Abstract
In this work, the site-saturation engineering of lysine 47 in cyclodextrin glycosyltransferase (CGTase) from Paenibacillus macerans was conducted to improve the specificity of CGTase towards maltodextrin, which can be used as a cheap and easily soluble glycosyl donor for the enzymatic synthesis of 2-O-d-glucopyranosyl-l-ascorbic acid (AA-2G) by CGTase. When using maltodextrin as glycosyl donor, four mutants K47F (lysine→ phenylalanine), K47L (lysine→ leucine), K47V (lysine→ valine) and K47W (lysine→ tryptophan) showed higher AA-2G yield as compared with that produced by the wild-type CGTase. The transformation conditions (temperature, pH and the mass ratio of l-ascorbic acid to maltodextrin) were optimized and the highest titer of AA-2G produced by the mutant K47L could reach 1.97 g/l, which was 64.2 % higher than that (1.20 g/l) produced by the wild-type CGTase. The reaction kinetics analysis confirmed the enhanced maltodextrin specificity, and it was also found that compared with the wild-type CGTase, the four mutants had relatively lower cyclization activities and higher disproportionation activities, which was favorable for AA-2G synthesis. The mechanism responsible for the enhanced substrate specificity was further explored by structure modeling and it was indicated that the enhancement of maltodextrin specificity may be due to the short residue chain and the removal of hydrogen bonding interactions between the side chain of residue 47 and the sugar at −3 subsite. Here the obtained mutant CGTases, especially the K47L, has a great potential in the production of AA-2G with maltodextrin as a cheap and easily soluble substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin C (VC), existing in vivo in its reduced form l-ascorbic acid (l-AA), plays vital roles in many biological processes. For instance, it can improve collagen formation, carnitine synthesis, iron absorption and prevent many chronic diseases (Englard and Seifter 1986; Jacob and Sotoudeh 2002; Naidu 2003). However, due to its extreme instability in aqueous solution especially in the presence of heat, light, Cu2+ and ascorbate oxidase, the application of VC has been greatly limited (Yamamoto et al. 1990).
To improve the stability of VC, many VC derivatives such as ascorbyl phosphate (Mima et al. 1970), ascorbyl palmitate (Hsieh et al. 2006) and ascorbyl glucoside (Mima et al. 1970) have been chemically or biologically synthesized. Among all the VC derivatives, 2-O-glucopyranosyl- l-ascorbic acid (AA-2G) is considered as superior to other VC derivatives (Jun et al. 2001). With its high non-reducibility, excellent antioxidant ability and effortless release of l-AA and glucose (Kouki et al. 1992; Mandai et al. 1992; Han et al. 2012), AA-2G has been widely used in cosmetics (Kumano et al. 1998), medicine (Miyai et al. 1996), husbandry and aquaculture fields (Muto et al. 1991; Yamamoto et al. 1993).
The enzymatic synthesis of AA-2G has been investigated using such enzymes as α-glucosidase (Muto et al. 1990), cyclodextrin glycosyltransferase (CGTase) (Aga et al. 1991; Zhang et al. 2011a,b), amylase (Lee et al. 2004), sucrose phosphorylase (Kwon et al. 2007) and α-isomaltosyl glucosaccharide-forming enzyme (Mukai et al. 2005). Among these enzymes, CGTase is considered to be most effective for AA-2G production (Jun et al. 2001; Tanaka et al. 1991). CGTase can catalyze three transglycosylation reactions: cyclization (cleavage of an α-glycosidic bond in amylase or starch and subsequent formation of a cyclodextrin), coupling (cleavage of an α-glycosidic bond of in a cyclodextrin ring and transfer of the resulting linear maltooligosaccharide to an acceptor substrate) and disproportionation (cleavage of an α-glycosidic bond of a linear maltooligosaccharide and transfer of one part to an acceptor substrate), and has a weak hydrolyzing activity (van der Veen et al. 2000a). CGTase can transfer one or more than one glycosyl residues from a glycosyl donor ring to the C-2 position of VC with α-1, 2-linkage to form AA-2G(n) (where n denotes the number of glycosyls attached to l-AA), which can be hydrolyzed to AA-2G by glucoamylase.
Many saccharides except glucose can be used as glycosyl donors (Zhang et al. 2011b). It has been demonstrated that α- and β-cyclodextrin are the best glycosyl donors for AA-2G production, while maltodextrin was a weaker one due to the lower binding capacity with CGTase (Jun et al. 2001; Tanaka et al. 1991; Zhang et al. 2011b). However, due to the high cost of α-cyclodextrin and low solubility of β-cyclodextrin in aqueous solution, both of them are unsuitable for production of AA-2G (Han et al. 2012). On the other hand, maltodextrin is cheap and easily dissolved in aqueous solution, and has a great potential in the production of AA-2G. To achieve this objective, the binding specificity of CGTase towards maltodextrin should be improved via protein engineering strategies.
Many site-directed mutations of CGTase have been conducted with a purpose of changing the cyclodextrin product specificity (Fujiwara et al. 1992; Li et al. 2009b; Penninga et al. 1995; Sin et al. 1994; van der Veen et al. 2000c; Costa et al. 2012). It was found that the residue 47 of CGTase played an important role in stabilizing the transition state that characterized the cyclization and coupling reactions specifically (van der Veen et al. 2000b), and affecting the activity of disproportionation and hydrolysis (Leemhuis et al. 2003). Therefore, in this work the Lys47 in the CGTase was selected as an engineering site to improve maltodextrin specificity.
First, the site-saturation engineering of Lys47 in the CGTase from Paenibacillus macerans was conducted, and four mutants K47F (lysine→ phenylalanine), K47L (lysine→ leucine), K47V (lysine→ valine) and K47W (lysine→ tryptophan) were found to produce higher AA-2G yield with maltodextrin as the glycosyl donor. The transformation conditions (temperature, pH and substrate ratio) were optimized to further improve the yield of AA-2G by the positive mutant CGTases. In addition, the reaction kinetics of the CGTases was investigated to confirm the enhanced maltodextrin specificity, and the mutation influence on the cyclization, disproportionation and hydrolysis activities of the CGTase was explored to clarify which reaction was mainly involved in AA-2G synthesis. Finally, the mechanism responsible for the increased maltodextrin specificity was unraveled at molecular level by modeling the complex structure of CGTase–maltononaose. To our best knowledge, this is the first report about the site-saturation engineering of CGTase for the enhancement of maltodextrin specificity. The obtained positive mutant CGTases have great potential in the production of AA-2G with maltodextrin as a cheap and easily soluble glycosyl donor.
Materials and methods
Bacterial strains, plasmids and materials
P. macerans strain JFB05-01 (CCTCC M203062) was used as the source of genomic DNA. Escherichia coli JM109 and E. coli BL 21 (DE3) purchased from TaKaRa (Dalian, China) were used as the host for plasmid construction and as the host for the expression of α-CGTase, respectively. The pMD19-T vector used for cgt gene cloning and the pET-20(+) vector used for expression of α-CGTase in E. coli BL 21 (DE3) were purchased from TaKaRa.
PrimeSTAR HS DNA polymerase, restriction endonucleases, PCR reagents, Genomic Extraction Kit and MutanBEST kit used for site-directed mutagenesis were purchased from TaKaRa. DNA sequencing was performed by Sangon (Shanghai, China). AA-2G was purchased from Wako Pure Chemical (Wako, Japan), and l-AA was purchased from Jiangshan Pharmaceutical (Jiangsu, China). Maltodextrin was purchased from Sangon. All other chemicals and reagents were of analytical grade.
Construction of the recombinant plasmid cgt/pET-20b(+)
The genomic DNA of P. macerans strain JFB05-01was extracted by the Genomic Extraction Kit and used as the DNA model for PCR. The primers were designed by Primer Premier 5 from the published cgt gene (GenBank accession no. AF047363.1). The restriction sites of BamHI and XhoI (underlined letters) were introduced into the forward primer (5′-CGCGGATCCGTCACCCGATACGAGCGTGGACA-3′) and the reverse primer (5′-CCGCTCGAGATTTTGCCAGTCCACCGTCACC-3′), respectively. The gel-purified PCR product was digested with BamHI and XhoI and then ligated into the similarly restriction-digested expression vector pET-20b(+) to construct recombinant plasmid cgt/pET-20b(+). The plasmid containing the correct insert was confirmed by DNA sequencing and used for site-directed mutation.
Site-directed mutagenesis
Site-directed mutagenesis was performed using the MutanBEST kit. One-step PCR method was carried out by PrimeSTAR HS DNA polymerase using the plasmid cgt/pET-20b(+) as the template DNA and oligonucleotide primers. The sequences of the mutagenic primers were shown in Supplementary materials (Table S1). The PCR products were treated with blunting kination enzyme and Ligation Solution I (TaKaRa), and ligated into circular plasmids, and then were transformed into E. coli JM109. These plasmids were confirmed by DNA sequencing and the correct ones were transformed into E. coli BL21 (DE3) for expression.
Preparation and purification of the mutant CGTases
The mutant CGTases were prepared according to what previously reported (Li et al. 2010). The recombinant E. coli BL 21 (DE3) were inoculated into 20 ml Luria–Bertani (LB) medium containing 100 μg/ml ampicillin and grown at 37 °C overnight. And then the seed culture was inoculated into the flask with a ratio of 4 % (v/v) for fermentation. The fermentation medium contained (g/l): glucose 8, lactose 0.5, peptone 12, yeast extract 24, K2HPO4 16.43, KH2PO4 2.31, CaCl2 0.28, glycerol 4, ampicillin 0.1, pH 7.0. The flask culture was incubated on a rotary shaker (200 rpm) at 25 °C for 90 h. The expression of CGTases was induced with 0.01 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) when the optical density at 600 nm (OD600) reached 0.6. The broth was centrifuged at 10,000 × g and 4 °C for 5 min, and then the supernatant was purified and used for the subsequent transformation. The purification of the crude enzyme solution was carried out by a Ni-NTA agarose column (Qiagen, Chatsworth, CA) as described by Li et al. (2009a), and a relatively high purity of CGTase (more than 95 %) can be obtained by this way.
Biosynthesis and analysis of AA-2G
The purified wild-type and mutant CGTases were diluted with acetic acid–sodium acetate buffer (pH 5.5) to a protein concentration of 1 mg/ml, and then were mixed with the substrates (l-AA and maltodextrin), the final concentration of which was 5 % (w/v). The mixture was incubated at 37 °C for 24 h under the dark and oxygen-free conditions. Due to the formation of the intermediate AA-2Gn, the glucoamylase (10 U/ml) was added to the reaction mixture and incubated at 65 °C and pH 5.5 for 24 h to hydrolyze the AA-2Gn to AA-2G and glucose. AA-2G was analyzed using the method described previously (Zhang et al. 2011b).
On the basis of the initial transformation conditions (temperature 37 °C, pH 5.5 and mass ratio of l-AA to maltose, 1:1), the influence of reaction temperature (20–52 °C), pH (4.5–7.0) and mass ratio of l-AA to maltose (1:5 to 5:1) on the biosynthesis of AA-2G by the wild-type and mutant CGTases were also investigated.
The influence of incubation time on the biosynthesis of AA-2G by the wild-type and mutant CGTases were investigated at the optimal pH, temperature and substrates ratio conditions. After a regular interval of 4 h incubation, the glucoamylase (10 U/ml) was finally added to the reaction mixture and incubated at 65 °C and pH 5.5 for 24 h to hydrolyze the reaction intermediate AA-2Gn to AA-2G.
The kinetic analysis of the wild-type and mutant CGTases for AA-2G biosynthesis (using l-AA and maltodextrin as substrates) was performed by measuring the amount of AA-2G with different concentrations of one substrate while fixing the concentration of the other substrate, and the obtained results were subjected to kinetic analysis using SigmaPlot (Jandel Scientific, America).
Analysis of CGTase
The α-cyclodextrin forming activity was determined using the methyl orange method described by Li et al. (2010). Thar is, 0.1 ml of the purified wild-type or mutant CGTase diluted with 50 mM phosphate buffer to 1 mg/ml was added into 0.9 ml of 3 % (w/v) soluble starch in 50 mM phosphate buffer (pH 6.0). After incubating at 40 °C for 10 min, the reaction was terminated by the addition of 1.0 M HCl (1.0 ml). Finally, 1.0 ml of 0.1 mM methyl orange in 50 mM phosphate buffer (pH 6.0) was added, and the absorbance at 505 nm was measured after incubation at 16 °C for 20 min. One unit of α-cyclodextrin-forming activity was defined as the amount of enzyme that was able to produce 1 μmol α-cyclodextrin/min.
The hydrolyzing activity was analyzed by the starch-degrading method (van der Veen et al. 2000b). The purified CGTase (1 mg/ml) was incubated with 1 % soluble starch solution at 50 °C for 10 min. One unit of hydrolyzing activity was defined as the amount of enzyme producing 1 μmol of reducing sugar (determined by 3, 5-dinitrosalicylic acid method) per minute.
The disproportionation activity was determined as described previously (van der Veen et al. 2000b), using 1 mM 4-nitrophenyl-α-d-maltoheptaoside-4-6-O-ethylidene (EPS: Megazyme, County Wicklow, Ireland) and 10 mM maltose as donor and acceptor substrates, respectively. One unit of activity was defined as the amount of enzyme converting 1 μmol EPS/min.
Protein concentrations were determined with the Bradford method using Bradford Protein Assay Kit and bovine serum albumin as a standard (Beyotime, Jiangsu, China).
Structure modeling of the (mutant) CGTases
The homology modeling of wild-type and mutant CGTases form P. macerans was performed using the crystal structure of B. circulans strain 251 CGTase (PDB 1D3C, 1.81 Å resolution; Uitdehaag et al. 1999b) as the template by the SWISS-MODEL protein-modeling sever (http://swissmodel.expasy.org/) (Arnold et al. 2006). The similarity of P. macerans CGTase with the template was 68.37 %. Structural alignment was done according to the combinatorial extension method by using the server http://cl.sdsc.edu/ (Shindyalov and Bourne 1998). The root mean square deviation between the template and model alpha carbon backbones (RMSD, 0.1 Å) was calculated by the combinatorial extension method (Shindyalov and Bourne 1998). All graphical molecular representations were generated using Accelrys Discovery Studio Client 2.5. The proposed complex structures of the wild-type and mutant CGTases with maltohexaose were modeled by means of superpositioning of the above theoretical structure and the complex structure of B. circulans strain 251 CGTase E257Q/D229N with a maltononaose inhibitor in the active site (PDB accession codes 1CXK; Uitdehaag et al. 1999a) and B. circulans strain 251 CGTase with a γ-cyclodextrin inhibitor in the active site (PDB accession codes 1D3C, Uitdehaag et al. 1999b) followed by least-squares fitting of the Cα atoms (Li et al. 2009a). Finally, an energy minimization of the enzyme–substrate interaction was carried out with the Amber-based energy minimization method. The correctness of the model was also assessed by ProQ (Wallner and Elofsson 2003).
Results
The construction of plasmid cgt/pET-20b(+) and site-directed mutagenesis
The cgt gene amplified from the genomic DNA of P. macerans strain JFB05-01 by PCR was ligated into the vector pET-20b(+) with the restriction sites of BamHI and XhoI, and the obtained recombinant plasmid cgt/pET-20b(+) contained the pelB signal peptide upstream and six histidine codons downstream. The constructed recombinant plasmid was further confirmed by DNA sequencing, and the result showed that the 2,061 bp open reading frame of cgt gene (without the stop codon) corresponded to the published cgt gene (GenBank accession no. AF047363. 1). In addition, the 687 amino acid residues encoded by the cgt gene was the same with the published CGTase amino acid sequence (NCBI accession number: P04830), indicating that the plasmid cgt/pET-20b(+) was successfully constructed.
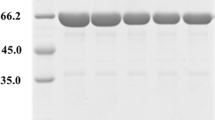
All mutants were successfully constructed by site-directed mutagenesis and verified by DNA sequencing. When the wild-type and mutant CGTases were expressed in the host E. coli BL21 (DE3), it was found that there was no difference in the expression levels of the enzymes (data not shown). After SDS-polyacrylamide gel electrophoresis (SDS-PAGE), it was found that there was no difference in the molecular mass (about 75 kDa) between the wild-type and mutant CGTases, and this result was similar to that previously reported (Li et al. 2010).
Synthesis of AA-2G by the wild-type and mutant CGTases
The wild-type and mutant CGTases were used for AA-2G biosynthesis. The yield of AA-2G produced by the wild-type CGTase with maltodextrin as the glycosyl donor was 1.07 g/l. As shown in Table 1, compared to the wild-type CGTase, four mutations of lysine 47 into valine (K47V), phenylalanine (K47F), leucine (K47L) and tryptophan (K47W) exhibited higher AA-2G yield with maltodextrin as the glycosyl donor among all of the mutants. The AA-2G yield was increased by 47.7 %, 29.9 %, 57.6 % and 24.2 % by the mutant K47V, K47F, K47L and K47W, respectively. All the other mutants produced lower AA-2G production than the wild-type CGTase with maltodextrin as the glycosyl donor (data not shown). Interestingly, with β-cyclodextrin as the glycosyl donor, the AA-2G yield by the mutants K47V, K47F, K47L and K47W decreased by 7 %, 5 %, 6 % and 5 %, respectively, as compared with the wild-type CGTase (Table 1). To further increase the titer of AA-2G, three factors including reaction temperature, pH and substrate ratio were further optimized.
Influence of reaction temperature, pH and substrate ratio on the enzymatic synthesis of AA-2G
The optimal temperature of the wild-type and mutant CGTases for the enzymatic synthesis of AA-2G were 36 °C, which was also the optimal temperature for α-CGTase-catalyzed AA-2G production with β-cyclodextrin as the glycosyl donor (Zhang et al. 2011b), whereas different from that of the recombinant α-CGTase for the cyclization activity (45 °C) (Li et al. 2010). The AA-2G titer decreased significantly when the temperature was lower than 20 °C, and above 60 % of the highest titer was kept within the range from 28 °C to 44 °C.
Both the wild-type and mutant CGTases showed the highest AA-2G yield at pH 6.5 with maltodextrin as the glycosyl donor. Nevertheless, the optimal pH of this recombinant α-CGTase for cyclization (activity level) and AA-2G biosynthesis (product level) with β-cyclodextrin as the glycosyl donor was 5.5 (Li et al. 2010; Zhang et al. 2011b). The production of AA-2G decreased rapidly below pH 5.0 or above pH 6.5, suggesting that the enzymes were instable at these pH values.
The influence of substrate ratio (l-AA/maltodextrin) on AA-2G biosynthesis was also examined. As shown in Fig. 1, for the wild-type and the four mutant CGTases (K47V, K47F, K47L and K47W), the maximal AA-2G yield was attained at a 1:1 mass ratio of l-AA to maltodextrin.
Figure 2 shows the time profiles of AA-2 G synthesis by the wild-type and mutant CGTases under the obtained optimal conditions. At the initial stage of the reaction, the amount of AA-2G increased dramatically. The mutant K47V produced the highest titer of AA-2 G of 1.80 g/l at 8 h. The yield of AA-2G by K47F and K47W reached the highest rate of 1.60 and 1.49 g/l at 12 h, respectively. The yield of AA-2G produced by the wild-type and K47L reached the highest of 1.20 and 1.97 g/l at 20 and 24 h, respectively. The yield of AA-2 G produced by K47L was 1.64-fold of that produced by the wild-type CGTase.
Influence of mutation on the reaction kinetics of CGTase for AA2G synthesis
The parallel lines in the Lineweaver–Burke plot (Fig. 3) for the wild-type CGTase indicated a normal ping-pong type of kinetics. As shown in Fig. 3, the linear regression of the experimental data of the wild-type CGTase corresponded well to the calculated values by Eq. 1. In addition, the experimental data of mutant CGTases were also best fitted by Eq. 1 (data not shown), and the detailed kinetic parameters were listed in Table 2. It could be seen that the maximal reaction rate (V max) of the mutant CGTases (K47L, K47V, K47F and K47W) was higher than that of the wild-type CGTase. Meanwhile, compared to the wild-type CGTase, the K m of the mutants K47L, K47V, K47F and K47W with maltodextrin as substrate decreased by 23 %, 25 %, 19 % and 14 %, respectively, while the K cat/K m increased by 96 %, 78 %, 54 % and 41 %, respectively. The kinetic results indicated that the affinity and catalytic efficiency of these mutants towards maltodextrin increased compared to the wild-type CGTase. This might be due to the fact that there was larger room and less obstruction for binding the linear saccharides when the long side chain lysine was replaced by short chain amino acids especially like leucine and valine. Furthermore, it was previously reported that the increase of hydrolysis activity of CGTase can be achieved by decreasing the cyclization or disproportionation activities (Leemhuis et al. 2003). Therefore, the decreased cyclization or hydrolysis activities in this work enabled the increase of disproportionation activity (Table 1). Although the K m values of the mutants towards l-AA showed somewhat increase compared to that of the wild-type CGTase, the K cat/K m values had little changes, suggesting that the catalytic efficiency was not negatively influenced though the affinity of mutants towards l-AA decreased.
Lineweaver–Burk plots of the AA-2G biosynthesis by the wild-type CGTase. (The reciprocal of the AA-2G yield (1/AA-2G) is plotted against the reciprocal of the l-AA concentration (1/l-AA) at fixed maltodextrin concentrations [in mM]; filled square 0.23, filled circle 0.46, filled upright triangle 1.60, empty square 23.25). Linear regression of the experimental data is represented by dotted lines, and the calculated values from Eq. 1 is represented by solid lines
where v is the reaction rate (the amount of AA-2G formed by 1 mg enzyme/h, mM mg−1 h−1), V max is the maximal reaction rate (mM mg−1h−1), a and b are the acceptor (l-AA) and donor (maltodextrin) concentrations (mM), respectively, and K mA and K mB are the affinity constants for the substrates l-AA and maltodextrin, respectively.
Previous reports (van der Veen et al. 2000a, 2001) indicated that the disproportionation reaction of CGTase belonged to a ping-pong mechanism. In addition, the inhibition relationship may exist between the acceptor and donor substrate because the binding sites of the linear donor and acceptor substrates overlapped at least partially (van der Veen et al. 2000a). In the present work, the parallel lines in the Lineweaver–Burke plot (Fig. 3) for the wild-type and mutant CGTases indicated that there was no inhibition relationship between the acceptor (l-AA) and donor substrate (maltodextrin). This indicated that the CGTase active site was perfectly suited for the successive binding of donor and acceptor. The fact that mutation of the residue 47 of CGTase did not affect the ping-pong mechanism suggested that the residue 47 (at −3 subsite) did not affect the acceptor binding site, which was near the +3 subsite.
The influence of mutations on the cyclization, hydrolysis and disproportionation activities of the CGTase was investigated. As shown in Table 1, the wild-type CGTase had a relatively higher cyclization (α-cyclodextrin-forming) activity than the four mutants, and the cyclization activity of K47V, K47F, K47L and K47W decreased by 80 %, 45 %, 45 % and 82.5 %, respectively. Compared with the wild-type CGTase, the mutant K47V and K47L showed a decreased hydrolysis (starch-degrading) activity by 56.2 % and 52.7 %, respectively, while the mutant K47F and K47W had an increased hydrolysis activity by 6.5 % and 18.9 %, respectively. All four mutants (K47V, K47F, K47L and K47W) showed an increased disproportionation activity by 47 %, 30.7 %, 57.6 % and 29.2 %, respectively. The increase in the disproportionation activity of the four mutants was in accordance with the increase of AA-2G yield with maltodextrin as the glycosyl donor, indicating that the disproportionation of CGTase was the main reaction for AA-2G biosynthesis with linear oligosaccharides as glycosyl donors.
Discussion
In this work, the maltodextrin specificity for AA-2G biosynthesis was enhanced when the lysine at the 47 position was respectively replaced by phenylalanine, leucine, valine and tryptophan. Interestingly, three of them (phenylalanine, leucine and valine) were hydrophobic amino acids, and their residue chains were much shorter than that of lysine. Although tryptophan was a hydrophilic amino acid, its hydrophilicity was much lower than lysine and its residue side chain was also shorter than that of lysine. Therefore, there was not hydrogen-bonding between the amino acid residue at the 47 position and the sugar at −3 subsite (Fig. 4). Meanwhile, a dramatic decrease in α-cyclodextrin-forming activity was observed for these mutants compared to the wild-type CGTase (here α-cyclodextrin-forming activity was used to represent the cyclization activity because the CGTase from P. macerans belonged to α-CGTase). The possible explanation was that these hydrophobic amino acids removed all the possible hydrogen-bonding interactions between the 47 residue and the sugar and affected the stabilization of the bent conformation.
Close-up the wild-type and mutant CGTases theoretical structure with superpositioning of γ-cyclodextrin structure (PDB accession codes 1D3C). (Atom display style: ball and stick amino acid, line γ-cyclodextrin inhibitor, dotted lines hydrogen bond between the amino residue at the 47 position and the sugar at −3 subsite; a wild-type CGTase, b mutant K47L, c mutant K47F, d mutant K47W, e mutant K47V)
It was reported that Arg47 of B. circulans CGTase could interact with cyclodextrins but not linear oligosaccharides, and the residue 47 might affect CGTase reactions with cyclic or linear substrates (Uitdehaag et al. 1999b; van der Veen et al. 2000b). Therefore, as shown in Fig. 5a, a hydrogen bond existed between the Lys 47 residue and the cyclodextrin but not linear oligosaccharide. Thus we could suppose that the preference of the Lys 47 in the CGTase from P. macerans was also the cyclic substrates such as cyclodextrins but not linear oligosaccharides such as maltodextrin due to the hydrogen-bonding interactions. This might explain why the most CGTases had higher transformation efficiency for AA-2G production with cyclodextrins than maltodextrin as glycosyl donors. When the lysine was replaced by the hydrophobic amino such as leucine (Fig. 5b), the affinity between the amino acid at position 47 and the cyclodextrin became weak due to the removal of the possible hydrogen-bonding interactions. This may be the reason why the AA-2G yields of the mutants with β-cyclodextrin as the glycosyl donor were lower than the wild-type CGTase (Table 1).
Close-up the wild-type (a) and K47L (b) theoretical structure with superpositioning of maltononaose structure (PDB accession codes 1CXK) and γ-cyclodextrin structure (PDB accession codes 1D3C). Dotted lines, hydrogen bond between the amino residue at the 47 position and the sugar at −3 subsite; amino acid: ball and stick, maltononaose: blue line, γ-cyclodextrin: yellow and red line
Table 3 shows the different AA-2G yields produced by CGTases from different source and under different conditions. Here we made a simple economic evaluation for AA-2G synthesis with α-cyclodextrin, β-cyclodextrin and maltodextrin as glycosyl donors. The detailed calculation method was listed in supplementary materials (Table S2). It could be seen that to produce 1 kg AA-2G, the production cost was US$439 for α-cyclodextrin, US$451.5 for β-cyclodextrin, and US$224 for maltodextrin. The increase in AA-2G yield from 1.2 g/l to 1.9 g/l made the production cost decreased from US$224/kg AA-2G to US$189/kg AA-2G. Though the current AA-2G yield with maltodextrin as the glycosyl donor was much lower than that with α-cyclodextrin or β-cyclodextrin, the production cost can be significantly reduced when using the inexpensive and easily soluble maltodextrin. In addition, there is much room for the increase of AA-2G by engineering the other binding sites of CGTase and the systematic optimization of transformation conditions. From this point of view, maltodextrin is a preferable substrate for AA-2G synthesis than α-cyclodextrin or β-cyclodextrin.
In summary, in this work four CGTase mutants with enhanced maltodextrin specificity were obtained by site-saturation engineering strategies and the AA-2G yield was significantly improved when using these mutant CGTase as biocatalysts. The enhanced substrate specificity towards maltodextrin was confirmed by reactions kinetics and the inherent mechanism was revealed by structure modeling. It should be noted that much work including the systems engineering of the other substrate binding sites of CGTase, enzyme immobilization and transformation optimization should be conducted to further improve the AA-2G yield with maltodextrin as substrate.
References
Aga H, Yoneyama M, Sakai S, Yamamoto I (1991) Synthesis of 2-O-α-d-glucopyranosyl l-ascorbic acid by cyclomaltodextrin glucanotransferase from Bacillus stearothermophilus. Agric Biol Chem 55:1751–1756
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Costa H, Distefano AJ, Marino-Buslje C, Hidalgo A, Berenguer J, de Jimenez B, Bonino M, Ferrarotti SA (2012) The residue 179 is involved in product specificity of the Bacillus circulans DF 9R cyclodextrin glycosyltransferase. Appl Microbiol Biotechnol 94:123–130
Englard S, Seifter S (1986) The biochemical functions of ascorbic acid. Annu Rev Nutr 6:365–406
Fujiwara S, Kakihara H, Sakaguchi K, Imanaka T (1992) Analysis of mutations in cyclodextrin glucanotransferase from Bacillus stearothermophilus which affect cyclization characteristics and thermostability. J Bacteriol 174:7478–7481
Han R, Liu L, Li J, Du G, Chen J (2012) Functions, applications and production of 2-O-d-glucopyranosyl- l-ascorbic acid. Appl Microbiol Biotechnol 95:313–320
Hsieh HJ, Nair GR, Wu WT (2006) Production of ascorbyl palmitate by surfactant-coated lipase in organic media. J Agric Food Chem 54:5777–5781
Jacob RA, Sotoudeh G (2002) Vitamin C function and status in chronic disease. Nutr Clin Care 5:66–74
Jun HK, Bae KM, Kim SK (2001) Production of 2-O-α-d-glucopyranosyl l-ascorbic acid using cyclodextrin glucanotransferase from Paenibacillus sp. Biotechnol Lett 23:1793–1797
Kouki M, Norio M, Kyoko F, Itaru Y (1992) Comparison of ascorbic acid and ascorbic acid 2-O-α-glucoside on the cytotoxicity and bioavailability to low density cultures of fibroblasts. Biochem Pharm 44:2191–2197
Kumano Y, Sakamoto T, Egawa M, Iwai I, Tanaka M, Yamamoto I (1998) In vitro and in vivo prolonged biological activities of novel vitamin C derivative, 2-O-α-d-glucopyranosyl- l-ascorbic acid (AA-2G), in cosmetic fields. J Nutr Sci Vitaminol (Tokyo) 44:345–359
Kwon T, Kim CT, Lee JH (2007) Transglucosylation of ascorbic acid to ascorbic acid 2-glucoside by a recombinant sucrose phosphorylase from Bifidobacterium longum. Biotechnol Lett 29:611–615
Lee SB, Nam KC, Lee SJ, Lee JH, Inouye K, Park KH (2004) Antioxidative effects of glycosyl-ascorbic acids synthesized by maltogenic amylase to reduce lipid oxidation and volatiles production in cooked chicken meat. Biosci Biotechnol Biochem 68:36–43
Leemhuis H, Rozeboom HJ, Wilbrink M, Euverink GJ, Dijkstra BW, Dijkhuizen L (2003) Conversion of cyclodextrin glycosyltransferase into a starch hydrolase by directed evolution: the role of alanine 230 in acceptor subsite +1. Biochemistry 42:7518–7526
Li ZF, Zhang JY, Sun Q, Wang M, Gu ZB, Du GC, Wu J, Chen J (2009a) Mutations of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans enhance β-cyclodextrin specificity. J Agric Food Chem 57:8386–8391
Li ZF, Zhang JY, Wang M, Gu ZB, Du GC, Li JK, Wu J, Chen J (2009b) Mutations at subsite-3 in cyclodextrin glycosyltransferase from Paenibacillus macerans enhancing α-cyclodextrin specificity. Appl Microbiol Biotechnol 83:483–490
Li Z, Li B, Gu Z, Du G, Wu J, Chen J (2010) Extracellular expression and biochemical characterization of α-cyclodextrin glycosyltransferase from Paenibacillus macerans. Carbohydr Res 345:886–892
Mandai T, Yoneyama M, Sakai S, Muto N, Yamamoto I (1992) The crystal structure and physicochemical properties of l-ascorbic acid 2-glucoside. Carbohydr Res 232:197–205
Mima H, Nomura H, Imai Y, Takashima H (1970) Chemistry and application of ascorbic acid phosphate. Vitamin 41:387
Miyai E, Yanagida M, Akiyama J, Yamamoto I (1996) Ascorbic acid 2-O-α-glucoside, a stable form of ascorbic acid, rescues human keratinocyte cell line, SCC, from cytotoxicity of ultraviolet light B. Biol Pharm Bull 19:984–987
Mukai K, Tsusaki K, Kubota M, Fukuda S, Miyake T (2005) Process for producing 2-O-α-d-glucopyranosyl-l-ascorbic acid. EP patent application no. 1553186
Muto N, Suga S, Fujii K, Goto K, Yamamoto I (1990) Formation of a stable ascorbic acid 2-glucoside by specific transglucosylation with rice seed α-glucosidase. Agric Biol Chem 54:1697–1703
Muto N, Ban Y, Akiba M, Yamamoto I (1991) Evidence for the in vivo formation of ascorbic acid 2-O-α-glucoside in guinea pigs and rats. Biochem Pharmacol 42:625–631
Naidu KA (2003) Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2:7
Penninga D, Strokopytov B, Rozeboom HJ, Lawson CL, Dijkstra BW, Bergsma J, Dijkhuizen L (1995) Site-directed mutations in tyrosine 195 of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 affect activity and product specificity. Biochemistry 34:3368–3376
Prousoontorn MH, Pantatan S (2007) Production of 2-O-α-glucopyranosyl l-ascorbic acid from ascorbic acid and β-cyclodextrin using immobilized cyclodextrin glycosyltransferase. J Incl Phenom Macro 57:39–46
Shindyalov IN, Bourne PE (1998) Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng 11(9):739–747
Sin KA, Nakamura A, Masaki H, Matsuura Y, Uozumi T (1994) Replacement of an amino acid residue of cyclodextrin glucanotransferase of Bacillus ohbensis doubles the production of γ-cyclodextrin. J Biotechnol 32:283–288
Tanaka M, Muto N, Yamamoto I (1991) Characterization of Bacillus stearothermophilus cyclodextrin glucanotransferase in ascorbic acid 2-O-α-glucoside formation. Biochim Biophys Acta 1078:127–132
Uitdehaag JC, Mosi R, Kalk KH, van der Veen BA, Dijkhuizen L, Withers SG, Dijkstra BW (1999a) X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the α-amylase family. Nat Struct Biol 6:432–436
Uitdehaag JC, Kalk KH, van Der Veen BA, Dijkhuizen L, Dijkstra BW (1999b) The cyclization mechanism of cyclodextrin glycosyltransferase (CGTase) as revealed by a γ-cyclodextrin-CGTase complex at 1.8-A resolution. J Biol Chem 274:34868–34876
van der Veen BA, van Alebeek GJ, Uitdehaag JC, Dijkstra BW, Dijkhuizen L (2000a) The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem 267:658–665
van der Veen BA, Uitdehaag JC, Dijkstra BW, Dijkhuizen L (2000b) The role of arginine 47 in the cyclization and coupling reactions of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 implications for product inhibition and product specificity. Eur J Biochem 267:3432–3441
van der Veen BA, Uitdehaag JC, Penninga D, van Alebeek GJ, Smith LM, Dijkstra BW, Dijkhuizen L (2000c) Rational design of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 to increase α-cyclodextrin production. J Mol Biol 296:1027–1038
van der Veen BA, Leemhuis H, Kralj S, Uitdehaag JC, Dijkstra BW, Dijkhuizen L (2001) Hydrophobic amino acid residues in the acceptor binding site are main determinants for reaction mechanism and specificity of cyclodextrin-glycosyltransferase. J Biol Chem 276:44557–44562
Wallner B, Elofsson A (2003) Can correct protein models be identified? Protein Sci 12:1073–1086
Yamamoto I, Muto N, Murakami K, Suga S, Yamaguchi H (1990) l-Ascorbic acid α-glucoside formed by regioselective transglucosylation with rat intestinal and rice seed α-glucosidases: its improved stability and structure determination. Chem Pharm Bull (Tokyo) 38:3020–3023
Yamamoto I, Tanaka M, Muto N (1993) Enhancement of in vitro antibody production of murine splenocytes by ascorbic acid 2-O-α-glucoside. Int J Immunopharmacol 15:319–325
Zhang ZC, Li JH, Liu L, Sun J, Hua ZZ, Du GC, Chen J (2011a) Enzymatic transformation of 2-O-α-d-glucopyranosyl- l-ascorbic acid (AA-2G) by immobilized α-cyclodextrin glucanotransferase from recombinant Escherichia coli. J Mol Catal B-Enzym 68:223–229
Zhang ZC, Li JH, Liu L, Sun J, Hua ZZ, Du GC, Chen JA (2011b) Enzymatic transformation of 2-O-α-d-glucopyranosyl- l-ascorbic acid by α-cyclodextrin glucanotransferase from recombinant Escherichia coli. Biotechnol Bioproc E 16:107–111
Acknowledgments
This project was financially supported by the Key Technologies R & D Program of Jiangsu Province, China (SBE201170459), Priority Academic Program Development of Jiangsu Higher Education Institutions, 863 Program (2012AA022202), 111 Project (111-2-06), and 973 Project (2012CB720802 and 2012CB720806).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 113 kb)
Rights and permissions
About this article
Cite this article
Han, R., Liu, L., Shin, Hd. et al. Site-saturation engineering of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans to enhance substrate specificity towards maltodextrin for enzymatic synthesis of 2-O-d-glucopyranosyl-l-ascorbic acid (AA-2G). Appl Microbiol Biotechnol 97, 5851–5860 (2013). https://doi.org/10.1007/s00253-012-4514-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4514-1