassi A, Boularbah A, Jaouad A et al (2006) A comparison of olive oil mill wastewaters (OMW) from three different process in morocco. Proc Biochem 41:74–78
Bengtsson S, Hallquist J, Werker A, Welander T (2008) Acidogenic fermentation of industrial wastewaters: effects of chemostat retention time and pH on volatile fatty acids production. Biochem Eng J 40:492–499
Bonrath W, Medlock J, Schutz J et al (2012) Hydrogenation in the vitamins and fine chemicals industry-an overview. Intech. doi:10.5772/48751
Brown SD, Guss AM, Karpinets TV et al (2011) Mutant alcohol dehydrogenase leads to improved ethanol tolerance in Clostridium thermocellum. Proc Natl Acad Sci USA 108:13752–13757
Buhr H, Andrews J (1977) The thermophilic anaerobic digestion process. Water Res 11:129–143
Cardona CA, Sanchez OJ, Gutierrez LF (2010) Process synthesis for fuel ethanol production. CRC Press, Boca Raton
Carioca J, Leal MRLV (2011) Ethanol production from sugar-based feedstocks. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic Press, Burlington, pp 27–35
Cavinato C, Bolzonella D, Fatone F et al (2011) Optimization of two-phase thermophilic anaerobic digestion of biowaste for hydrogen and methane production through reject water recirculation. Bioresour Technol 102:8605–8611
Cavinato C, Bolzonella D, Pavan P, Cecchi F (2016) Two-phase anaerobic digestion of food wastes for hydrogen and methane production. In: Marcello De Falco, Angelo Basile (eds) Enriched methane, green energy and technology. Springer, Switzerland. doi:10.1007/978-3-319-22192-2_5
Chatzipaschali AA, Stamatis AG (2012) Biotechnological utilization with a focus on anaerobic treatment of cheese whey: current status and prospects. Energies 5:3492–3525
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064
Cheung H, Tanke RS, Torrence GP (2000) Acetic acid. Ullmann’s Encyclopedia of Industrial Chemistry, Wiley, New York
Chung KT (1976) Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl Environ Microbiol 31:342–348
Cirne D, Paloumet X, Björnsson L, Alves M, Mattiasson B (2007) Anaerobic digestion of lipid-rich waste—effects of lipid concentration. Renew Energy 32:965–975
Corbitt RA (1998) Standard handbook of environmental engineering, vol 2. Mc Graw-Hill, Pennsylvania
Dahiya S, Sarkar O, Swamy YV, Venkata Mohan S (2015) Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour Technol 182:103–113
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol 81:1119–1129
Davidsson Å, Gruvberger C, Christensen TH et al (2007) Methane yield in source-sorted organic fraction of municipal solid waste. Waste Manag 27:406–414
Davila-Vazquez G, de León-Rodríguez A, Alatriste-Mondragón F, Razo-Floresa E (2011) The buffer composition impacts the hydrogen production and the microbial community composition in non-axenic cultures. Biomass Bioenergy 25:3174–3181
de Almeida EF, Bomtempo JV, De Souza e Silva C (2007) The performance of Brazilian biofuels: an economic, environmental and social analysis. OECD/ITF Joint Transport Research Centre Discussion Papers, No. 2007/05, OECD Publishing, Paris. doi:10.1787/234818225330
De Bere L (2000) Anaerobic digestion of solid waste: state-of-the-art. Water Sci Technol 41:283–290
De la Rubia M, Perez M, Romero L, Sales D (2006) Effect of solids retention time (SRT) on pilot scale anaerobic thermophilic sludge digestion. Process Biochem 41:79–86
Demirel B, Yenigün O (2002) Two-phase anaerobic digestion processes: a review. J Chem Technol Biotechnol 77:743–755
Denac M, Miguel A, Dunn I (1988) Modeling dynamic experiments on the anaerobic degradation of molasses wastewater. Biotechnol Bioeng 31:1–10
Department for Environment Food and Rural Affair (Defra) (2012) Wastewater treatment in the United Knigdom-2012. Crown Copyright 2012
Dias JM, Lemos PC, Serafim LS et al (2006) Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol Biosci 6:885–906
Dionisi D, Majone M, Vallini G et al (2006) Effect of the applied organic load rate on biodegradable polymer production by mixed microbial cultures in a sequencing batch reactor. Biotechnol Bioeng 93:76–88
Dionisi D, Anderson JA, Aulenta F et al (2015) The potential of microbial processes for lignocellulosic biomass conversion to ethanol: a review. J Chem Technol Biotechnol 90:366–383
Dwidar M, Park J, Mitchell RJ, Sang B (2012) The future of butyric acid in industry. Sci World J. http://dx.doi.org/10.1100/2012/471417
Eastman JA, Ferguson JF (1981) Solubilization of particulate organic carbon during the acid phase of anaerobic digestion. J Water Pollut Control Fed 53:352–366
Ethanol Producer Magazine (2015) US Ethanol Production. http://www.ethanolproducer.com/plants/listplants/US/Existing/Sugar-Stach. Accessed Dec 2015
European Commission (2014) Large scale demonstration of refuelling infrastructure for road vehicles. Call FCH-01.7-2014
Fan Y, Zhang Y, Zhang S et al (2006) Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Bioresour Technol 97:500–505
Fang HH, Li C, Zhang T (2006) Acidophilic biohydrogen production from rice slurry. Int J Hydrog Energy 31:683–692
Feng L, Chen Y, Zheng X (2009) Enhancement of waste activated sludge protein conversion and volatile fatty acids accumulation during waste activated sludge anaerobic fermentation by carbohydrate substrate addition: the effect of pH. Environ Sci Technol 43:4373–4380
Fillaudeau L, Blanpain-Avet P, Daufin G (2006) Water, wastewater and waste management in brewing industries. J Clean Prod 14:463–471
Frohning CD, Kohlpaintner CW, Bohnen H-W (2002) Carbon monoxide and synthesis gas chemistry. In: Cornils B, Herrmann WA (eds) Applied homogeneous catalysis with organometallic compounds, 2nd edn. Wiley, Weinheim. doi:10.1002/9783527618231.ch2a
Galbe M, Zacchi G (2012) Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 46:70–78
Gerin PA, Vliegen F, Jossart JM (2008) Energy and CO2 balance of maize and grass as energy crops for anaerobic digestion. Bioresour Technol 99:2620–2627
Gilroyed BH, Chang C, Chu A, Hao X (2008) Effect of temperature on anaerobic fermentative hydrogen gas production from feedlot cattle manure using mixed microflora. Int J Hydrog Energy 33:4301–4308
Global Methane Initiative (2013) Successful applications of anaerobic digestion from across the world. https://www.globalmethane.org/documents/GMI%20Benefits%20Report.pdf
Guo XM, Trably E, Latrille E, Carrère H, Steyer J (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrog Energy 35:10660–10673
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567
Helsel RW (1977) Removing carboxylic acids from aqueous wastes. Chem Eng Progr 73:55–59
Hoornweg D, Bhada-Tata P (2012) What a waste. A global review of solid waste management. No. Urban Development Series. Knowledge papers. World Bank
Huang YL, Wu Z, Zhang L et al (2002) Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour Technol 82:51–59
Inamdar STA (2012) Biochemical engineering: principles and concepts. PHI Learning Pvt. Ltd, New Delhi
Joseck F, Sutherland E (2014) Early market hydrogen cost target calculation. DOE Fuel Cell Technologies Office Record. Report No. 14013, Department of Energy, USA
Jung K, Kim D, Shin H (2010) Continuous fermentative hydrogen production from coffee drink manufacturing wastewater by applying UASB reactor. Int J Hydrog Energy 35:13370–13378
Karlsson A, Vallin L, Ejlertsson J (2008) Effects of temperature, hydraulic retention time and hydrogen extraction rate on hydrogen production from the fermentation of food industry residues and manure. Int J Hydrogen Energy 33:953–962
Kawabata N, Yasuda S, Yamazaki T (1982) Process for Recovering a Carboxylic Acid. Patent US4323702 A
Kent JA (2010) Kent and Riegel’s Handbook of industrial chemistry and biotechnology, vol 1. Springer, Berlin
Kleerebezem R, van Loosdrecht MCM (2007) Mixed culture biotechnology for bioenergy production. Curr Opin Biotechnol 18:207–212
Kleerebezem R, Joosse B, Rozendal R, van Loosdrecht MCM (2015) Anaerobic digestion without biogas? Rev Environ Sci Biotechnol. doi:10.10007/s11157-015-9374-6
Kongjan P, Min B, Angelidaki I (2009) Biohydrogen production from xylose at extreme thermophilic temperatures (70 & #xB0;C) by mixed culture fermentation. Water Res 43:1414–1424
Kongjan P, Kotay M, Min B, Angelidaki I (2010) Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol Bioeng 105:899–908
Koopmans A, Koppejan J (1997) Agricultural and forest residues. Generation, utilization and availability. Paper presented at the Regional Consultation on Modern Applications of Biomass Energy, Kuala Lumpur, Malaysia, 6–10 January
Kubitschke J, Lange H, Strutz H (1986) Carboxylic acids, aliphatic. Ullmann’s Encyclopaedia of Industrial Chemistry, Wiley, New York
Kumar S (2011) Composting of municipal solid waste. Crit Rev Biotechnol 31:112–136
Kumar S, Babu B (2008) Propionic acid production via fermentation route using renewable sources. Chem Ind Dig 9:76–81
Kwiatkowski JR, McAloon AJ, Taylor F, Johnston DB (2006) Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind Crops Prod 23:288–296
Lawrence AW, McCarty PL (1969) Kinetics of methane fermentation in anaerobic treatment. J Water Pollut Control Fed 41:R1–R17
Lay J, Li Y, Noike T (1997) Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res 31:1518–1524
Lay C, Wu J, Hsiao C et al (2010) Biohydrogen production from soluble condensed molasses fermentation using anaerobic fermentation. Int J Hydrog Energy 35:13445–13451
Lee G, McCain J, Bhasin M (2007) Synthetic organic chemicals. In: James A Kent (ed) Kent and Riegel’s handbook of industrial chemistry and biotechnology. Springer, Berlin
Levy PF, Sanderson JE, Kispert RG, Wise DL (1981) Biorefining of biomass to liquid fuels and organic chemicals. Enzyme Microbial Technol 3:207–215
Li C, Fang HH (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39
Licht FO (2008) World fuel ethanol production. Renew Fuels Assoc. http://www.ethanolrfa.org/resource/facts/trade/
Lin C, Lay C, Sen B et al (2012) Fermentative hydrogen production from wastewaters: a review and prognosis. Int J Hydrog Energy 37:15632–15642
Litchfield JH (1996) Microbiological production of lactic acid. Adv Appl Microbiol 42:45–95
Liu D, Liu D, Zeng RJ, Angelidaki I (2006) Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res 40:2230–2236
Liu D, Zeng RJ, Angelidaki I (2008) Effects of pH and hydraulic retention time on hydrogen production versus methanogenesis during anaerobic fermentation of organic household solid waste under extreme-thermophilic temperature (70° C). Biotechnol Bioeng 100:1108–1114
Ma Q, Lu H (2011) Wind energy technologies integrated with desalination systems: review and state-of-the-art. Desalination 277:274–280
Majone M, Aulenta F, Dionisi D et al (2010) High-rate anaerobic treatment of Fischer-Tropsch wastewater in a packed-bed biofilm reactor. Water Res 44:2745–2752
Martinez FAC, Balciunas EM, Salgado JM et al (2013) Lactic acid properties, applications and production: a review. Trends Food Sci Technol 30:70–83
Mata-Alvarez J, Mace S, Llabres P (2000) Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour Technol 74:3–16
Miller C, Fosmer A, Rush B et al (2011) Industrial production of lactic acid. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic Press, Burlington, pp 179–188
Mizuno O, Dinsdale R, Hawkes FR et al (2000) Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour Technol 73:59–65
NASDAQ stock market (2015). Retrieved February 2015. www.nasdaq.com
National Hydrogen Association (2010) Hydrogen and fuel cells. The U.S. market report. http://www.hydrogenassociation.org/market report
Newsome DS (1980) The water-gas shift reaction. Catal Rev Sci Eng 21:275–318
Nges IA, Liu J (2010) Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophilic and thermophilic conditions. Renew Energy 35:2200–2206
Ogilvie D (1998) National study of the composition of sewage sludge. New Zealand Water and Wastes Association, Wellington
Ojumu T, Yu J, Solomon B (2004) Production of polyhydroxyalkanoates, bacterial biodegradable polymers. Afr J Biotechnol 3:18–24
Olson DG, McBride JE, Joe Shaw A, Lynd LR (2012) Recent progress in consolidated bioprocessing. Curr Opin Biotechnol 23:396–405
Orbichem Tecnon (2013) Chem-net facts-acetic acid. Chemical market insight and foresight. http://www.orbichem.com/userfiles/CNF%20Samples/aac_13_11.pdf
Rajendran K, Taherzadeh MJ (2014) Pretreatment of lignocellulosic materials. In: Bisaria VS, Kondo A (eds) Bioprocessing of renewable resources to commodity bioproducts. Wiley, Hoboken. doi:10.1002/9781118845394.ch3
Ramsay IR, Pullammanappallil PC (2001) Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation 12(4):247–256
Randhawa MA, Ahmed A, Akram K (2012) Optimization of lactic acid production from cheap raw material: sugarcane molasses. Pak J Bot 44:333–338
Ren N, Li J, Li B et al (2006) Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int J Hydrog Energy 31:2147–2157
Rogers P, Lee K, Skotnicki M, Tribe D (1982) Ethanol production by Zymomonas mobilis. In: Fiechter A (ed) Microbial reactions. Springer, Berlin, pp 37–84
Rosales-Colunga LM, de León Rodríguez A (2015) Escherichia coli and its application to biohydrogen production. Rev Environ Sci Biotechnol 14:123–135
Rostrup-Nielsen T (2005) Manufacture of hydrogen. Catal Today 106:293–296
Roychowdhury S, Cox D, Levandowsky M (1988) Production of hydrogen by microbial fermentation. Int J Hydrog Energy 13:407–410
Ruzicka M (1996) The effect of hydrogen on acidogenic glucose cleavage. Water Res 30:2447–2451
Sans C, Mata-Alvarez J, Cecchi F et al (1995) Volatile fatty acids production by mesophilic fermentation of mechanically-sorted urban organic wastes in a plug-flow reactor. Bioresour Technol 51:89–96
Satchatippavarn S, Martinex-Hernandez E, Leung Pah Hang MY et al (2015) Urban biorefinery for waste processing. Chem Eng Res Design. doi:10.1016/j.cherd.2015.09.022
Scottish Environmental Protection Agency (SEPA) (2014). Household waste summary data and commentary text. http://sepa.org.uk/environment/waste/waste-data/waste-data-reporting/household-waste-data/. Accessed Dec 2015
Sebastiani E, Lacquaniti L (1967) Acetic acid—water system thermodynamical correlation of vapor—liquid equilibrium data. Chem Eng Sci 22:1155–1162
Sharma SK, Mishra IM, Sharma MP, Saini JS (1988) Effect of particle size on biogas generation from biomass residues. Biomass 17:251–263
Shea TG, Pretorius W, Cole R, Pearson E (1968) Kinetics of hydrogen assimilation in the methane fermentation. Water Res 2:833–848
Siegert I, Banks C (2005) The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem 40:3412–3418
Siso MG (1996) The biotechnological utilization of cheese whey: a review. Bioresour Technol 57:1–11
Smejkal Q, Linke D, Baerns M (2005) Energetic and economic evaluation of the production of acetic acid via ethane oxidation. Chem Eng Process 4:421–428
Sousa DZ, Pereira MA, Stams AJ et al (2007) Microbial communities involved in anaerobic degradation of unsaturated or saturated long-chain fatty acids. Appl Environ Microbiol 73:1054–1064
Steffen R, Szolar O, Braun R (1998) Feedstocks for anaerobic digestion. Report No. 1998-09-30 Institute of Agrobiotechnology Tulin, University of Agricultural Sciences, Vienna
Sunley GJ, Watson DJ (2000) High productivity methanol carbonylation catalysis using iridium: the cativa™ process for the manufacture of acetic acid. Catal Today 58:293–307
Tang G, Huang J, Sun Z et al (2008) Biohydrogen production from cattle wastewater by enriched anaerobic mixed consortia: influence of fermentation temperature and pH. J Biosci Bioeng 106:80–87
Temudo MF, Kleerebezem R, van Loosdrecht M (2007) Influence of the pH on (open) mixed culture fermentation of glucose: a chemostst study. Biotechnol Bioeng 98:69–79
Temudo MF, Mato T, Kleerebezem R, van Loosdrecht MC (2009) Xylose anaerobic conversion by open-mixed cultures. Appl Microbiol Biotechnol 82:231–239
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Timmer J, Kromkamp J, Robbertsen T (1994) Lactic acid separation from fermentation broths by reverse osmosis and nanofiltration. J Membr Sci 92:185–197
Traverso P, Pavan P, Bolzonella D et al (2000) Acidogenic fermentation of source separated mixtures of vegetables and fruits wasted from supermarkets. Biodegradation 11:407–414
Turick CE, Peck MW, Chynoweth DP (1991) Methane fermentation of woody biomass. Bioresour Technol 37:141–147
Veeken A, Kalyuzhnyi S, Scharff H, Hamelers B (2000) Effect of pH and VFA on hydrolysis of organic solid waste. J Environ Eng 126:1076–1081
Villano M, Beccari M, Dionisi D et al (2010) Effect of pH on the production of bacterial polyhydroxyalkanoates by mixed cultures enriched under periodic feeding. Process Biochem 45:714–723
Visser A, Gao Y, Lettinga G (1993) Effects of pH on methanogenesis and sulphate reduction in thermophilic (55 & #xB0;C) UASB reactors. Bioresour Technol 44:113–121
Wadhwa M, Bakshi MPS (2013) Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. FAO RAP Publication 2013/04
Wardell JM, King CJ (1978) Solvent equilibriums for extraction of carboxylic acids from water. J Chem Eng Data 23:144–148
Wee Y, Kim J, Ryu H (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Weimer PJ (2015) Ruminal fermentations to produce liquid and gaseous fuels. In: Anil Kumar Puniya, Rameshwar Singh, Devki Nandan Kamra (eds) Rumen microbiology: from evolution to revolution, vol 18. Springer India, pp 265–280
Wiegel J (1980) Formation of ethanol by bacteria. A pledge for the use of extreme thermophilic anaerobic bacteria in industrial ethanol fermentation processes. Experentia 36:1434–1446
Wiegel J, Ljungdahl LG, Rawson JR (1979) Isolation from soil and properties of the extreme thermophile clostridium thermohydrosulfuricum. J Bacteriol 139:800–810
Williams TI, Combs JC, Lynn BC, Strobel HJ (2007) Proteomic profile changes in membranes of ethanol-tolerant Clostridium thermocellum. Appl Microbiol Biotechnol 74:422–432
Winter C (2009) Hydrogen energy—abundant, efficient, clean: a debate over the energy-system-of-change. Int J Hydrog Energy 34:S1–S52
Wu H, Yang D, Zhou Q, Song Z (2009) The effect of pH on anaerobic fermentation of primary sludge at room temperature. J Hazard Mater 172:196–201
Xu Z, Jiang L (2011) Butyric acid. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic press, Burlington, pp 207–215
Yang P, Zhang R, McGarvey JA, Benemann JR (2007) Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int J Hydrogen Energy 32:4761–4771
Yang S, El-Ensashy H, Thongchul N (2013) Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Wiley, New York
Yang F, Liu Z, Afzal W et al (2015) Pretreatment of miscanthus giganteus with lime and oxidants for biofuels. Energy Fuels 29:1743–1750
Yokoyama H, Waki M, Moriya N et al (2007) Effect of fermentation temperature on hydrogen production from cow waste slurry by using anaerobic microflora within the slurry. Appl Microbiol Biotechnol 74:474–483
Yoneda N, Kusano S, Yasui M et al (2001) Recent advances in processes and catalysts for the production of acetic acid. Appl Catal A 221:253–265
Yu H, Zhu Z, Hu W, Zhang H (2002) Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int J Hydrog Energy 27:1359–1365
Zacharof MP, Lovitt RW (2013) Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valorisation 4:557–581
Zero Waste Scotland, Natural Scotland. (2010). The composition of municipal solid waste in Scotland. Final Report, Project Code EVA098-001
Zhang B, Zhang L, Zhang S et al (2005) The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ Technol 26:329–340
Zhang M, Fan Y, Xing Y et al (2007) Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 31:250–254
Zhang Y, Banks CJ, Heaven S (2012a) Anaerobic digestion of two biodegradable municipal waste streams. J Environ Manag 104:166–174
Zhang Y, Banks CJ, Heaven S (2012b) Co-digestion of source segregated domestic food waste to improve process stability. Bioresour Technol 114:168–178
Zoetemeyer RJ, Vandenheuvel JC, Cohen A (1982a) pH influence on acidogenic dissimilation of glucose in an anaerobic digester. Water Res 16:303–311
Zoetemeyer RJ, Arnoldy P, Cohen A, Boelhouwer C (1982b) Influence of temperature on the anaerobic acidification of glucose in a mixed culture forming part of a two-stage digestion process. Water Res 16:313–321
Zong W, Yu R, Zhang P et al (2009) Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenergy 33:1458–1463
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dionisi, D., Silva, I.M.O. Production of ethanol, organic acids and hydrogen: an opportunity for mixed culture biotechnology?. Rev Environ Sci Biotechnol 15, 213–242 (2016). https://doi.org/10.1007/s11157-016-9393-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-016-9393-y