Abstract
A putative recombinant enzyme from Dictyoglomus turgidum was characterized and immobilized on Duolite A568 beads. The native enzyme was a 46 kDa tetramer. Its activity was highest for l-rhamnose, indicating that it is an l-rhamnose isomerase. The maximum activities of both the free and immobilized enzymes for l-rhamnose isomerization were at pH 8.0 and 75 °C in the presence of Mn2+. Under these conditions, the half-lives of the free and immobilized enzymes were 28 and 112 h, respectively. In a packed-bed bioreactor, the immobilized enzyme produced an average of 130 g l-rhamnulose l−1 from 300 g l-rhamnose l−1 after 240 h at pH 8.0, 70 °C, and 0.6 h−1, with a productivity of 78 g l−1 h−1 and a conversion yield of 43 %. To the best of our knowledge, this is the first report describing the enzymatic production of l-rhamnulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-Rhamnulose (6-deoxy-l-sorbose) is a precursor of furaneol that has been used in the flavor industry because of its sweet strawberry aroma (Hecquet et al. 1996). l-Rhamnose isomerase catalyzes the isomerization of l-rhamnose to l-rhamnulose (Menavuvu et al. 2006) and participates in the metabolism of sugars such as mannose and fructose (Kanehisa and Goto 2000). l-Rhamnose isomerase produce rare sugars, such as l-lyxose, l-mannose, l-talose, d-gulose, and d-allose, and have been isolated and characterized from various microorganisms including Escherichia coli (Badia et al. 1991), Pseudomonas stutzeri (Bhuiyan et al. 1999; Leang et al. 2004a, b; Menavuvu et al. 2006), Bacillus pallidus (Poonperm et al. 2007), Bacillus halodurans (Prabhu et al. 2011), Thermotoga maritima (Park et al. 2010), Mesorhizobium loti (Takata et al. 2011), and Caldicellulosiruptor saccharolyticus (Lin et al. 2011). However, the production of l-rhamnulose, the authentic product for l-rhamnose isomerase, has not yet been reported.
In this study, a thermostable recombinant l-rhamnose isomerase from Dictyoglomus turgidum was characterized and immobilized. The reaction conditions of the free and immobilized enzymes were optimized. Under optimized conditions, the continuous production of l-rhamnulose from l-rhamnose was performed in a packed-bed bioreactor containing immobilized enzyme.
Materials and methods
Bacterial strains, plasmid, and culture conditions
Dictyoglomus turgidum DSMZ 6724 (DSMZ, Brauschweig, Germany), E. coli ER2566 (New England Biolabs, Herfordshire, UK), and pET-28a(+) (Novagen, Darmstadt, Germany) were used as the source of l-rhamose isomerase gene, host cells, and expression vector, respectively. The recombinant E. coli for l-rhamose isomerase expression was cultured in a 2 l flask containing 500 ml of Luria–Bertani medium and 25 μg kanamycin ml−1 at 37 °C with agitation at 200 rpm. When the OD600 reached 0.6, IPTG was added to give 0.1 mM to induce enzyme expression, after which the culture was incubated with shaking at 150 rpm at 16 °C for 16 h.
Gene cloning and expression
The gene encoding a putative l-rhamose isomerase was amplified by PCR using genomic DNA isolated from D. turgidum as a template. The sequence of the oligonucleotide primers used for gene cloning was based on the DNA sequence of the putative l-rhamose isomerase from D. turgidum (GenBank accession number, CP001251.1). Forward (5′-TTCATATGAAGAATTATGAAAAGAGATATGAAA-3′) and reverse primers (5′-AACTCGAGTTATCCTCTCTCTTTAATCACTTTTTCC-3′) were designed to introduce the NdeI and XhoI restriction sites (underlined), respectively. To obtain C-terminal His-tag sequence, stop codon of the reverse primer was removed. The PCR product was subcloned into the pET-28a(+) plasmid digested with the same restriction enzymes and then transformed into E. coli ER2566.
Enzyme purification
The cells were harvested and disrupted using a sonicator in 50 mM phosphate buffer (pH 8.0) containing 300 mM NaCl and 1 mg lysozyme ml−1. The crude enzyme obtained from disrupted cells was applied to a His-Trap HP column (Amersham Biosciences, Uppsala, Sweden) equilibrated with 50 mM phosphate buffer (pH 8.0). The bound protein was subsequently eluted with the same buffer containing 250 mM imidazole at 1 ml min−1. The active fractions were collected and dialyzed at 4 °C for 16 h against 50 mM citrate/phosphate buffer (pH 6.5). The resulting solution was used as a purified enzyme. The purification using the column was carried out by a fast protein liquid chromatography system (Bio-Rad, Hercules, CA) at 4 °C.
Enzyme assay
Unless otherwise stated, the reaction was performed in 50 mM EPPS buffer (pH 8.0) containing 10 mM l-rhamnose and 1.5 U enzyme per ml in the presence of 1 mM Mn2+ at 75 °C for 10 min. One unit (U) of enzymatic activity was defined as the amount of enzyme required to produce 1 μmol l-rhamnulose from l-rhamnose per min at 75 °C and pH 8.0.
Immobilization of l-rhamose isomerase
Duolite A568 resins were used as a carrier for immobilization. To immobilize l-rhamose isomerase on the beads, 20 mg enzyme was absorbed onto 1 g (wet wt) beads, and the mixture was stirred at 4 °C for 4 h. The beads were then collected by filtration and washed three times with 50 mM EPPS buffer (pH 8.0).
Analytical methods
The concentrations of monosaccharides were determined using a Bio-LC system (Dionex ICS-3000, Sunnyvale, CA) with an electrochemical detector and a CarboPac PAI column. The column was elution at 30 °C with 0.1 M NaOH (0–5 min), followed by a linear gradient (5–35 min) of sodium acetate (0–0.2 M) at 1 ml min−1.
Results and discussion
Gene cloning and enzyme purification
A gene encoding a non-characterized protein from D. turgidum, previously proposed to be l-rhamnose isomerase, with the same sequence as that reported in GenBank (accession number CP001251.1), was cloned and expressed in E. coli. The amino acid sequence of the resulting enzyme showed 59, 59, 58, and 56 % identity with those of l-rhamnose isomerase from T. maritima, Ktedonobacter racemifer, Thermobaculum terrenum, and B. licheniformis, respectively.
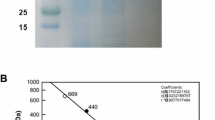
The expressed enzyme was purified from the crude extract by His-Trap HP affinity chromatography to yield a soluble protein with a specific activity of 38 U mg−1. The molecular mass of the purified enzyme, determined by SDS-PAGE, was approx. 47 kDa (Fig. 1a), which is consistent with the calculated value of 46,695 Da based on the 403 amino acids, including six histidine residues. The native enzyme was estimated to exist as a tetramer with a total molecular mass of 185 kDa as determined by gel-filtration chromatography (Fig. 1b). l-Rhamnose isomerase from T. maritima and C. saccharolyticus were 46 and 48 kDa tetramers, respectively (Park et al. 2010; Lin et al. 2011).
a SDS-PAGE analysis of l-rhamnose isomerase from D. turgidum. Lane 1, prestained marker proteins (170, 130, 100, 72, 55, 43, and 34 kDa); lane 2, His-Trap HP column chromatography product (purified enzyme), b Determination of molecular mass of l-rhamnose isomerase from D. turgidum by gel-filtration chromatography. The molecular mass of native enzyme was determined using a Sephacryl S-300 preparative grade column HR 16/60. The column was calibrated with apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), and albumin (66 kDa), as reference proteins (GE Healthcare) and the molecular mass of the native enzyme was calculated by comparing with the migration length of reference proteins
Substrate specificity of the purified enzyme
The specific activity of the putative l-rhamnose isomerase from D. turgidum was investigated with the d- and l-forms of pentose and hexose. Among the tested sugars, the highest activity was observed with l-rhamnose, indicating that the putative enzyme is an l-rhamnose isomerase. The K m , k cat, and k cat/K m values for l-rhamnose at pH 8.0 and 75 °C were 24.6 mM, 195 s−1 and 7.93 mM−1 s−1, respectively. The catalytic efficiency of the enzyme followed the order l-rhamnose > l-lyxose > l-mannose > l-xylulose > l-fructose > d-allose > d-ribose (Table 1). This substrate specificity was similar to that of l-rhamnose isomerases from E. coli, P. stutzeri, B. pallidus, T. maritima, and C. saccharolyticus (Badia et al. 1991; Leang et al. 2004b; Poonperm et al. 2007; Park et al. 2010; Lin et al. 2011).
Effects of metal ions, pH, and temperature on the activities of free and immobilized enzymes
l-Rhamnose isomerase from D. turgidum was immobilized on Duolite A568 resin to evaluate its effectiveness in continuous l-rhamnulose production. The activities of both the free and immobilized enzymes were maximal at pH 8.0 and 75 °C in the presence of 1 mM Mn2+ (data not shown) and the conversion yields of l-rhamnose to l-rhamnulose were approx. 45 %. l-Rhamnose isomerases from E. coli, P. stutzeri, B. pallidus, C. saccharolyticus, and T. maritima have been reported as Mn2+-dependent enzymes (Badia et al. 1991; Leang et al. 2004b; Poonperm et al. 2007; Park et al. 2010; Lin et al. 2011). The activities of l-rhamnose isomerase from E. coli, P. stutzeri, B. pallidus, T. maritima, and C. saccharolyticus were maximal at 60, 60, 65, 85, and 90 °C, respectively (Badia et al. 1991; Leang et al. 2004b; Poonperm et al. 2007; Park et al. 2010; Lin et al. 2011). The maximum activities for all reported l-rhamnose isomerase were observed at pH 7.0, except for l-rhamnose isomerase from T. maritima, which was maximal at pH 8.0 (Park et al. 2010).
The thermostability of the free and immobilized enzymes in the production of l-rhamnulose from l-rhamnose was determined by measuring enzyme activity as a function of time and temperature. Thermal inactivation in both enzymes followed first-order kinetics. The half-lives of the free enzyme at 65, 70, 75, 80, and 85 °C were 71.3, 52.7, 28.0, 12.7, and 4.5 h, respectively. The half-lives of the immobilized enzyme were 362, 236, 112, 45.1, and 15.4 h, respectively, which were 5.1-, 4.5-, 4.0-, 3.5-, and 3.4-fold higher than those of the free enzyme, respectively. These results show that the thermostability of the enzyme was enhanced by immobilization (Fig. 2).
Thermal inactivation of l-rhamnose isomerase from D. turgidum of 65 (open circle), 70 (open triangle), 75 (open square), 80 (closed diamond), and 85 °C (closed square). a Free enzyme, b immobilized enzyme. The enzymes were incubated at temperatures ranging from 65 to 80 °C for varying periods of time. A sample was withdrawn at each time interval and was assayed for the remaining enzyme activity in 50 mM EPPS buffer (pH 8.0) containing 10 mM l-rhamnose and 1 mM Mn2+ at 75 °C for 10 min. The experimental data for thermal deactivation of enzyme were fitted to a first order curve and the half-lives of the enzyme calculated using Sigma Plot 10.0 Software (Systat Software, San Jose, CA, USA). The relative activity of 100 % was 2.5 U enzymes per ml. Data represent the means of three experiments and error bars represent standard deviation
Continuous l-rhamnulose production in a packed-bed reactor containing immobilized enzyme
l-Rhamnulose production by the immobilized enzyme was maximal at 75 °C with a half-life of 112 h. However, the operation temperature of the packed-bed reactor was decreased to 70 °C (enzyme half-life, 236 h) to increase operational stability. l-Rhamulose production was monitored at dilution rates ranging from 0.075 to 2.25 h−1 using a 300 g l-rhamnose l−1 solution in the packed-bed reactor (Fig. 3). As the dilution rate increased to 0.6 h−1, the productivity of l-rhamnulose increased with constant conversion yield. The conversion yield began to decrease dilution rates greater than 0.6 h−1. Thus, the optimal dilution rate for continuous l-rhamnulose production was 0.6 h−1.
Effect of the dilution rate on l-rhamnulose production (closed circle) and productivity (open circle) from l-rhamnose in a packed-bed reactor. Duolite A568 beads (40 g wet weight) harboring l-rhamose isomerase were packed into a reactor (XK 16, Amersham Pharmacia Biotech, Uppsala, Sweden). The working volume of the reactor was 40 ml. A solution of 50 mM EPPS buffer (pH 8.0) containing 300 g l-rhamose l−1 in the feeding reservoir was fed continuously into the reactor, and the effluent flowed out of the reactor to the outside reservoir using a peristaltic pump (Watson–Marlow 101 U/R, Cornwall, UK). The temperature was maintained at 70 °C using a water circulator (VTRC-620, Jeio Tech, Daejon, Korea)
The long-term stability of the immobilized enzyme with continuous l-rhamnulose production in the packed-bed reactor was evaluated with 300 g l-rhamnose l−1 at pH 8.0, 70 °C, and 0.6 h−1 for 360 h. The immobilized enzyme produced an average of 130 g l-rhamnulose l−1 from 300 g l-rhamnose l−1 within 240 h, with a productivity of 78 g l−1 h−1 and a conversion yield of 43 %. The l-rhamnulose concentration decreased to approx. 50 % at 312 h. Therefore, increased volumetric productivity and long-term operational stability of the immobilized enzyme were achieved in a continuous reaction in a packed-bed bioreactor. Only one report has described the immobilization of l-rhamnose isomerase. The enzyme was isolated from P. stutzeri and was immobilized by cross-linking with glutaraldehyde to enable d-allose production from d-psicose (Menavuvu et al. 2006) (Fig. 4).
Continuous l-rhamnulose production from l-rhamnose by the immobilized enzyme in a packed-bed reactor. A solution of 50 mM EPPS buffer (pH 8.0) containing 300 g l-rhamose l−1 in the feeding reservoir was fed continuously into the reactor, and the effluent flowed out of the reactor with a dilution rate of 0.6 h−1. The reaction was performed at 70 °C for 360 h
In summary, the substrate specificity of the putative enzyme from D. turgidum identified it as an l-rhamnose isomerase. The enzyme was immobilized on Duolite A568 resin for continuous l-rhamnulose production. The immobilized enzyme in a packed-bed bioreactor produced an average of 130 g l-rhamnulose l−1 from 300 g l-rhamnose l−1 within an operation time of 240 h, with a productivity of 78 g l−1 h−1 and a conversion yield of 43 %. To the best of our knowledge, this is the first report describing the enzymatic production of l-rhamnulose, a precursor of the strawberry aroma furaneol. This system provides enhanced l-rhamnulose productivity from l-rhamnose and offers long-term, continuous l-rhamnulose production. These results provide insight into the commercial production of l-form sugars via biological processes.
References
Badia J, Gimenez R, Baldoma L, Barnes E, Fessner WD, Aguilar J (1991) l-Lyxose metabolism employs the l-rhamnose pathway in mutant cells of Escherichia coli adapted to grow on l-lyxose. J Bacteriol 173:5144–5150
Bhuiyan SH, Itami Y, Takada G, Izumori K (1999) Preparation of l-talose and d-gulose from l-tagatose and d-sorbose, respectively, using immobilized l-rhamnose isomerase. J Biosci Bioeng 88:567–570
Hecquet L, Bolte J, Demuynck C (1996) Enzymatic synthesis of “natural-labeled” 6-deoxy-l-sorbose precursor of an important food flavor. Tetrahedron 52:8223–8232
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Leang K, Takada G, Fukai Y, Morimoto K, Granstrom TB, Izumori K (2004a) Novel reactions of l-rhamnose isomerase from Pseudomonas stutzeri and its relation with d-xylose isomerase via substrate specificity. Biochim Biophys Acta 1674:68–77
Leang K, Takada G, Ishimura A, Okita M, Izumori K (2004b) Cloning, nucleotide sequence, and overexpression of the l-rhamnose isomerase gene from Pseudomonas stutzeri in Escherichia coli. Appl Environ Microbiol 70:3298–3304
Lin CJ, Tseng WC, Fang TY (2011) Characterization of a thermophilic l-rhamnose isomerase from Caldicellulosiruptor saccharolyticus ATCC 43494. J Agric Food Chem 59:8702–8708
Menavuvu BT, Poonperm W, Leang K, Noguchi N, Okada H, Morimoto K, Granstrom TB, Takada G, Izumori K (2006) Efficient biosynthesis of d-allose from d-psicose by cross-linked recombinant l-rhamnose isomerase: separation of product by ethanol crystallization. J Biosci Bioeng 101:340–345
Park CS, Yeom SJ, Lim YR, Kim YS, Oh DK (2010) Characterization of a recombinant thermostable l-rhamnose isomerase from Thermotoga maritima ATCC 43589 and its application in the production of l-lyxose and l-mannose. Biotechnol Lett 32:1947–1953
Poonperm W, Takata G, Okada H, Morimoto K, Granstrom TB, Izumori K (2007) Cloning, sequencing, overexpression and characterization of l-rhamnose isomerase from Bacillus pallidus Y25 for rare sugar production. Appl Microbiol Biotechnol 76:1297–1307
Prabhu P, Doan TT, Jeya M, Kang LW, Lee JK (2011) Cloning and characterization of a rhamnose isomerase from Bacillus halodurans. Appl Microbiol Biotechnol 89:635–644
Takata G, Uechi K, Taniguchi E, Kanbara Y, Yoshihara A, Morimoto K, Izumori K (2011) Characterization of Mesorhizobium loti l-rhamnose isomerase and its application to l-talose production. Biosci Biotechnol Biochem 75:1006–1009
Acknowledgments
This work was supported by the Basic Research Lab program (No. 2010-0019306), the National Research Foundation, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YS., Shin, KC., Lim, YR. et al. Characterization of a recombinant l-rhamnose isomerase from Dictyoglomus turgidum and its application for l-rhamnulose production. Biotechnol Lett 35, 259–264 (2013). https://doi.org/10.1007/s10529-012-1069-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1069-2