Abstract
Unprotected l-phenylalanine was derivatized by an innovative enzymatic method by means of laccases from Pycnoporus cinnabarinus and Myceliophthora thermophila. During the incubation of l-phenylalanine with para-hydroquinones using laccase as biocatalyst, one or two main products were formed. Dependent on the substitution grade of the hydroquinones mono- and diaminated products were detected. Differences of the used laccases are discussed. The described reactions are of interest for the derivatization of amino acids and a synthesis of pharmacological-active amino acid structures in the field of white biotechnology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzyme catalyzed reactions are currently of considerable interest for new biotechnological purposes. A great advantage of these reactions is the utilization of mild and environmental-friendly conditions, for example none or less toxic educts, ambient temperature and atmospheric pressure. Thus, also substances which are sensitive towards chemical synthesis procedures, e.g. amino acids can be derivatized. The so far known chemical derivatization methods of amino acids are complicate and mostly connected with a use of dangerous compounds, for example the usage of sodium periodate (Lai et al. 1996). The laccase-catalyzed reaction is an environmental-friendly method for the derivatization of unprotected amino acids.
Laccases [E.C. 1.10.3.2] are polyphenoloxidases, which oxidize aromatic phenols to reactive radicals, which can undergo non-enzymatic coupling reactions (Davin and Lewis 2005; Burton 2003; Claus 2003; Gianfreda et al. 1999; Thurston 1994; Bollag 1992). Therefore, interest in the potential of these enzymes in biotransformations used in organic synthesis has increased (Mikolasch et al. 2006; Niedermeyer et al. 2005; Schauer et al. 2001) especially to show great promise for the derivatization of amino acids (Manda et al. 2006).
To further determine the potential of laccases for amino acid derivatization, laccases of Pycnoporus cinnabarinus and Myceliophthora thermophila were used for reactions of l-phenylalanine with different para-hydroquinones. In this study, we show that l-phenylalanine can be derivatized by different substituted para-hydroquinones under laccase-catalyzed conditions. Depending on the substituents mono- or diaminated products were formed.
Material and methods
Chemicals
l-phenylalanine was purchased from Serva Feinbiochemica GmbH & Co. (Heidelberg, Germany). 1,4-Hydroquinone and methyl-1,4-hydroquinone were products of Sigma-Aldrich Chemie GmbH (Steinheim, Germany) and 2,3-dimethyl-1,4-hydroquinone was obtained from Arcos Organics (New Jersey, USA). Trimethyl-1,4-hydroquinone was purchased from Alfa Aesar GmbH & Co. KG (Karlsruhe, Germany).
Enzymes
The laccase was obtained from Pycnoporus cinnabarinus SBUG-M 1044. The white rot fungus was isolated from an oak tree in northern Germany and is deposited at the strain collection of the Department of Biology of the University of Greifswald (SBUG).
Cultivation of Pycnoporus cinnabarinus SBUG-M 1044 and crude preparation of laccase as we reported previously (Jonas et al. 1998).
Myceliophthora thermophila laccase (expressed in genetically modified Aspergillus sp.) was obtained from Novozymes (Bagsværd, Denmark).
Measurement of laccase activity
The activity of laccase was determined spectrophotometrically at 420 nm using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) as substrate (Bourbonnais and Paice 1990) and the method described by Jonas et al. (1998).
Experimental procedures
For analytical experiments the educts l-phenylalanine (1 or 100 mM) and the respective 1,4-hydroquinone (1 mM) were incubated with laccase (activity 487 nmol ml−1 min−1). For the reaction with Pycnoporus cinnabarinus laccase, 20 mM sodium acetate buffer (SAB) pH 5 was used and the reaction with the laccase of Myceliophthora thermophila proceeded in citrate phosphate buffer (CPB, 18 mM citrate, 165 mM phosphate) pH 7. The reaction mixture was incubated in 5-ml-brown-glass-bottles with 2 ml sample volume at room temperature with agitation at 200 rpm.
Analytical HPLC
For routine analysis, the reaction mixtures were analyzed using an HPLC system LC-10AT VP (Shimadzu, Germany) consisting of a FCV-10AL VP pump, SPD-M10A VP diode array detector, and a SCL-10A VP control unit controlled by Class-VP version 6.12 SP5. The separation of the substances was achieved on an endcapped, 5-μm, LiChroCART® 125-4 RP18 column (Merck, Darmstadt, Germany) at a flow rate of 1 ml/min. A solvent system consisting of methanol (eluent A) and 0.1% phosphoric acid (eluent B), starting from an initial ratio of 10% A and 90% B and reaching 100% methanol within 14 min, was used.
Isolation of transformation products
At first, a liquid–liquid-extraction with ethylacetate was performed to remove undesired byproducts.
Then a RP18 silicagel column (polypropylene 6 ml, 2000 mg adsorbent material, Merck, Darmstadt, Germany) was charged with 1 ml of the aqueous phase from the liquid–liquid-extraction. The amino acid l-phenylalanine was eluted with 30 ml citrate phosphate buffer, 1 ml 5%, 2 ml 10% methanol in water and 1 ml 100% methanol. The cross-coupling products were eluted with additional 1 ml 100% methanol. For mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy the isolated products (3c and 4a) were dried by lyophilization.
Characterization
The products were characterized by liquid chromatography/mass spectrometry (LC/MS). The standard atmospheric pressure ionization (API) mass spectrometry experiments were performed using an Agilent Series 1100 HPLC system and an Agilent 1946C quadrupole mass spectrometer (Waldbronn, Germany). The MS was used with both, atmospheric pressure chemical ionization (APCI) and electrospray ionization (API-ES) sources. HPLC separation was performed on a LiChroCART® 125-4, LiChrosphere® 100 RP-18e column (Merck, Darmstadt, Germany) at 25°C at a flow rate of 1 mL/min within a 14-min gradient from 10 to 100% methanol in 0.1% aqueous formic acid. APCI/API-ES MS conditions (positive and negative ion mode) were as follows: nebulizer and drying gas, nitrogen; nebulizer pressure, 30 psig; drying gas flow, 10 L/min; vaporizer temperature (for APCI), 350°C; drying gas temperature, 250°C; capillary voltage, 4 kV; corona current (for APCI), 4 μA.
The LC high-resolution mass spectrometry experiments were performed on an Agilent Series 1200 HPLC system and an Agilent 1969A time-of-flight mass spectrometer (Waldbronn, Germany). The TOF-MS conditions (neg. and pos. ion mode) with a dual sprayer API-ES source were as follows: nebulizer and drying gas, nitrogen; nebulizer pressure, 40 psig; drying gas flow, 10 L/min; drying gas temperature, 350°C; capillary voltage, 4 kV; fragmentor voltage, 175 V; skimmer voltage, 60 V; octopole voltage, 250 V; mass reference (m/z), 121.05087 and 922.00979 in pos. ion mode, and 112.98558 and 1033.98810 in neg. ion mode. HPLC separation was performed on a Zorbax Eclipse XDB-C8, 4.6 × 50 mm, 3.5 μm, column (Agilent, Germany) at 25°C at a flow rate of 0.5 mL/min within a 14-min gradient from 10 to 100% methanol in 0.1% aqueous formic acid.
The nuclear magnetic resonance (NMR) spectra for product 3c and 4a (1H-, 13C-NMR, DEPT) were recorded on different Bruker Avance 600 instruments (Karlsruhe, Germany) at 600 MHz in MeOH-d4. Tetramethylsilane was used as internal standard.
2-(3,6-Dioxocyclohexa-1,4-dienylamino)-3-phenylpropionic acid (3a)
Synthesis and isolation as described above. 3a was only present in a mixture with 4a, due to the fast reaction of 3a to 4a. Yield for 3a and 4a 66.8%. Rf (HPLC) 10.52 min, UV/VIS λ max 211, 260, 477 nm.
MS (LC/MS): m/z (relative intensity): API-ES, pos. ion mode 272 ([M + H]+, 65), 226 ([M − CO2H–H]+, 100).
HRMS for 3a: [M + H]+ (C15H14NO4): calcd.: 272.0917; found: 272.0911 (−2.4 ppm).
2-[4-(1-Carboxy-2-phenylethylamino)-3,6-dioxocyclohexa-1,4-dienylamino]-3-phenylpropionic acid (4a)
Synthesis and isolation as described above. Red brown solid. Yield 71.3%. Rf (HPLC) 12.95 min, UV/VIS λ max 205, 342, 460 nm; 600 MHz 1H-NMR (MeOH-d4), see Table 1.
MS (LC/MS): m/z (relative intensity): API-ES, pos. ion mode 1325 ([3 M + Na]+, 28), 891 ([2 M + Na]+, 42), 457 ([M + Na]+, 68), 435 ([M + H]+, 100); APCI, pos. ion mode 435 ([M + H]+, 15), 391 ([M − COO + H]+, 100).
HRMS for 4a: [M + H]+ (C24H23N2O6): calcd.: 435.1551; found: 435.1549 (−0.4 ppm).
[M − H]− (C24H21N2O6): calcd.: 433.1405; found: 433.1411 (+1.3 ppm).
2-(5-Methyl-3,6-dioxocyclohexa-1,4-dienylamino)-3-phenylpropionic acid (3b)
Rf (HPLC) 11.75 min, UV/VIS λ max 208, 275, 477 nm.
MS (LC/MS): m/z (relative intensity): API-ES, pos. ion mode 286 ([M + H]+, 100), 240 ([M − CO2H–H]+, 83).
HRMS for 3b: [M + H]+ (C16H16NO4): calcd.: 286.1074; found: 286.1081 (+2.7 ppm).
2-[4-(1-Carboxy-2-phenylethylamino)-5-methyl-3,6-dioxocyclohexa-1,4-dienylamino]-3-phenylpropionic acid (4b)
Rf (HPLC) 13.57 min, UV/VIS λ max 346, 515 nm.
MS (LC/MS): m/z (relative intensity): API-ES, pos. ion mode 919 ([2 M + Na]+, 68), 471 ([M + Na]+, 29), 449 ([M + H]+, 100), 403 ([M− CO2H–H]+, 76).
HRMS for 3b: [M + H]+ (C24H23N2O6): calcd.: 449.1707; found: 449.1705 (−0.4 ppm).
[M + Na]+ (C24H22N2O6Na): calcd.: 471.1527; found: 471.1524 (−0.7 ppm).
2-(4,5-Dimethyl-3,6-dioxocyclohexa-1,4-dienylamino)-3-phenylpropionic acid (3c)
Synthesis and isolation as described above. Dark red to purple solid. Yield 60.5%. Rf (HPLC) 12.95 min, UV/VIS λ max 204, 289, 477 nm. 1H-NMR δ 7.20 (m, 3J = 6.7 Hz, 4H, H-2′, H-3′, H-5′, H-6′), 7.15 (t, 3J = 6.7 Hz, 1H, H-4′), 5.26 (s, 1H, H-2″), 3.97 (dd, 3J = 4.6 Hz, 3J = 7.7 Hz, 1H, H-2), 3.24 (dd, 3J = 4.6 Hz, 2J = −13.8 Hz, 1H, H-3), 3.04 (dd, 3J = 7.7 Hz, 2J = −13.8 Hz, 1H, H-3), 1.96 (d (s), 5J = 1.0 Hz, 3H, aromatic methyl group), 1.95 (d (s), 5J = 1.0 Hz, 3H, aromatic methyl group).
13C-NMR δ 183.46 (C-3″); 180.60 (C-6″); 172.89 (C-1); 143.79; 141.18; 135.19; 133.91; 126.63; 126.19; 125.54; 124.58; 123.79; 94.05 (C-2″); 56.22 (C-2); 35.18 (C-3); 8.91 (aromatic methyl group); 7.99 (aromatic methyl group).
MS (LC/MS): m/z (relative intensity): APCI, pos. ion mode 300 ([M + H]+, 26), 256 ([M − CO2 + H]+, 100); API-ES, neg. ion mode 298 ([M − H]−, 30), 254 ([M − COO-H]−, 100).
HRMS for 3c: [M + H]+ (C17H18NO4): calcd.: 300.1230; found: 300.1228 (−0.6 ppm).
[M + Na]+ (C17H17NO4Na): calcd.: 322.1050; found: 322.1051 (+0.6 ppm).
3-Phenyl-2-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienylamino)propionic acid (3d)
Rf (HPLC) 13.53 min, UV/VIS λ max 203, 287, 494 nm.
MS (LC/MS): m/z (relative intensity): API-ES, pos. ion mode 336 ([M + Na]+, 12), 256 ([M + H]+, 63); 270 ([M − COO-H]−, 100).
HRMS for 3c: [M + Na]+ (C18H19NO4Na): calcd.: 336.1206; found: 336.1199 (−2.2 ppm).
Results
General observations
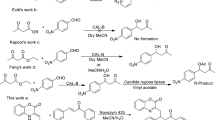
The heteromolecular transformation of 1,4-hydroquinone and methyl-1,4-hydroquinone with l-phenylalanine (educts 1a, 1b and 2, Fig. 1) in the presence of lacccase from Pycnoporus cinnabarinus and Myceliophthora thermophila resulted in the formation of two main products for each reaction 3a, 3b and 4a, 4b (Fig. 1). Whereas in the reaction of 2,3-dimethyl-1,4-hydroquinone (1c) and l-phenylalanine only one main coupling product (3c) was formed. Due to their characteristic UV/VIS-spectra, formation of products and decrease of substrates could easily be detected by HPLC using a diode array detector (200–595 nm).
In all performed heteromolecular transformations, the product yield was low with an educt concentration of 1:1 mM, and in the reaction mixture of trimethyl-1,4-hydroquinone (1d) no products were detected. Through a rise of amino acid concentration from 1:1 to 1:100 mM the reaction rate and the product yield could be increased. The highest product yield was achieved with the laccase of M. thermophila at a pH of 7 and a 1:100 mM concentration of the educts.
Using M. thermophila laccase (1:100 mM, 1a:2) the concentration of 3a (first product) was approx. 13-fold higher after 20 min and those of 4a (second product) approx. 3-fold higher after 24 h than in the reaction with P. cinnabarinus laccase (1:100 mM) (Fig. 2). By means of the laccase of M. thermophila the concentration of product 3c was 27-fold higher in the 1:100 mM attempt (2,3-dimethyl-1,4-hydroquinone:l-phenylalanine) after 24 h compared with the 1:1 mM reaction.
In the reaction mixture of 1d and 2 (1:100 mM) only traces of one coupling product (3d) were detected, due to the characteristic UV/VIS-spectra and MS measurements (for data see “Characterization” section).
In all laccase-catalyzed reactions of 1a-d, respectively, one product was detected, which was identified through the retention time (HPLC) and UV/VIS-spectrum as the corresponding quinonoid form of the substrate (data not shown), as described for other para-hydroquinones (Mikolasch et al. 2006; Manda et al. 2005; Niedermeyer et al. 2005). In all cross-coupling reactions, the respective hydroquinone was converted within 20 min completely into the corresponding quinone.
The concentration of these quinones decreased during the incubation of the reaction mixture, under simultaneous formation of heteromolecular coupling products. This means that the reactions proceeded in two steps. First the quinones are formed, which then react with the amino acid resulting in the development of a monoaminated heteromolecular coupling product, as recently described (Mikolasch et al. 2006; Manda et al. 2005; Niedermeyer et al. 2005). Further reactions with the amino partner to diaminated products are dependent on the substitution grade of the p-hydroquinone. Whereas 3a reacted with 2 forming 4a, an diaminated product, for 3c no further reaction with 2 could be detected.
Due to the highest yield of the main coupling products 3a, 3c and 4a, the reaction with Myceliophthora thermophila laccase, 1:100 mM (1,4-hydroquinone/2,3-dimethyl-1,4-hydroquinone:l-phenylalanine) was chosen for the characterization of the products.
Detailed structural characterization of three products
By means of HPLC-MS the reaction product 3a was identified as quinonoid structure, arising from the oxidation of 1,4-hydroquinone and further coupling with one molecule l-phenylalanine.
In accordance with the first heteromolecular coupling product, the second coupling product 4a was identified as quinonoid structure. But while 3a was formed of one molecule benzoquinone and one molecule l-phenylalanine, product 4a was constituted of one molecule benzoquinone and two molecules of the amino acid l-phenylalanine. The structure was revealed by MS measurements and NMR analysis. In the API-ES positive ion mode the typical fragment ion of the decarboxylated form of the product was found. The analysis of product 3a by high-resolution mass spectrometry (HRMS) in positive ion mode showed the expected ions [M + H]+ at m/z = 272.0911. This measured value, with an error of −2.4 ppm, was in adequate conformance with the calculated values for the formula C15H14NO4. The HRMS of product 4a in the positive ion mode showed a signal at m/z = 435.1549 for the quasi-molecular ion [M + H]+. This value confirmed the calculated mass of the quasi-molecular ion [M + H]+ with a deviation of −0.4 ppm. Additionally, an intensive signal was detected in negative ion mode at m/z = 433.1411 for the [M − H]− ion, in good accordance with the supposed value m/z = 433.1405, too.
1H-NMR spectral data of 4a showed characteristic signals for both educts (Table 1). Multiplicity of the H5″ and H2″ protons indicated substituents at the C1″ and the C4″ position. In the 13C-NMR spectrum of 4a, the signal for C3″ and C6″ in the range of 180 ppm indicated a quinonoid character of the product. The HMBC spectrum of 4a (Table 1) showed correlations between the protons H5″/H2″ and the quinone carbonylcarbons confirming the oxidation of the p-hydroquinone to a quinone as described previously (Mikolasch et al. 2006; Manda et al. 2005; Niedermeyer et al. 2005; Tatsumi et al. 1994a, b). All facts together proved 4a to be aminated at the C1″ and the C4″ position of the quinone ring.
The heteromolecular product 3c has a monoaminated quinonoid structure, as shown with MS and NMR measurements. In the API-ES (neg. ion mode) and APCI (pos. ion mode) measurements the anticipated quasi-molecular ions [M − H]− at m/z = 298 and [M + H]+ at m/z = 300 were detected. The investigations concerning product 3c by high-resolution mass spectrometry in positive ion mode showed signals for the [M + H]+ ion at m/z = 300.1228 and for the [M + Na]+ ion at m/z = 322.1051 which were in good accordance with the theoretical values of m/z = 300.1230 (−0.6 ppm) and m/z = 322.1050 (+0.6 ppm), respectively.
The final confirmation of the structure was accomplished with different NMR measurements in deuterated methanol-d4 (1H-, 13C-NMR, DEPT). In evaluation of the 1H- and 13C-NMR-spectra it could be stated that both the number and the multiplicity of resonance signals within the aromatic range changed in comparison with 1c. In the 1H-NMR-spectrum of 3c appears a signal at 5.26 ppm, which could be assigned to the H6″. This could be attributed to the coupling of one molecule 2 at C1. The aromatic signals of 2 are high-field-shifted in the coupling product. Additionally all signals show more varied couplings due to the substitution at the amino group caused by steric effects of 1c. In the 13C-NMR-spectrum, two signals in the range of 180 ppm indicated a quinonoid character of the product. Including the results of DEPT the structure of 3c could be confirmed, as 2-(4,5-dimethyl-3,6-dioxocyclohexa-1,4-dienylamino)-3-phenylpropionic acid (Fig. 1).
Discussion
Structural characterization of three reaction products showed that in the case of laccase-catalyzed reaction, derivatization of the amino acid l-phenylalanine with para-dihydroxylated compounds depends on the number and position of substituents of the substrates.
The p-hydroquinone-substrates can be divided into two groups. There are on the one hand alkylated p-hydroquinones as used in this study and on the other hand 2,5-dihydroxybenzoic acid derivatives as used by Mikolasch et al. (2006), Manda et al. (2005) and Niedermeyer et al. (2005).
We pointed out that enzymatic derivatization of amino acids is possible with alkylated p-hydroquinones, namely 1,4-hydroquinone as the basic structure, methyl-1,4-hydroquinone, 2,3-dimethyl-1,4-hydroquinone and trimethyl-1,4-hydroquinone, while Manda et al. (2006) derivatized l-tryptophane with 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide only. 2,5-Dihydroxy-N-(2-hydroxyethyl)-benzamide is a substance of the 2,5-dihydroxybenzoic acid derivative group.
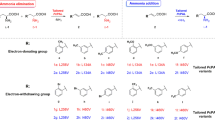
As mentioned above the laccase catalyzes a radical formation. The radicals can be transformed into quinones, and then a nucleophilic attack of the amino acid is possible. The nuclear amination resulting in the formation of a C–N-coupled heteromolecular product, consisting of the respective quinone and the amino acid l-phenylalanine, as described for other aminations (Mikolasch et al. 2006; Manda et al. 2005; Niedermeyer et al. 2005; Michalek and Szarkowska 1959).
In the reaction of hydroquinones with amines, two aminations dependent on the substitution grade are conceivable. First one amine is coupled to the hydroquinone, confirmed by the structural characterization of the products 3a and 3c. A further amine molecule can then react with the para-position to the first amine group, if there is no other substituent. That was confirmed by the structural analysis of 4a (Fig. 1). The same reaction mechanism could be observed with 1b and 2. Nevertheless, the ortho-position for the diamination would be possible, but it is not very likely due to the steric covering of the ortho-position of the first amino substituent. The preferential substitution is in para-position (Yamaoka and Nagakura 1971), as described likewise for other diaminations (Niedermeyer et al. 2005; Berger 1988; Finley 1988) (Fig. 3).
Remarkable is the fact that small amounts of 3d were formed from 1d under the used reaction conditions (1:100 mM, 1d:2), whereas Niedermeyer et al. (2005) did not detect any products in the reactions of trimethyl-1,4-hydroquinone and aromatic amines with a concentration of 1:5 mM. Our results suggest that changes in molarity can initiate the formation of products.
Altogether the amination of unsubstituted and monomethylated 1,4-hydroquinone leads to two coupling products, heteromolecular di- and trimer. In contrast to this reaction pattern, the heteromolecular dimer is the final product in the transformation reactions of 2,3-dimethyl-1,4-hydroquinone and trimethyl-1,4-hydroquinone respectively with l-phenylalanine, developed after the first nucleophilic addition. Due to the methyl groups at C2 and C3 at the aromatic ring of 1c and additionally at C5 of 1d a second amination is hindered. A diamination of substituted 1,4-benzoquinones is however not impossible in principle. With hydroxy, alkoxy or halogen substituents the reaction happens usually under substitution of the functional group and also alkyl groups can be replaced in some cases by amino groups (Ulrich and Richter 1977). Chakraborty et al. (1998) determined in the reaction of 2,3-dimethyl-1,4-hydroquinone and octadecylamine, a substitution of a methyl group, which was in para-position to the first bound octadecylamine.
Here it is to be pointed out that these reactions were chemically catalyzed, without participation of laccase. Due to the mild reaction conditions in laccase-catalyzed synthesis, compared to chemical methods, no diamination was determined with laccase.
The reactions of 1a and 1c, respectively, with 2 showed many parallels.
Concerning product concentration the heteromolecular transformation reactions were affected by laccase and pH-value of the used buffer solution respectively. Moreover, the product yields were depended on the quantity of the amino acid.
The highest product yield was reached with the laccase of M. thermophila and an excess of amino acid. This corresponds with the experiments of Niedermeyer et al. 2005. There it was stated that with Trametes spec. laccase (pH-optima 5) the product formation happens slower and with lower yield than with M. thermophila laccase (pH-optima 7). Furthermore, increasing the ratio of the amino partner in comparison with the alkylated hydroquinone of 5:1 led to faster turnover rates and higher product yields (Niedermeyer et al. 2005).
Nevertheless, an influence of organic acids of the buffer cannot be excluded, since these can already cause an oxidation of the substrate (Jonas 1997).
In summary, the experiments confirmed, that a derivatization of unprotected amino acids, like l-phenylalanine catalyzed by laccase is possible. The presented method is a new one pot synthesis for the production of molecules with probably new properties. So these derivatized amino acids could be the basis of new pharmaceuticals. Especially in the connection with quinones this could be promising. 1,4-Quinones are components of important bioactive compounds, for example antibiotics or chemotherapeutics (Pachatouridis et al. 2002; Mizushina et al. 2000; Fleck et al. 1978; Falci et al. 1977).
Our described synthesis method is suitable to render the properties of the amino acids and/or of the used hydroquinone. The biological characteristics of the new compounds will be prospectively tested in future.
The application possibilities of the new enzymatically synthesized compounds is manifold and the laccase could be specifically chosen for the respective utilization, for example temperature-resistance or pH-stability. In the future the applicability of laccase will be optimized and enhanced to improve the utilization of the coupling products.
References
Berger S, Hertl P, Rieker A (1988) Physical and chemical analysis of quinones. In: Patai S, Rappoport Z (eds) The chemistry of the quinoid compounds, 2 edn. John Wiley and Sons Ltd., New York, pp 29–86
Bollag JM (1992) Enzymes catalyzing oxidative coupling reactions of pollutants. In: Sigel H, Sigel A (eds) Metal ions in biological systems, 28th edn. Marcel Dekker Inc, New York, pp 205–217
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates—an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Burton SG (2003) Laccases and phenol oxidases in organic synthesis—a review. Curr Org Chem 7:1317–1331
Chakraborty M, McConville DB, Niu Y, Tessier CA, Youngs WJ (1998) Reactions of primary and secondary amines with substituted hydroquinones: nuclear amination, side-chain amination, and indolequinone formation. J Org Chem 63:7563–7567
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Davin LB, Lewis NG (2005) Dirigent phenoxy radical coupling: advances and challenges. Curr Opin Biotechnol 16:398–406
Falci KJ, Franck RW, Smith GP (1977) Approaches to the mitomycins: photochemistry of aminoquinones. J Org Chem 42:3317–3319
Finley K (1988) In: Patai S, Rappoport Z (eds) The chemistry of the quinoid compounds. 2 edn. John Wiley and Sons Ltd., New York, pp 537–717
Fleck WF, Strauss DG, Meyer J, Porstendorfer G (1978) Fermentation, isolation, and biological activity of maduramycin: a new antibiotic from Actinomadura rubra. Z Allg Mikrobiol 18:389–398
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediat J 3:1–25
Jonas U (1997) Biotransformation von Biarylverbindungen durch Weißfäulepilze unter besonderer Berücksichtigung des ligninolytischen Enzymsystems von Pycnoporus cinnabarinus und Trametes versicolor. Dissertation, Mat. Nat. Fakultät. E.-M.-Arndt-Universität, Greifswald
Jonas U, Hammer E, Schauer F, Bollag JM (1998) Transformation of 2-hydroxydibenzofuran by laccases of white rot fungi Trametes versicolor and Pycnoporus cinnabarinus and characterization of oligomerization products. Biodegradation 8:321–328
Lai JH, Pham H, Hangauer DG (1996) Synthesis of a vicinal tricarbonyl amide derivative of l-phenylalanine. J Org Chem 61:1872–1874
Manda K, Hammer E, Mikolasch A, Gördes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid l-tryptophan by coupling with p-hydroquinone 2, 5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31:409–419
Manda K, Hammer E, Mikolasch A, Niedermeyer T, Dec J, Jones AD, Benesi AJ, Schauer F, Bollag JM (2005) Laccase-induced cross-coupling of 4-aminobenzoic acid with para-dihydroxylated compounds 2, 5-dihydroxy-N-(2-hydroxyethyl)-benzamide and 2, 5-dihydroxybenzoic acid methyl ester. J Mol Catal B Enzym 35:86–92
Michalek H, Szarkowska L (1959) The quinone–amino acid complexes and polyphenolase. Acta Biochimica Polonica 6:399–409
Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Jülich WD, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull 54:632–638
Mizushina Y, Ueno T, Oda M, Yamaguchi T, Saneyoshi M, Sakaguchi K (2000) The biochemical mode of inhibition of DNA polymerase β by α rubromycin. Biochem Biophys Acta 1523:172–181
Niedermeyer THJ, Mikolasch A, Lalk M (2005) Nuclear amination catalyzed by fungal laccases: reaction products of p-hydroquinones and primary aromatic amines. J Org Chem 70:2002–2008
Pachatouridis C, Iakovidou Z, Myoglou E, Mourelatos D, Pantazaki AA, Papageorgiou VP, Kotsis A, Liakopoulou-Kyriakides M (2002) Synthesis and cytogenetic effects of aminoquinone derivatives with a di- and tripeptide. Anti Cancer Drugs 13:367–372
Schauer F, Lindequist U, Hammer E, Jülich WD, Schäfer A, Jonas U (2001) Biotransformation von biologisch aktiven Verbindungen aus verschiedenen chemischen Stoffklassen mittels der Enzyme Laccase und Manganperoxidase. Patentschrift. WO 01/98518 A2
Tatsumi K, Freyer A, Minard RD, Bollag JM (1994a) Enzymatic coupling of chloroanilines with syringic acid, vanillic acid and protocatechuic acid. Soil Biol Biochem 26:735–742
Tatsumi K, Freyer A, Minard RD, Bollag JM (1994b) Enzyme-mediated coupling of 3, 4-dichloroanilin and ferulic acid: a model for pollutant binding to humic materials. Environ Sci Technol 28:210–215
Thurston CF (1994) The structure and function of fungal laccases. Microbiol 140:19–26
Ulrich H, Richter R (1977) Die para-Chinone der Benzol- und Naphthalin-Reihe. In: Houben-Weyl (eds) Methoden der organischen Chemie. 4. Aufl. (Band VII/3a), Chinone, Teil 1. Thieme-Verlag, Stuttgart, pp 402–413
Yamaoka T, Nagakura S (1971) Reactions of aliphatic amines with p-benzoquinone and its chloro derivatives. Bull Chem Soc Jpn 44:2971–2975
Acknowledgments
Financial support by means of a scholarship from the government of Mecklenburg-Vorpommern is gratefully acknowledged. We thank M. Lalk (Institute of Pharmacy, University of Greifswald), K. Weisz (Institute of Biochemistry, University of Greifswald) for providing NMR data and R. Jack (Institute of Immunology, University of Greifswald) for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hahn, V., Mikolasch, A., Manda, K. et al. Laccase-catalyzed carbon–nitrogen bond formation: coupling and derivatization of unprotected l-phenylalanine with different para-hydroquinones. Amino Acids 37, 315–321 (2009). https://doi.org/10.1007/s00726-008-0154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0154-2