Abstract
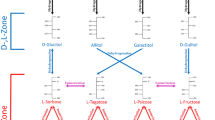
l-Rhamnose isomerase (L-RI, EC 5.3.1.14), catalyzing the isomerization between l-rhamnose and l-rhamnulose, plays an important role in microbial l-rhamnose metabolism and thus occurs in a wide range of microorganisms. It attracts more and more attention because of its broad substrate specificity and its great potential in enzymatic production of various rare sugars. In this article, the enzymatic properties of various reported L-RIs were compared in detail, and their applications in the production of l-rhamnulose and various rare sugars including d-allose, d-gulose, l-lyxose, l-mannose, l-talose, and l-galactose were also reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-Rhamnose isomerase (L-RI, EC 5.3.1.14) is an aldose isomerase reversibly catalyzing the isomerization between l-rhamnose and l-rhamnulose. L-RI exists in a wide range of microorganisms due to its important role in l-rhamnose metabolism. L-RI exhibits very broad specificity toward various aldoses and ketoses and thus displays a great potential in biological production of many expensive rare sugars (Leang et al. 2004b). Rare sugars are defined by International Society of Rare Sugars as monosaccharides and their derivatives existing in nature in very limited quantities (Izumori 2002). Recently, plenty of literatures have focused on the unique physiological effects and medical potential of rare sugars, and they are proven to be of paramount significance in the food industry, nutraceuticals, pharmaceuticals, and other applications. d-Tagatose (Kim 2004), d-psicose (Mu et al. 2012), and some polyols have been formally approved by US Food and Drug Administration (FDA) as Generally Recognized As Safe (GRAS) and allowed to be used in food and medical industries.
Microbial L-RI has been used for enzymatic production of d-allose and various l-monosaccharides. d-Allose, a C3 epimer of d-glucose, has been proven to be of potential medical benefits, including cryoprotective (Sui et al. 2007), anti-oxidative (Nakamura et al. 2011), anti-hypertensive (Kimura et al. 2005), immunosuppressant (Hossain et al. 2000), anti-inflammatory (Gao et al. 2013), anti-tumor (Malm et al. 2015), and anti-cancer activities (Indo et al. 2014). L-form monosaccharides attract increasing attention because they can be potentially used as important starting materials to synthesize many high-value pharmaceutical compounds (Ahmed 2001). l-Sorbose has been used as the industrial precursor for chemical synthesis of l-ascorbic acid for decades (Pappenberger and Hohmann 2014) and was used as a substrate for synthesis of the potential glycosidase inhibitor 1-deoxygalactonojirimycin (Furneaux et al. 1993). l-Ribose is an important precursor for the synthesis of L-nucleoside analogs (Hu et al. 2011).
Although L-RI displays great potential for use in production of rare sugars, there is still no available literature reviewing the recent studies of L-RIs. In this work, all the reported microbial L-RIs are compared in detail, and the biotechnological production of various rare sugars by L-RI is reviewed.
Role in microbial sugar metabolism
l-Rhamnose is an important component of mycobacterial cell walls of some microorganisms (Southard et al. 1959). The anabolism and catabolism of l-rhamnose have been studied. It is normally generated by microorganism through d-fructose and d-mannose metabolism (Kanehisa and Goto 2000). L-RI plays a major role in microbial catabolism of l-rhamnose. Three structural genes are involved in l-rhamnose metabolism in Escherichia coli, including rhaA, rhaB, and rhaD, encoding L-RI, l-rhamnulose kinase (EC 2.7.1.5), and l-rhamnulose-1-phosphate aldolase (EC 4.1.2.19), respectively (Egan and Schleif 1993; Moralejo et al. 1993; Power 1967). L-RI catalyzes l-rhamnose to l-rhamnulose firstly; l-rhamnulose is further phosphorylated by l-rhamnulose kinase; and finally, l-rhamnose-1-phosphate is hydrolyzed by l-rhamnulose-1-phosphate aldolase to dihydroxyacetone phosphate and l-lactaldehyde (Fig. 1). The former product enters tricarboxylic acid (TCA) cycle for glycolysis. In E. coli, l-lactaldehyde is converted to l-lactate by lactaldehyde dehydrogenase (EC 1.2.1.22) under aerobic conditions, while it is reduced to l-1,2-propanediol by propanediol oxidoreductase (EC 1.1.1.77) to be transported extracellularly (Baldoma and Aguilar 1988).
Comparison of various L-RIs
So far, L-RI has been characterized from E. coli (Takagi and Sawada 1964), Lactobacillus plantarum (Domagk and Zech 1966), Pseudomonas sp. strain LL172 (Bhuiyan et al. 1997b), Pseudomonas stutzeri (Leang et al. 2004b; Leang et al. 2004c), Bacillus pallidus Y25 (Poonperm et al. 2007), Thermoanaerobacterium saccharolyticum NTOU1 (Lin et al. 2010), Thermotoga maritima ATCC 43589 (Park et al. 2010), Caldicellulosiruptor saccharolyticus ATCC 43494 (Lin et al. 2011), Bacillus halodurans ATCC BAA-125 (Prabhu et al. 2011), Mesorhizobium loti Tono (Takata et al. 2011), Dictyoglomus turgidum DSMZ 6724 (Kim et al. 2013), Bacillus subtilis ATCC 23857 (Park 2014), and B. subtilis str. 168 (Bai et al. 2015). The enzymatic properties of the different L-RIs are listed in Table 1.
Comparison of the amino acid sequences
The comparison of amino acid sequences of various microbial L-RIs was shown in Table S1. Based on the comparison data, the L-RIs could be divided into two groups. Group I members, including L-RIs from E. coli, B. halodurans ATCC BAA-125, B. subtilis str. 168, T. saccharolyticum NTOU1, C. saccharolyticus ATCC 43494, and B. pallidus Y25 exhibited 40–80 % amino acid residue identity with each other. Group II members, including L-RIs from P. stutzeri, M. loti Tono, and D. turgidum DSMZ 6724 also displayed 40–80 % identity with each other. However, interestingly, relatively low identity (only 15–25 %) was shown between groups I and II members (Table S1). In addition, the phylogenetic tree of L-RIs also provided two regions with groups I and II members (Fig. 2). Multiple sequence alignment of various L-RIs was shown in Fig. S1. Although the reported L-RIs showed much difference in the residue sequence with each other, especially between groups I and II, there were still some residues that are completely identical in all of the displayed sequences. The crystal structures of L-RIs from E. coli (Korndorfer et al. 2000), B. halodurans ATCC BAA-125 (Doan et al. 2010), and P. stutzeri (Yoshida et al. 2007) have been determined and released in Protein Data Bank (PDB) database with no. of 1D8W, 3UXI, and 2I57. The available structural information suggested that L-RI catalyzes the isomerization by a metal-mediated hydride-shift mechanism, like d-xylose isomerase. According to the E. coli L-RI structure, two metal ions were observed. One was “structural” metal to help substrate binding, coordinated by Glu234, Asp267, His294, and Asp334, and the other was “catalytic” metal to help the hydride shift, coordinated by Asp294 and His262 (Korndorfer et al. 2000). It was found that these six residues were completely conserved in all reported L-RIs (Fig. S1).
A phylogenetic tree of the already identified L-RIs with known GenBank accession number. The tree was generated by the neighbor-joining method using ClustalW software. The amino acid substitution per position was indicated by scale bar. The bootstrap values were indicated by the numbers on each clade. The GenBank numbers of various enzymes were shown after each microbial source of L-RI
Effect of metal ions
In general, L-RIs require Mn2+ or Co2+ as a divalent metal cofactor for activation (Table 1). The three-dimensional structures of both E. coli (Korndorfer et al. 2000) and P. stutzeri L-RI (Yoshida et al. 2007; Yoshida et al. 2010) reveal the clear existence of metal-binding sites and bound metals, and the mechanism of aldose-ketose isomerization by L-RI has been proposed as a metal-mediated hydride-shift biocatalysis process based on the reported structure information.
Effect of pH
The L-RIs from B. halodurans ATCC BAA-125 (Prabhu et al. 2011), C. saccharolyticus ATCC 43494 (Lin et al. 2011), T. saccharolyticum NTOU1 (Lin et al. 2010), and B. pallidus Y25 (Poonperm et al. 2007) showed pH optima at neutral pH (7.0), and others showed pH optima at slightly alkaline side up to pH 9.0 (Table 1). However, for practical applications, aldose isomerases are expected to have slightly acidic pH optima, because acidic pH conditions may reduce non-enzymatic browning reaction leading to the unwanted byproducts (Friedman 1996). Many researches have focused on the isolation of acidic aldose isomerase and ketose epimerases or the molecular modification to reduce the pH optima of these enzymes, such as d-glucose isomerase (Bhosale et al. 1996), l-arabinose isomerase (Fan et al. 2015; Xu et al. 2014), and d-psicose epimerase (Zhang et al. 2015).
Effect of temperature
A major consideration for the practical use of biotransformation is the development and improvement of the satisfied enzymes (Polizzi et al. 2007; Plou and Ballesteros 1999). Reaction at high temperatures can enhance the solubility of substrates and products, provide a higher reaction rate, and reduce the microbial contamination (Mozhaev 1993).
As shown in Table 1, most of the L-RIs show highest activities at relatively high temperatures (not less than 60 °C); however, significant difference of thermostability is seen among different sources of L-RIs. The one from M. loti Tono retains 70 % of initial activity after incubation at 50 °C for 1 h (Takata et al. 2011). The ones from B. pallidus Y25 (Poonperm et al. 2007) and B. subtilis str. 168 (Bai et al. 2015) show half-lives at 65 °C of 1 and 6 h, respectively. The one from B. halodurans ATCC BAA-125 can retain >90 % of activity after 15 h of incubation at 60 °C but only shows half-lives at 70 and 80 °C of 25 and 5 min, respectively (Prabhu et al. 2011). The one from B. subtilis ATCC 23857 has a half-life of approximately 2 h at 70 °C (Park 2014). By comparison, the ones from some thermophiles exhibit very good thermostability (Table 1). The half-lives of D. turgidum L-RI at 75, 80, and 85 °C are 28, 12.7, and 4.5 h, respectively (Kim et al. 2013). The one from T. maritima ATCC 43589 has a half-life of 773 h at 75 °C (Park et al. 2010). The one from C. saccharolyticus ATCC 43494 is strongly stable at 80 and 85 °C and exhibits a half-life of 65 min at 90 °C (Lin et al. 2011).
Substrate specificity and kinetics
L-RI has recently attracted much attention due to its wide substrate specificity toward various aldoses and ketoses and its potential applications for production of various rare sugars (Tables 2 and 3). All the reported L-RIs showed the optimum substrate as l-rhamnose, and among them, the C. saccharolyticus L-RI had the highest specific activity (380 U mg−1) toward l-rhamnose. l-Lyxose was the second favored substrate of the reported L-RIs because l-lyxose had similar configuration with l-rhamnose, and the enzyme from T. saccharolyticum NTOU1 showed much higher specific activity (130 U mg−1) toward l-lyxose than other L-RIs.
E. coli L-RI was specific for l-rhamnose, l-lyxose, and l-mannose (Korndorfer et al. 2000) while it showed null activity toward d-allose and d-ribose, but other reported L-RIs had broader specificity including d-allose and d-ribose (Table 2). From the crystal structure information, both E. coli (Korndorfer et al. 2000) and P. stutzeri (Yoshida et al. 2007) L-RIs showed highly conserved amino acid residues involved in the interactions between the protein and the O1, O2, and O3 of the l-rhamnose, which were responsible for the metal-binding and catalytic mechanism, while significant structural differences were found in the recognition of the atoms at 4, 5, and 6 positions of the substrate, which were responsible for the substrate specificity. In E. coli L-RI, Val53, Leu63, Ile67, and Phe336 created a unique hydrophobic pocket to recognize the substrate and it thus led to the narrow substrate specificity (Korndorfer et al. 2000). The residues of P. stutzeri L-RI involved in the interactions with substrate at 4, 5, and 6 positions (Yoshida et al. 2007) were interestingly very highly conserved compared to Astragalus missouriensis d-xylose isomerase (Jenkins et al. 1992), which had relatively loose substrate recognition and was able to isomerize a wide variety of substrates.
The comparison of kinetic parameters of various reported L-RIs was shown in Table 3. The L-RI from B. subtilis str. 168 exhibited the lowest K m (0.49 mM) toward l-rhamnose (Bai et al. 2015); however, the enzyme from C. saccharolyticus ATCC 43494 showed the highest catalytic efficiency (k cat/K m) toward l-rhamnose, which reached 100.32 mM−1 s−1 (Lin et al. 2011). In addition, the kinetic parameters toward various substrates significantly varied among various reported L-RIs (Table 3).
Biotechnological applications of L-RI for production of rare sugars
Production of d-allose from d-psicose
As mentioned above, the rare sugar d-allose attracts increasing attention because of its physiological effects and commercial interest. d-Allose can be produced from another rare sugar d-psicose, which is easily produced from d-fructose by ketose 3-epimerase (Mu et al. 2012). Bioconversion of d-psicose to d-allose can be catalyzed by d-galactose-6-phosphate isomerase, d-ribose-5-phosphate isomerase, and L-RI (Mu et al. 2015). Lactococcus lactis d-galactose-6-phosphate isomerase efficiently converts d-psicose to d-allose and d-altrose (Park et al. 2007b). The reported d-allose-producing d-ribose-5-phosphate isomerases include the ones from Clostridium thermocellum (Park et al. 2007a), Clostridium difficile (Yeom et al. 2010), Thermotoga maritime (Yeom et al. 2010), and Thermotoga lettingae TMO (Feng et al. 2013), and they generate no byproduct but only d-allose from d-psicose.
The biological production of d-allose was first reported using L-RI from P. stutzeri LL172 (Bhuiyan et al. 1997b). The immobilized whole cells of P. stutzeri were used to convert d-psicose to within 20 days, with the conversion yield of 40 % (Bhuiyan et al. 1998). d-Allose production was also performed by the recombinant P. stutzeri L-RI, which was immobilized by cross-linking with glutaraldehyde and l-lysine; however, it produced 25 % d-allose together with 8 % d-altrose as byproduct from d-psicose after bioconversion and ethanol crystallization (Menavuvu et al. 2006). Large-scale production of d-allose from d-psicose was performed using a continuous column bioreactor containing the recombinant P. stutzeri L-RI immobilized on BCW-2510 Chitopearl beads. When 50 % (W/W) d-psicose was applied to the column, approximately 30 % d-psicose was isomerized to d-allose for 17 days but still with a very small amount of byproducts (Morimoto et al. 2006).
Enzymatic production of d-allose from d-psicose has also been studied using the L-RIs from B. pallidus Y25 (Poonperm et al. 2007), T. saccharolyticum NTOU1 (Lin et al. 2010), C. saccharolyticus ATCC 43494 (Lin et al. 2011), and B. subtilis str. 168 (Bai et al. 2015), and they produce d-allose without any byproduct, with maximal conversion yield of 35, 34, 33, and 37.5 %, respectively.
Enzymatic production of d-gulose from d-sorbose
d-Gulose has been produced from d-sorbose by both the free (Leang et al. 2004b) and the immobilized P. stutzeri L-RI (Bhuiyan et al. 1999), with the same of conversion yield of 10 %.
Enzymatic production of L-sugars
l-Rhamnulose
l-Rhamnulose (6-deoxy-l-sorbose) is a precursor of the strawberry aroma furaneol and has been used in the flavor industry (Hecquet et al. 1996). Most of L-RIs show the optimal substrate as l-rhamnose producing l-rhamnulose. Free L-RI from B. pallidus Y25 produces l-rhamnulose from l-rhamnose with a turnover ratio of 45 % (Poonperm et al. 2007). l-Rhamnulose has also been continuously produced by immobilized L-RI from D. turgidum DSMZ 6724 in a packed bed bioreactor, and an average of 130 g L−1 l-rhamnulose can be produced from 300 g L−1 l-rhamnose, with a productivity of 78 g L−1 h−1 and a conversion yield of 43 % (Kim et al. 2013).
l-Lyxose
The recombinant P. stutzeri L-RI catalyzed the isomerization of l-xylose to l-xylulose and l-lyxose, with the final equilibrium between l-xylose/l-xylulose/l-lyxose of 61:35:4 (Leang et al. 2004b); however, the equilibrium ratio was 26:53:21 when the same enzyme was used in immobilized form using l-xylulose as substrate (Granstrom et al. 2005). The immobilized recombinant P. stutzeri L-RI produced 4.06 g L−1 l-lyxose and 4.94 g L−1 l-xylose from 19.2 g L−1 l-xylulose (Granstrom et al. 2005). Unlike P. stutzeri L-RI, the ones from B. subtilis ATCC 23857 (Park 2014) and T. maritima ATCC 43589 (Park et al. 2010) produced l-lyxose from l-xylulose without any byproduct, and the final equilibrium ratio between l-lyxose and l-xylulose was 40:60 and 45:55, respectively. The B. subtilis ATCC 23857 L-RI produced 40 g L−1 l-lyxose from 100 g L−1 l-xylulose (Park 2014), and the one from T. maritima ATCC 43589 produced 225 g L−1 l-lyxose from 500 g L−1 l-xylulose (Park et al. 2010).
l-Mannose
The enzymatic production of l-mannose from l-fructose was first studied using immobilized P. stutzeri L-RI with turnover yield of 30 % (Bhuiyan et al. 1997a). Recently, l-mannose production was also reported using free L-RIs from B. subtilis ATCC 23857 and T. maritima ATCC 43589. Twenty-five grams per liter of l-mannose was produced from 100 g L−1 l-fructose by 15 U mL−1 B. subtilis L-RI after 80 min (Park 2014), while 175 g L−1 l-mannose was produced from 500 g L−1 l-fructose by 100 U mL−1 T. maritima L-RI after 5 h (Park et al. 2010).
l-Talose
Enzymatic production of l-talose from l-tagatose was performed by P. stutzeri L-RI immobilized on Chitopearl beads BCW 2603 (Bhuiyan et al. 1999) and M. loti L-RI immobilized on BCW 2510 (Takata et al. 2011), and both could convert l-tagatose to l-talose with production yield of 12 %. P. stutzeri L-RI produced l-galactose as byproduct (Leang et al. 2004b), while M. loti L-RI produces no byproduct but only l-talose from l-galactose (Takata et al. 2011).
l-Galactose
Leang et al. reported the production of l-galactose from l-tagatose by P. stutzeri L-RI immobilized on BCW 2510, and the conversion ratio was 60:30:10 (l-tagatose/l-galactose/l-talose) at equilibrium. In addition, the authors also performed the l-galactose from l-sorbose by two reactions catalyzed by d-tagatose 3-epimerase followed by P. stutzeri L-RI, with an overall yield of 7.5 % l-galactose obtained from l-sorbose (Leang et al. 2004a).
References
Ahmed Z (2001) Production of natural and rare pentoses using microorganisms and their enzymes. Electron J Biotechnol 4:103–111
Bai W, Shen J, Zhu Y, Men Y, Sun Y, Ma Y (2015) Characteristics and kinetic properties of L-rhamnose isomerase from Bacillus subtilis by isothermal titration calorimetry for the production of D-allose. Food Sci Technol Res 21:13–22
Baldoma L, Aguilar J (1988) Metabolism of L-fucose and L-rhamnose in Escherichia coli: aerobic-anaerobic regulation of L-lactaldehyde dissimilation. J Bacteriol 170:416–421
Bhosale SH, Rao MB, Deshpande VV (1996) Molecular and industrial aspects of glucose isomerase. Microbiol Res 60:280–300
Bhuiyan SH, Itami Y, Izumori K (1997a) Immobilization of L-rhamnose isomerase and its application in L-mannose production from L-fructose. J Ferment Bioeng 84:558–562
Bhuiyan SH, Itami Y, Izumori K (1997b) Isolation of an L-rhamnose isomerase-constitutive mutant of Pseudomonas sp. strain LL172: Purification and characterization of the enzyme. J Ferment Bioeng 84:319–323
Bhuiyan SH, Itami Y, Rokui Y, Katayama T, Izumori K (1998) D-allose production from D-psicose using immobilized L-rhamnose isomerase. J Ferment Bioeng 85:539–541
Bhuiyan SH, Itami Y, Takada G, Izumori K (1999) Preparation of L-talose and D-gulose from L-tagatose and D-sorbose, respectively, using immobilized L-rhamnose isomerase. J Biosci Bioeng 88:567–570
Doan TN, Prabhu P, Kim JK, Ahn YJ, Natarajan S, Kang LW, Park GT, Lim SB, Lee JK (2010) Crystallization and preliminary X-ray crystallographic analysis of L-rhamnose isomerase with a novel high thermostability from Bacillus halodurans. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:677–680
Domagk GF, Zech R (1966) L-Rhamnose isomerase. Method Enzymol 9:579–582
Egan SM, Schleif RF (1993) A regulatory cascade in the induction of rhaBAD. J Mol Biol 234:87–98
Fan C, Xu W, Zhang T, Zhou L, Jiang B, Mu W (2015) Engineering of Alicyclobacillus hesperidum L-arabinose isomerase for improved catalytic activity and reduced pH optimum using random and site-directed mutagenesis. Appl Biochem Biotech 7:1480–1492
Feng Z, Mu W, Jiang B (2013) Characterization of ribose-5-phosphate isomerase converting D-psicose to D-allose from Thermotoga lettingae TMO. Biotechnol Lett 35:719–724
Friedman M (1996) Food browning and its prevention: an overview. J Agric Food Chem 44:631–653
Furneaux RH, Tyler PC, Whitehouse LA (1993) Synthesis of 1,5-dideoxy-1,5-imino-D-galactitol from L-sorbose. Tetrahedron Lett 34:3609–3612
Gao D, Kawai N, Nakamura T, Lu F, Fei Z, Tamiya T (2013) Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol Med Chir 53:365–374
Granstrom TB, Takata G, Morimoto K, Leisola M, Izumori K (2005) L-Xylose and L-lyxose production from xylitol using Alcaligenes 701B strain and immobilized L-rhamnose isomerase enzyme. Enzym Microb Technol 36:976–981
Hecquet L, Bolte J, Demuynck C (1996) Enzymatic synthesis of “natural-labeled” 6-deoxy-L-sorbose precursor of an important food flavor. Tetrahedron 52:8223–8232
Hossain MA, Wakabayashi H, Goda F, Kobayashi S, Maeba T, Maeta H (2000) Effect of the immunosuppressants FK506 and D-allose on allogenic orthotopic liver transplantation in rats. Transplant Proc 32:2021–2023
Hu C, Li L, Zheng Y, Rui L, Hu C (2011) Perspective of biotechnological production of L-ribose and its purifcaiton. Appl Microbiol Biotechnol 92:449–455
Indo K, Hoshikawa H, Kamitori K, Yamaguchi F, Mori T, Tokuda M, Mori N (2014) Effects of D-allose in combination with docetaxel in human head and neck cancer cells. Int J Oncol 45:2044–2050
Izumori K (2002) Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89:120–124
Jenkins J, Janin J, Rey F, Chiadmi M, Vantilbeurgh H, Lasters I, Demaeyer M, Vanbelle D, Wodak SJ, Lauwereys M, Stanssens P, Mrabet NT, Snauwaert J, Matthyssens G, Lambeir AM (1992) Protein engineering of xylose (glucose) isomerase from Actinoplanes missouriensis. 1. Crystallography and site-directed mutagenesis of metal-binding sites. Biochemistry 31:5449–5458
Kanehisa M, Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30
Kim P (2004) Current studies on biological tagatose production using L-arabinose isomerase: a review and future perspective. Appl Microbiol Biotechnol 65:243–249
Kim YS, Shin KC, Lim YR, Oh DK (2013) Characterization of a recombinant L-rhamnose isomerase from Dictyoglomus turgidum and its application for L-rhamnulose production. Biotechnol Lett 35:259–264
Kimura S, Zhang GX, Nishiyama A, Nagai Y, Nakagawa T, Miyanaka H, Fujisawa Y, Miyatake A, Nagai T, Tokuda M, Abe Y (2005) D-Allose, an all-cis aldo-hexose, suppresses development of salt-induced hypertension in Dahl rats. J Hypertens 23:1887–1894
Korndorfer IP, Fessner WD, Matthews BW (2000) The structure of rhamnose isomerase from Escherichia coli and its relation with xylose isomerase illustrates a change between inter and intra-subunit complementation during evolution. J Mol Biol 300:917–933
Leang K, Maekawa K, Menavuvu BT, Morimoto K, Granström TB, Takada G, Izumori K (2004a) A novel enzymatic approach to the massproduction of L-galactose from L-sorbose. J Biosci Bioeng 97:383–388
Leang K, Takada G, Fukai Y, Morimoto K, Granstrom TB, Izumori K (2004b) Novel reactions of L-rhamnose isomerase from Pseudomonas stutzeri and its relation with D-xylose isomerase via substrate specificity. Biochim Biophys Acta 1674:68–77
Leang K, Takada G, Ishimura A, Okita M, Izumori K (2004c) Cloning, nucleotide sequence, and overexpression of the L-rhamnose isomerase gene from Pseudomonas stutzeri in Escherichia coli. Appl Environ Microbiol 70:3298–3304
Lin CJ, Tseng WC, Fang TY (2011) Characterization of a thermophilic L-rhamnose isomerase from Caldicellulosiruptor saccharolyticus ATCC 43494. J Agric Food Chem 59:8702–8708
Lin CJ, Tseng WC, Lin TH, Liu SM, Tzou WS, Fang TY (2010) Characterization of a thermophilic L-rhamnose isomerase from Thermoanaerobacterium saccharolyticum NTOU1. J Agric Food Chem 58:10431–10436
Malm SW, Hanke NT, Gill A, Carbajal L, Baker AF (2015) The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. J Exp Clin Cancer Res 34
Menavuvu BT, Poonperm W, Leang K, Noguchi N, Okada H, Morimoto K, Granstrom TB, Takada G, Izumori K (2006) Efficient biosynthesis of D-allose from D-psicose by cross-linked recombinant L-rhamnose isomerase: separation of product by ethanol crystallization. J Biosci Bioeng 101:340–345
Moralejo P, Egan SM, Hidalgo E, Aguilar J (1993) Sequencing and characterization of a gene cluster encoding the enzymes for L-rhamnose metabolism in Escherichia coli. J Bacteriol 175:5585–5594
Morimoto K, Park CS, Ozaki M, Takeshita K, Shimonishi T, Granstrom TB, Takata G, Tokuda M, Izumori K (2006) Large scale production of D-allose from D-psicose using continuous bioreactor and separation system. Enzym Microb Technol 38:855–859
Mozhaev VV (1993) Mechanism-based strategies for protein thermostabilization. Trends Biotechnol 11:88–95
Mu W, Yu L, Zhang W, Zhang T, Jiang B (2015) Isomerases for biotransformation of D-hexoses. Appl Microbiol Biotechnol 99:6571–6584
Mu W, Zhang W, Feng Y, Jiang B, Zhou L (2012) Recent advances on applications and biotechnological production of D-psicose. Appl Microbiol Biotechnol 94:1461–1467
Nakamura T, Tanaka S, Hirooka K, Toyoshima T, Kawai N, Tamiya T, Shiraga F, Tokuda M, Keep RF, Itano T, Miyamoto O (2011) Anti-oxidative effects of D-allose, a rare sugar, on ischemia-reperfusion damage following focal cerebral ischemia in rat. Neurosci Lett 487:103–106
Park CS (2014) Characterization of a recombinant L-rhamnose isomerase from Bacillus subtilis and its application on production of L-lyxose and L-mannose. Biotechnol Bioproc Eng 19:18–25
Park CS, Yeom SJ, Kim HJ, Lee SH, Lee JK, Kim SW, Oh DK (2007a) Characterization of ribose-5-phosphate isomerase of Clostridium thermocellum producing D-allose from D-psicose. Biotechnol Lett 29:1387–1391
Park CS, Yeom SJ, Lim YR, Kim YS, Oh DK (2010) Characterization of a recombinant thermostable L: -rhamnose isomerase from Thermotoga maritima ATCC 43589 and its application in the production of L-lyxose and L-mannose. Biotechnol Lett 32:1947–1953
Park HY, Park CS, Kim HJ, Oh DK (2007b) Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces D-allose from D-psicose. J Biotechnol 132:88–95
Pappenberger G, Hohmann HP (2014) Industrial production of L-ascorbic acid (Vitamin C) and D-isoascorbic acid. In: Zorn H, Czermak P (eds) Biotechnology of food and feed additives. Springer, Berlin, pp. 143–188
Plou FJ, Ballesteros A (1999) Stability and stabilization of biocatalysts. Trends Biotechnol 17:304–306
Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF (2007) Stability of biocatalysts. Curr Opin Chem Biol 11:220–225
Poonperm W, Takata G, Okada H, Morimoto K, Granstrom TB, Izumori K (2007) Cloning, sequencing, overexpression and characterization of L-rhamnose isomerase from Bacillus pallidus Y25 for rare sugar production. Appl Microbiol Biotechnol 76:1297–1307
Power J (1967) The L-rhamnose genetic system in Escherichia coli K-12. Genetics 55:557–568
Prabhu P, Thanh Thi Ngoc D, Jeya M, Kang LW, Lee JK (2011) Cloning and characterization of a rhamnose isomerase from Bacillus halodurans. Appl Microbiol Biotechnol 89:635–644
Southard WH, Hayashi JA, Barkulis SS (1959) Studies of streptococcal cell walls. IV The conversion of D-glucose to cell wall L-rhamnose. J Bacteriol 78:79–81
Sui L, Nomura R, Dong Y, Yamaguchi F, Izumori K, Tokuda M (2007) Cryoprotective effects of D-allose on mammalian cells. Cryobiology 55:87–92
Takagi Y, Sawada H (1964) The metabolism of L-rhamnose in Escherichia coli. I. L-rhamnose isomerase. Biochim Biophys Acta 92:10–17
Takata G, Uechi K, Taniguchi E, Kanbara Y, Yoshihara A, Morimoto K, Izumori K (2011) Characterization of Mesorhizobium loti L-rhamnose isomerase and its application to L-talose production. Biosci Biotechnol Biochem 75:1006–1009
Xu Z, Li S, Feng XH, Zhan YJ, Xu H (2014) Function of aspartic acid residues in optimum pH control of L-arabinose isomerase from Lactobacillus fermentum. Appl Microbiol Biotechnol 98:3987–3996
Yeom SJ, Kim BN, Park CS, Oh DK (2010) Substrate specificity of ribose-5-phosphate isomerases from Clostridium difficile and Thermotoga maritima. Biotechnol Lett 32:829–835
Yoshida H, Yamada M, Ohyama Y, Takada G, Izumori K, Kamitori S (2007) The structures of L-rhamnose isomerase from Pseudomonas stutzeri in complexes with L-rhamnose and D-allose provide insights into broad substrate specificity. J Mol Biol 365:1505–1516
Yoshida H, Yamaji M, Ishii T, Izumori K, Kamitori S (2010) Catalytic reaction mechanism of Pseudomonas stutzeri L-rhamnose isomerase deduced from X-ray structures. FEBS J 277:1045–1057
Zhang W, Li H, Zhang T, Jiang B, Zhou L, Mu W (2015) Characterization of a d-psicose 3-epimerase from Dorea sp. CAG317 with an acidic pH optimum and a high specific activity. J Mol Catal B Enzym 120:68–74
Acknowledgments
This work was supported by the NSFC Project (No. 21276001), the 863 Project (No. 2013AA102102), the Support Project of Jiangsu Province (No. BK20130001 and 2015-SWYY-009), and the project of Outstanding Scientific and Technological Innovation Group of Jiangsu Province (Jing Wu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 731 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Zhang, W., Zhang, T. et al. l-Rhamnose isomerase and its use for biotechnological production of rare sugars. Appl Microbiol Biotechnol 100, 2985–2992 (2016). https://doi.org/10.1007/s00253-016-7369-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7369-z