Abstract
2-O-α-D-glucopyranosyl-l-ascorbic acid (AA-2G) is a stable derivative of l-ascorbic acid (l-AA), which has been widely used in food and cosmetics industries. Sugar molecules, such as glucose and maltose produced by cyclodextrin glycosyltransferase (CGTase) during AA-2G synthesis may compete with l-AA as the acceptors, resulting in low AA-2G yield. Multiple sequence alignment combined with structural simulation analysis indicated that residues at positions 191 and 255 of CGTase may be responsible for the difference in substrate specificity. To investigate the effect of these two residues on the acceptor preference and the AA-2G yield, five single mutants Bs F191Y, Bs F255Y, Bc Y195F, Pm Y195F and Pm Y260F of three CGTases from Bacillus stearothermophilus NO2 (Bs), Bacillus circulans 251 (Bc) and Paenibacillus macerans (Pm) were designed for AA-2G synthesis. Under optimal conditions, the AA-2G yields of the mutants Bs F191Y and Bs F255Y AA-2G were 34.3% and 7.9% lower than that of Bs CGTase, respectively. The AA-2G yields of mutant Bc Y195F, Pm Y195F and Pm Y260F were 45.8%, 36.9% and 12.6% higher than those of wild-type CGTases, respectively. Kinetic studies revealed that the residues at positions 191 and 255 of the three CGTases were F, which decreased glucose and maltose specificity and increased l-AA specificity. This study not only proposes for the first time that the AA-2G yield can be improved by weakening the acceptor specificity of CGTase toward sugar byproducts, but also provides new insight on the modification of CGTase that catalyze the double-substrate transglycosylation reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
2-O-α-D-glucopyranosyl-l-ascorbic acid (AA-2G), as a glucosylated derivative of l-ascorbic acid (l-AA), is an excellent substitute for l-AA, and cannot only maintain stable properties but also produce l-AA by α-glucosidase, which can exert the normal physiological functions of l-AA [1]. Therefore, it has wide applications in the fields of food and cosmetics industries[2]. Currently, there are five glycosyltransferases, including α-glucosidase, cyclodextrin glycosyltransferase (CGTase) [3], amylase [4], sucrose phosphorylase [5] and α-isomaltosyl glucosaccharide-forming enzyme [6] that can catalyze AA-2G synthesis. CGTase is considered the most suitable enzyme for AA-2G synthesis due to its excellent substrate specificity and high AA-2G yield [7].
CGTase can catalyze the synthesis of AA-2G with α-cyclodextrin, β-cyclodextrin and starch derivatives (maltodextrin, maltose) as glucosyl donors, among which maltodextrin has the advantages of low cost and high solubility in aqueous solution, so it has the potential to industrially prepare AA-2G [7]. The disproportionation reaction is the key CGTase activity for AA-2G synthesis utilizing maltodextrin as the glucosyl donor [8]. After maltodextrin binds to the substrate binding site of CGTase, CGTase first cleaves maltodextrin between + 1 and – 1 subsite to release glucose or maltose at the acceptor-binding site, forming a covalent enzyme bound oligosaccharide intermediate [9], followed by l-AA binds to enzyme bound oligosaccharide at the acceptor-binding site to form oligosaccharides l-AA. Due to the weak hydrolytic activity of CGTase, maltodextrin can also be hydrolyzed into carbohydrate molecules such as glucose and maltose. CGTase can utilize glucose and maltose as acceptor substrates in disproportionation reactions [10]. The glucose conversion rate observed using Bacillus circulans DF 9R CGTase glucose as an acceptor was 60%[11], and the l-AA conversion rate observed using Pm CGTase was only 9% [12]. Therefore, glucose and maltose might compete with l-AA as acceptor substrates. In a previous study, AA-2G was prepared by displaying CGTase on the surface of Saccharomyces cerevisiae [13]. Saccharomyces cerevisiae consumed the byproduct glucose during AA-2G production, so that the glucose concentration was always kept at a low level, and the final yield of AA-2G was 37% higher than that produced by free CGTase. The result provides effective evidence for the competitive inhibitory effect of glucose. Therefore, it is speculated that reducing the specificity of CGTase toward glucose and maltose can weaken the competitive effect with l-AA and thus improve the AA-2G yield.
Currently, the molecular modification of CGTase mainly focused on improving glucosyl donor specificity and acceptor l-AA specificity to increase the AA-2G conversion rate. Han Ruizhi and Liu Long et al. selected the residues in the + 1 [14], + 2 [15], − 3 [16] and − 6 [17] subsites of Pm CGTase for site-directed saturation mutation to improve maltodextrin specificity. Our previous study improved l-AA specificity by saturation mutation [18, 19]. However, there is no research on improving the AA-2G yield by reducing the competitive inhibitory effect of CGTase on the byproduct carbohydrate molecules as an acceptor substrate. The literature reports and analysis of the crystal structure of CGTase found that positions 179, 191, 255 and 279 (B. stearothermophilus NO2 amino acids) in the acceptor-binding sites of the active center are highly conserved in CGTase, but do not exist in α-amylase and play an important role in cyclization and disproportionation [20, 21]. The previous researches also reported that these four aromatic residues have an important influence on the affinity towards glucose or maltose as acceptors [20]. The other residues at acceptor sites of CGTase are L190, A226, K228, H229 and M230 (according to the Bs CGTase amino acid number). Han et al. selected L190, A226, K228 and H229 for site-directed saturation mutation to directly improve the binding affinity of CGTase towards maltodextrin [14, 15]. In our previous research, K228 and M230 were selected for directed evolution to enhance the binding affinity of CGTase towards l-AA [18]. The purpose of this study is to enhance the l-AA specificity by weakening the competition of sugar byproducts with l-AA; thereby, improving the yield of AA-2G. Based on the above analysis, we hypothesized that these four aromatic residues have a greater effect on disproportionation activity and the affinity towards sugar byproducts of CGTase; thus, affecting the synthesis of AA-2G. Therefore, these four aromatic residues were preferentially selected in this study. Sequence alignment analysis of six CGTases with different AA-2G conversion rates showed that positions 179 and 279 were phenylalanine (F), while positions 191 and 255 were phenylalanine (F) or tyrosine (Y). It is speculated that these two residues may lead to differences in the acceptor specificity of CGTase.
Three CGTases from P. macerans, B. circulans 251 and B. stearothermophilus NO2 were selected to investigate the effect of F/Y mutation at positions 191 and 255 (according to the Bs CGTase amino acid number) on the glucose, maltose and l-AA catalytic efficiency, respectively. We found that the glucose and maltose specificity were reduced when position 191 was Phe (F) in these CGTases, and the AA-2G yield increased. When position 255 of Bs CGTase and Pm CGTase is F, the glucose specificity was reduced, and the AA-2G yield increased. The results indicated that the acceptor specificity of CGTase for l-AA was improved by weakening the acceptor specificity of CGTase toward carbohydrate molecules such as glucose and maltose, thus increasing the AA-2G yield.
Materials and methods
Bacterial strains, plasmids and chemicals
Escherichia coli JM109 and E. coli BL21 (DE3) were utilized as the host strains for recombinant DNA manipulations and enzyme expression, respectively. Three recombinant plasmids, pET-20b (+)/Bs-cgt, pET-20b (+)/Pm-cgt and pET-20b (+)/Bc-cgt with genes encoding B. stearothermophilus NO2 CGTase, P. macerans JFB05-01 CGTase, and B. circulans 251 CGTase were constructed in our previous work [18, 22, 23]. AA-2G was supplied by Shanghai TCI (Shanghai, China). 4-Nitrophenyl-α-D-maltoheptaoside-4-6-O-ethylene (EPS) was purchased from Yixin Biotechnology Co. Ltd. (Shanghai, China). Maltodextrin (dextrose equivalent [DE] 5–7) was provided by TianBang Biological Technology Co. Ltd (ShanDong, China).
Site-directed mutagenesis
Site-directed mutagenesis of Bs CGTase, Pm CGTase and Bc CGTase was performed using one-step PCR. The plasmids pET-20b ( +)/Bs-cgt, pET-20b ( +)/Bc-cgt and pET-20b ( +)/Pm-cgt were used as templates and all primers with the target sites are shown in Table 1. After Dpn I digestion, the PCR product was transformed into E. coli JM109 for DNA cloning. The intended mutations of various mutants were confirmed by DNA sequencing. The confirmed mutant plasmids were transferred to E. coli BL21 (DE3) for protein production.
Expression and purification of CGTase and mutants
The expression of Bs CGTase, Pm CGTase, Bc CGTase and their mutants followed our previous study [24]. Twenty microliters of recombinant E. coli BL21 containing natural or a variant plasmid was incubated in LB medium supplemented with 100 μg/mL ampicillin at 37 °C for 8 h. The cultured seed solution was transferred to 50 mL of TB medium supplemented with 100 μg/mL ampicillin at a 5% (v/v) inoculum. The E. coli BL21(DE3) expressing Bs CGTase and Pm CGTase and their mutant were incubated at 25 °C for 60 h. Although the E. coli BL21(DE3) expressing Bc CGTase and its mutant were incubated at 37 °C for 2 h until the OD600 reached approximately 1.0, IPTG was added with a final concentration of 0.05 mM to induce CGTase expression, and incubation was continued at 25 °C for 60 h. The crude enzyme solution was obtained by centrifugation at 10,000×g and 4 °C for 15 min, and then the samples were purified by an anion exchange chromatography column using DEAE (where Bc CGTase and its mutants use a Mono Q anion exchange column) as described in the literature [24]. The determination of CGTase disproportionation activity was performed as described previously [18].
Kinetic analysis of CGTase and mutants using glucose and maltose as acceptors
EPS (5.2 g/L) was used as the glycosyl donor while glucose (1, 1.8, 3.6, 5.4, 7.2, 9, 18 and 36 g/L), maltose (0.34, 0.68, 1.0, 1.4, 1.7, 3.4, 6.8, 17.1 and 34.2 g/L) were used as the acceptors to determine the disproportionation activity of CGTase. The experimental data were fitted according to the Ping − Pong mechanism [9] using GraphPad Prism 6.
Kinetic analysis of CGTase for AA-2G synthesis
The kinetic parameters of AA-2G synthesis of wild type and mutant CGTases were measured at pH 5.0 and 30 °C. Bs CGTase used l-AA (1, 5, 10, 25, and 50 g/L) as the acceptor and maltodextrin (25 g/L) as the donor. Bc CGTase and Pm CGTase used l-AA (1, 5, 10, 25, 50, 100 g/L) as the acceptor and maltodextrin (25 g/L) as the donor was used as a substrate to measure the kinetic parameters of AA-2G synthesis of wild type and mutant CGTase at pH 5.0 and 30 °C. When the concentration of one of the substrates is constant, the effect of the concentration of the other substrate on AA-2G synthesis is examined. Finally, when the concentration of both substrates increases, the concentration of the product no longer increases. The kinetic parameters were obtained by fitting the experimental data using GraphPad Prism 6 according to the Ping − Pong mechanism.
Biosynthesis and analysis of AA-2G
Enzymatic production of AA-2G was performed from 50 g/L l-AA (pH 5.0) and 50 g/L maltodextrin (DE value 5–7) by natural and variant enzymes. Based on the initial reaction conditions (enzyme amount of 1000 U/g maltodextrin, pH 5.0), the effects of temperature (25–45 °C), pH (4.5–6.0) and enzyme concentration (500–3000 U/g maltodextrin) on AA-2G synthesis were investigated. The reaction was conducted under dark and oxygen-free conditions for 24 h. After that glucoamylase was added to convert AA-2Gn (n indicates the number of glucose residues linked to L-AA) in the initial reaction to AA-2G at 60 °C and pH 5.0 for 24 h. The amount of AA-2G was quantified using HPLC as previously described [18].
Structure modeling of the mutant proteins
Homology models of Bs F191Y, Bs F255Y, Bc Y195F, Pm Y195F and Pm Y260F were obtained using the Swiss Model (https://www.swissmodel.expasy.org). The crystal structures of Pm CGTase (PDB ID: 4JCL_A), Bs CGTase (PDB ID: 1CYG_A) and Bc CGTase (PDB ID: 1CDG_A) were used as templates. PyMOL was used to analyze the generated three-dimensional structural models as well as to construct illustrative graphics of the protein structures.
Results and discussion
Selection of mutation sites based on the sequence alignment and structural analysis
It was reported that ten CGTases have been used to produce AA-2G, and the conversion rates of AA-2G of these CGTases were significantly different [7]. Among them, the amino acid sequences of six CGTases were known. Then, the multiple sequence alignment of these six CGTases was performed by MEGA X. As shown in Fig. 1, the CGTase sequences were ranked according to the AA-2G yield from highest to lowest [7], and the four aromatic residues 179, 191, 255 and 279 (according to the Bs CGTase amino acid number) at the acceptor site of CGTase were analyzed. Positions 179 and 279 are completely conserved and are all F, while positions 191 and 255 are Y or F. Bs CGTase has the highest AA-2G yield, and residues 191 and 255 are F. Pm CGTase with the lowest conversion rate has two Y residues here. The AA-2G yield using Bc CGTase was between Bs CGTase and Pm CGTase, and the positions 195 and 259 are Y and F. Therefore, it is speculated that the two aromatic residues at positions 191 and 255 may have an impact on the AA-2G yield.
Based on the analysis of three structures of Pm CGTase, Bs CGTase and Bc CGTase with maltose complex (Fig. 2), there is hydrogen bonding with glucose and maltose when position 191 is Y, and it may also form hydrogen bonds with maltose when position 255 is Y. Thus, when the 191 and 255 positions are Y, it is favorable for glucose and maltose as acceptors, which may lead to the low AA-2G yield. To verify this hypothesis, the residues 191/195/195 and 255/259/260 of the three CGTases Bs CGTase, Bc CGTase and Pm CGTase were subjected to Y or F mutation, and the effect of these two residues on acceptor specificity was explored.
Construction, recombinant expression and purification of CGTase mutants
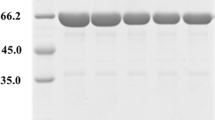
The Bs F191Y, Bs F255Y, Bc Y195F, Pm Y195F and Pm Y260F plasmids were constructed by one-step PCR using the recombinant plasmids pET-20b (+)/Bs-cgt, pET-20b (+)/Pm-cgt and pET-20b (+)/Bc-cgt as templates. The plasmids verified to be correct by DNA sequencing were transferred into E. coli BL21(DE3) for expression. The extracellular supernatant of CGTase was purified by a DEAE column or Mono Q anion exchange column, and the purity and molecular mass of the protein were detected by SDS-PAGE. As shown in Fig. 3, the molecular mass was approximately 66.2 kDa. As shown in Table 2, the specific activities of the mutants Bs F191Y and Bs F255Y were reduced to 78.2% and 71.7% of that of Bs CGTase, respectively. The specific activity of the mutant Bc Y195F was 1.1-fold that of Bc CGTase. This specific activity is basically consistent with the specific activity of Bc Y195F reported by Penninga et al. [25] The specific activities of the mutants Pm Y195F and Pm Y260F were significantly increased, which were 1.9- and 2.6-fold that of Pm CGTase, respectively.
Preparation of AA-2G by CGTases and mutants
Maltodextrin was used as glucosyl donor for AA-2G synthesis because of its low cost and high solubility in aqueous solution. The degree of polymerization of maltodextrin has an important influence on the synthesis of AA-2G. When the DE value is lower, the degree of polymerization is higher and the AA-2G yield is higher. Therefore, maltodextrin with low DE values of 5–7 was selected as glucosyl donor for AA-2G synthesis. The reaction conditions, including temperature, pH and enzyme amount were optimized to improve the AA-2G yield. The influence of reaction temperature on AA-2G synthesis by CGTases and their mutants was shown in Fig. 4A. The optimum temperatures for AA-2G synthesis by Bs CGTase, Bc CGTase, Pm CGTase and their mutants are all 30 °C. The AA-2G yield did not vary much under 25–35 °C conditions, and decreased significantly above 40 °C, probably due to the instability of the substrate l-AA under high temperature conditions. The optimum pH for AA-2G synthesis by CGTases and their mutants was investigated at pH 4.5, 5.0, 5.25, 5.5 and 6.0. All CGTases and their mutants displayed an optimal pH of 5.0 (Fig. 4B). The amount of enzyme for AA-2G synthesis by CGTases and their mutants was optimized. As shown in Fig. 4C, the optimal amount of Bs CGTase, Bc CGTase and their mutants was 2000 U/g maltodextrin, while the optimum amount of enzyme for Pm CGTase and its mutants was 2500 U/g maltodextrin.
Under the optimal conditions, the AA-2G yields of Bs CGTase, Bs F191Y and Bs F255Y were 29.97, 19.70 and 27.60 g/L, the AA-2G yields of Bc CGTase and Bc Y195F were 19.97 and 29.12 g/L, and the AA-2G yields of Pm CGTase, Pm Y195F and Pm Y260F were 14.94, 20.46 and 16.82 g/L. The results showed that the AA-2G yields of Bs F191Y and Bs F255Y decreased by 34.3% and 7.9% as compared to Bs CGTase, respectively. The AA-2G yields of Bc Y195F, Pm Y195F and Pm Y260F increased by 45.8%, 36.9% and 12.6% compared to their wild-type CGTases, respectively. The results demonstrated that the AA-2G yield is high when residues 191 and 255 are F, whereas the AA-2G yield is low when they are Y.
Kinetic analysis of CGTase and its mutants
When CGTase prepared AA-2G with maltodextrin as a glucosyl donor, glucose or maltose can be produced by either disproportionation or hydrolysis reactions. The natural acceptors of CGTase are carbohydrate molecules, thus glucose and maltose are more likely to serve as acceptors than l-AA, resulting glucose and maltose competing with l-AA. Therefore, the affinity and catalytic efficiency of CGTases and their mutants for glucose, maltose and l-AA were determined.
The results of kinetic analysis of CGTases and mutants with glucose as the acceptor are shown in Table 3. The Km values of Bs F191Y and Bs F255Y toward glucose decreased by 93.0% and 36.2%, respectively, while the kcat/Km values of Bs F191Y was 2.54-fold and 1.25-fold that of Bs CGTase. The Km values of Bc Y195F, Pm Y195F and Pm Y260F toward glucose increased by 52.0%, 66.7% and 256%, respectively, while the kcat/Km values of Bc Y195F, Pm Y195F and Pm Y260F were 0.59-, 0.88- and 1.12-fold, respectively, those of the wild-type. The experimental results showed that the catalytic efficiency of Bs F191Y and Bs F255Y using glucose as an acceptor increased, while the catalytic efficiency of Bc Y195F and Pm Y195F decreased. In addition, the catalytic efficiency of Pm Y260F toward glucose was slightly increased by 12.8%.
The kinetic parameters of CGTases and mutants with maltose as the acceptor are shown in Table 4. The Km values of Bs F191Y and Bs F255Y toward maltose decreased 37.4% and 25.3%, respectively, and the kcat/Km values of Bs F191Y and Bs F255Y for maltose was 0.78- and 0.82-fold that of the wild type. The Km values of Bc Y195F and Pm Y260F with maltose as the acceptor increased by 25.3% and 35.4%, respectively, while the Km values of Pm Y195F with maltose as the acceptor decreased by 14.6%. The kcat/Km values of Bc Y195F, Pm Y195F and Pm Y260F for maltose were 0.87-, 0.96- and 1.38-fold those of the wild type. The experimental results show that kcat/Km of Bs F191Y and Bs F255Y for maltose is decreased, while the kcat/Km of two mutants Bc Y195F and Pm Y195F for maltose is decreased, and the kcat/Km of Pm Y260F for maltose is increased.
Kinetic analysis of AA-2G synthesis by three CGTases and their mutants was performed. The kinetic parameters are shown in Table 5. The Km values of mutant Bs F191Y and Bs F255Y for l-AA increased by 28.1% and 20.2%, while the kcat/Km values of mutant Bs F191Y and Bs F255Y for l-AA were 0.37 and 0.72-fold that of Bs CGTase. The Km values of mutant Bc Y195F, Pm Y195F and Pm Y260F for l-AA decreased by 18.9%, 21.3% and 11.5%, respectively, whereas the kcat/Km values of mutant Bc Y195F, Pm Y195F and Pm Y260F for l-AA was 2.94-, 2.06- and 1.28-fold, respectively, those of wild-type CGTases.
Kinetic analysis of CGTases and their mutants with glucose, maltose and l-AA as acceptors showed that the affinity and the catalytic efficiency of Bs F191Y and Bs F255Y for glucose and maltose increased, but the affinity for l-AA was decreased and the catalytic efficiency for maltose and l-AA decreased. The decrease rate in the catalytic efficiency of Bs F191Y and Bs F255Y with l-AA as the acceptor was much greater than maltose. Thus, the AA-2G yield was decreased by Bs F191Y and Bs F255Y. The affinity of Bc Y195F and Pm Y260F for glucose and maltose decreased, and the affinity for l-AA increased, while the affinity of Pm Y195F for glucose, maltose and l-AA all increased. The catalytic efficiency of Bc Y195F and Pm Y195F for glucose and maltose decreased, and the catalytic efficiency for l-AA increased. Therefore, the yields of AA-2G from mutants Bc Y195F and Pm Y195F were improved. The catalytic efficiency of Pm Y260F for l-AA, glucose and maltose was improved by 28.5%, 12.8% and 38.1%. CGTase has the lowest ratio of improved catalytic efficiency to glucose as acceptor, so the glucose specificity was reduced and the AA-2G yield increased.
Structure analysis
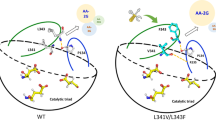
The protein structures of the mutants were obtained by structural simulation and fitted with the maltose complex. As shown in Fig. 5, F191/Y195 is located near the + 1 subsite of CGTase. When the amino acid at this site is F, the enzyme has no hydrogen bonding interaction with glucose and maltose; when this site is Y, the enzyme forms hydrogen bonding interactions with glucose and/or maltose. The mutant Bc Y195F resulted in the loss of hydrogen bonding with glucose at the + 1 subsite (Fig. 5B), leading to reduced affinity for glucose and maltose. The changes in affinity for glucose and maltose are consistent with the changes reported in the literature for the alkalophilic Bacillus sp. 1011 mutant Y195F [20]. The mutant Pm Y195F also decreased the affinity for glucose, but the affinity for maltose did not change. This unchanged affinity of Pm Y195F for maltose, probably due to the presence of hydrogen bonding between Y260 and maltose (Fig. 5C), resulting in a smaller effect of Pm Y195F on affinity for maltose but reduced catalytic efficiency. Therefore, when the 191 position is F, the glucose and maltose specificity decreased, and the l-AA specificity increased. Thus, the mutants Bc Y195F and Pm Y195F promoted the catalytic efficiency of CGTase using l-AA as acceptors and increased the AA-2G yield.
F255/Y260 is located near the + 2 subsite of CGTase, where the mutant Bs F255Y may form a hydrogen bond with maltose (Fig. 5A), increasing the maltose specificity. The mutant Pm Y260F leads to a larger distance between Y195 and glucose and maltose (Fig. 5D), especially the weakened hydrogen bond with glucose, resulting in poor affinity for glucose. When combined with the kinetic parameters, the specificity for glucose was reduced compared to maltose and l-AA, thus increasing the yield of AA-2G.
Conclusion
In this study, mutation of two aromatic amino acids of three CGTases (Bs CGTase, Bc CGTase, and Pm CGTase) suggested that the AA-2G yield could be improved by weakening the specificity of CGTase for byproducts carbohydrate molecules as acceptor substrate. This study is beneficial for a deeper understanding of the reaction mechanism of CGTase catalyzed-synthesis of AA-2G. Meanwhile, this study provides a new insight for the modification of CGTase that catalyze the double-substrate transglycosylation reactions.
Data availability
All relevant data are within the manuscript.
References
Yamamoto I, Muto N, Murakami K, Suga S, Yamaguchi H (1990) L-ascorbic acid alpha-glucoside formed by regioselective transglucosylation with rat intestinal and rice seed alpha-glucosidases: its improved stability and structure determination. Chem Pharm Bull 38:3020–3023. https://doi.org/10.1248/cpb.38.3020
Wenbin Z, Qicheng H, Ruijin Y, Wei Z, Xiao H (2021) 2-O-D-glucopyranosyl-L-ascorbic acid: properties, production, and potential application as a substitute for L-ascorbic acid. J Funct Foods 82:104481. https://doi.org/10.1016/j.jff.2021.104481
Song K, Sun J, Wang W, Hao J (2021) Heterologous expression of cyclodextrin glycosyltransferase my20 in Escherichia coli and its application in 2-O-alpha-d-glucopyranosyl-L-ascorbic acid production. Front Microbiol 12:664339. https://doi.org/10.3389/fmicb.2021.664339
Lee SB, Nam KC, Lee SJ, Lee JH, Inouye K, Park KH (2004) Antioxidative effects of glycosyl-ascorbic acids synthesized by maltogenic amylase to reduce lipid oxidation and volatiles—production in cooked chicken meat. Biosci Biotechnol Biochem 68:36–43. https://doi.org/10.1271/bbb.68.36
Zhou Y, Gan T, Jiang R, Chen H, Ma Z, Lu Y, Zhu L, Chen X (2022) Whole-cell catalytic synthesis of 2-O-alpha-glucopyranosyl-L-ascorbic acid by sucrose phosphorylase from Bifidobacterium breve via a batch-feeding strategy. Process Biochem 112:27–34. https://doi.org/10.1016/j.procbio.2021.11.023
Mukai K, Tsusaki K, Kubota M, Fukuda S, Miyake T (2014) Process for producing 2-O-alpha-D-glucopyranosyl-L-ascorbic acid
Tao X, Su L, Wu J (2019) Current studies on the enzymatic preparation 2-O-alpha-D-glucopyranosyl-L-ascorbic acid with cyclodextrin glycosyltransferase. Crit Rev Biotechnol 39:249–257. https://doi.org/10.1080/07388551.2018.1531823
Gudiminchi RK, Towns A, Varalwar S, Nidetzky B (2016) Enhanced synthesis of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid from alpha-cyclodextrin by a highly disproportionating CGTase. Acs Catal 6:1606–1615. https://doi.org/10.1021/acscatal.5b02108
van der Veen BA, van Alebeek G-JWM, Uitdehaag JCM, Dijkstra BW, Dijkhuizen L (2000) The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem 267:658–665. https://doi.org/10.1046/j.1432-1327.2000.01031.x
Leemhuis H, Dijkstra BW, Dijkhuizen L (2003) Thermoanaerobacterium thermosulfurigenes cyclodextrin glycosyltransferase—mechanism and kinetics of inhibition by acarbose and cyclodextrins. Eur J Biochem 270:155–162. https://doi.org/10.1046/j.1432-1033.2003.03376.x
Gaston JAR, Costa H, Rossi AL, Krymkiewicz N, Ferrarotti SA (2012) Maltooligosaccharides production catalysed by cyclodextrin glycosyltransferase from Bacillus circulans DF 9R in batch and continuous operation. Process Biochem 47:2562–2565. https://doi.org/10.1016/j.procbio.2012.08.008
Zhang Z, Li J, Liu L, Sun J, Hua Z, Du G, Chen J (2011) Enzymatic transformation of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid by alpha-cyclodextrin glucanotransferase from recombinant Escherichia coli. Biotechnol Bioprocess Eng 16:107–113. https://doi.org/10.1007/s12257-010-0161-5
Xiong Y, Su L, Wang L, Wu J, Chen S (2015) Anchorage of cyclodextrin glycosyltransferase on outer membrane of Saccharomyces cerevisiae to produce 2-O-alpha-D-glucopyranosyl-L-ascorbic acid. Acta Microbiol Sin 55:1305–1313
Xu Q, Han R, Li J, Du G, Liu L, Chen J (2014) Improving maltodextrin specificity by site-saturation engineering of subsite +1 in cyclodextrin glycosyltransferase from Paenibacillus macerans. Chin J Biotechnol 30:98–108
Han R, Liu L, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Systems engineering of tyrosine 195, tyrosine 260, and glutamine 265 in cyclodextrin glycosyltransferase from Paenibacillus macerans To enhance maltodextrin specificity for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. Appl Environ Microbiol 79:672–677. https://doi.org/10.1128/aem.02883-12
Liu L, Xu Q, Han R, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Improving maltodextrin specificity for enzymatic synthesis of 2-O-D-glucopyranosyl-L-ascorbic acid by site-saturation engineering of subsite-3 in cyclodextrin glycosyltransferase from Paenibacillus macerans. J Biotechnol 166:198–205. https://doi.org/10.1016/j.jbiotec.2013.05.005
Han R, Liu L, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Iterative saturation mutagenesis of-6 subsite residues in cyclodextrin glycosyltransferase from Paenibacillus macerans to improve maltodextrin specificity for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. Appl Environ Microbiol 79:7562–7568. https://doi.org/10.1128/aem.02918-13
Tao X, Wang T, Su L, Wu J (2018) Enhanced 2-O-alpha-D-glucopyranosyl-L-ascorbic acid synthesis through iterative saturation mutagenesis of acceptor subsite residues in Bacillus stearothermophilus NO2 cyclodextrin glycosyltransferase. J Agric Food Chem 66:9052–9060. https://doi.org/10.1021/acs.jafc.8b03080
Chen S, Xiong Y, Su L, Wang L, Wu J (2017) Position 228 in Paenibacillus macerans cyclodextrin glycosyltransferase is critical for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. J Biotechnol 247:18–24. https://doi.org/10.1016/j.jbiotec.2017.02.011
Nakamura A, Haga K, Yamane K (1994) Four aromatic residues in the active center of cyclodextrin glucanotransferase from Alkalophilic Bacillus sp. 1011: effects of replacements on substrate binding and cyclization characteristics? Biochemistry 33:9929–9936. https://doi.org/10.1021/bi00199a015
Kanai R, Haga K, Akiba T, Yamane K, Harata K (2004) Role of Phe283 in enzymatic reaction of cyclodextrin glycosyltransferase from alkalophilic Bacillus sp. 1011: substrate binding and arrangement of the catalytic site. Protein Sci 13:457–465. https://doi.org/10.1110/ps.03408504
Yang Y, Wang L, Chen S, Wu J (2014) Optimization of beta-cyclodextrin production by recombinant beta-cyclodextrin glycosyltransferase. Biotechnol Bull 0:175–181
Li Z, Li B, Gu Z, Du G, Wu J, Chen J (2010) Extracellular expression and biochemical characterization of alpha-cyclodextrin glycosyltransferase from Paenibacillus macerans. Carbohydr Res 345:886–892. https://doi.org/10.1016/j.carres.2010.02.002
Tao X, Su L, Wang L, Chen X, Wu J (2020) Improved production of cyclodextrin glycosyltransferase from Bacillus stearothermophilus NO2 in Escherichia coli via directed evolution. Appl Microbiol Biotechnol 104:173–185. https://doi.org/10.1007/s00253-019-10249-8
Penninga D, Strokopytov B, Rozeboom HJ, Lawson CL, Dijkstra BW, Bergsma J, Dijkhuizen L (1995) Site-directed mutations in tyrosine 195 of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 affect activity and product specificity. Biochemistry 34:3368–3376. https://doi.org/10.1021/bi00010a028
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (32001637, 31972032 and 31771916), the Natural Science Foundation of Jiangsu Province (BK20221536), the Agricultural independent innovation fund of Jiangsu Province (CX (21) 3039), and the Jiangnan University Basic Research Program (JUSRP122010).
Author information
Authors and Affiliations
Contributions
XT: conceptualization, methodology, data curation, visualization, writing-original draft; DK: methodology, visualization; HZ: formal analysis, writing—review and editing; LS: methodology, funding acquisition; SC: methodology, funding acquisition; DR: methodology; BW: methodology; JW: conceptualization, writing-review and editing; LW: conceptualization, supervision, writing-review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, X., Kong, D., Zhang, H. et al. Enhancing 2-O-α-D-glucopyranosyl-l-ascorbic acid synthesis by weakening the acceptor specificity of CGTase toward glucose and maltose. Bioprocess Biosyst Eng 46, 903–911 (2023). https://doi.org/10.1007/s00449-023-02875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02875-4