Abstract
Recently, a gene for a 7-dimethylallyltryptophan synthase (7-DMATS) was identified in Aspergillus fumigatus and its enzymatic function was proven biochemically. In this study, the behaviour of 7-DMATS towards aromatic substrates was investigated and compared with that of the 4-dimethylallyltryptophan synthase FgaPT2 from the same fungus. In total, 24 simple indole derivatives were tested as potential substrates for 7-DMATS. With an exception of 7-methyltryptophan, all of the substances were accepted by 7-DMATS and converted to their prenylated derivatives, indicating a more flexible substrate specificity of 7-DMATS in comparison to that of FgaPT2. The relative activities of 7-DMATS towards these substrates were from 4% to 89% of that of l-tryptophan, much higher than that of FgaPT2. Structural elucidation of the isolated enzymatic products by nuclear magnetic resonance and mass spectrometry analysis proved unequivocally the prenylation at position C7 of the indole ring. Overnight incubation with eight substances showed that the conversion ratios were in the range of 55.9% to 99.7%. This study provided an additional example that prenylated indole derivatives can be effectively produced by using the overproduced and purified 7-DMATS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About one third of the clinically used drugs are natural products or natural product-derived molecules (Clardy and Walsh 2004; Newman and Cragg 2007). Structural modification of natural products are often necessary in drug development, e.g. for improvement in efficacy and pharmacokinetics (Walsh 2003). However, the complexity of many natural products, e.g. functional groups to be protected and the limited stereoselectivity of many chemical reactions, can limit the scope for making chemical modifications. In the last years, modifications of such molecules have been achieved by combinatorial biosynthesis, mutasynthesis or chemoenzymatic synthesis. Combinatorial biosynthesis was defined as the application of genetic engineering to modify biosynthetic pathways of natural products in order to produce new and altered structures using nature’s biosynthetic machinery (Floss 2006). Mutasynthesis or mutational biosynthesis combines genetic and chemical approaches and was successfully used in a number of bacterial systems to produce biologically active substances (Galm et al. 2004; Weissman 2007; Weist and Süssmuth 2005). Chemoenzymatic synthesis is an in vitro approach with the help of purified proteins and was used successfully in the synthesis of peptides (Grunewald and Marahiel 2006), glycopeptides (Buskas et al. 2006), nucleotide deoxysugars (Rupprath et al. 2005), glycan libraries (Blixt and Razi 2006) and glycosides (Langenhan et al. 2005). Availability of genome sequences of bacteria and fungi, which has been increased tremendously in the last years (Jones 2007; Raskin et al. 2006), accelerates the identification of genes and enzymes involved in the biosynthesis of natural products (Abe et al. 2006; Keller et al. 2005; Li and Unsöld 2006) and therefore provides basis for use of these approaches. For a successful application of these approaches, substrate flexibility of the biosynthetic enzymes is the prerequisite.
Our research interest focuses on the biosynthesis of prenylated indole derivatives from fungi. In general, prenylated indole derivatives belong to hybrid natural products containing both aromatic and isoprenoid moieties, which are widely distributed in plants, fungi and bacteria (Botta et al. 2005b). Prenylated indole derivatives from fungi carry diverse structures, show different biological activities and are found especially in the genera of Claviceps, Aspergillus and Penicillium (Stocking et al. 2000; Williams et al. 2000). Prenylation represents a critical step in the biosynthesis of these natural products of mixed biosynthetic origins. In the last years, progress has been achieved, especially, on the soluble prenyltransferases from bacteria and fungi, which catalyse the attachment of a prenyl moiety to an aromatic nucleus, known as “aromatic prenyltransferases”. Examples of such enzymes from bacteria are the members of the ABBA family of aromatic prenyltransferases (Tello et al. 2008): CloQ in the biosynthesis of clorobiocin, (Pojer et al. 2003), Orf2 (renamed NphB) in the biosynthesis of naphterpin (Kuzuyama et al. 2005; Tello et al. 2008) and Fnq26 in the biosynthesis of a prenylated naphthaquinone (Haagen et al. 2007). We have identified five different prenyltransferases from Aspergillus fumigatus, which catalyse different prenyl transfer reactions to different positions of indole derivatives (Grundmann and Li 2005; Kremer et al. 2007; Ruan et al. 2008; Unsöld and Li 2005, 2006; Yin et al. 2007). Some of these soluble prenyltransferases show broad substrate specificities: e.g. NphB from Streptomyces sp., CL 190 and Fnq26 from Streptomyces cinnnamonensis catalyse the conversion of some dihydroxynaphthalenes to their geranylated derivatives (Haagen et al. 2007; Kuzuyama et al. 2005). NphB accepted even flavonoids as substrates. Similar phenomena have also been observed with fungal prenyltransferases: the brevianamide F prenyltransferase FtmPT1 and the cyclic dipeptide N-prenyltransferase CdpNPT accepted all of the tested tryptophan-containing cyclic dipeptides as substrates (Grundmann and Li 2005; Ruan et al. 2008; Yin et al. 2007). This tolerance towards aromatic substrates makes the enzymes especially attractive for their use as tools for chemoenzymatic synthesis, because prenylation represents not only a critical step in the biosynthesis of these compounds, but also often results in the formation of biologically active substances (Sanz-Cervera et al. 2000; Zhao et al. 2002). Therefore, chemoenzymatic synthesis using prenyltransferases could be used as a novel strategy for the synthesis of known compounds of emerging pharmaceutical interest and could create chemical libraries of novel prenylated compounds for biological screenings (Botta et al. 2005a).
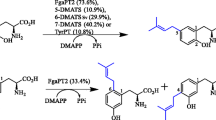
Investigation of the substrate specificity of the 4-dimethylallyltrytophan synthase FgaPT2 from A. fumigatus showed that 16 of 24 commercially available simple indole derivatives were accepted as substrates, in addition to its natural substrate l-tryptophan (Steffan et al. 2007). These compounds carry modifications at the indole moiety or at the side chain. Interestingly, indole derivatives lacking the amino or carboxyl group at the side chain were also accepted by FgaPT2 (Steffan et al. 2007). Eight substances were selected for chemoenzymatic synthesis and found to be converted to their prenylated derivatives with conversion ratios of up to 99.7% (Steffan et al. 2007). This finding encouraged us to investigate the substrate specificity of another dimethylallyltryptophan synthase, 7-dimethylallyltryptophan synthase (7-DMATS), from the same fungus (Kremer et al. 2007), because some simple prenylated indole derivatives were reported to be biologically active, e.g. flustramines with cytotoxic properties (Lysek et al. 2002). In contrast to the prenylation of tryptophan at position C4 of the indole moiety catalysed by FgaPT2, 7-DMATS catalyses the prenylation at position C7. Therefore, two different prenylated derivatives could be obtained from one substrate by using FgaPT2 and 7-DMATS. Here we report the substrate specificity of 7-DMATS and its potential to be used for production of prenylated indole derivatives.
Materials and methods
Chemicals
Trisammonium salt of dimethylallyl diphosphate (DMAPP) was synthesised in analogy to the synthesis of trisammonium geranyl diphosphate reported by Woodside et al. (1988). The other substrates were obtained from Sigma, Aldrich, Fluka, Bachem, Roth, Acros and Alfa Aesar, respectively, and of the highest purity available.
Cloning of Afua_3g12930, overproduction and purification of His6-7-DMATS
The coding region of Afua_3g12930 was amplified using PCR from cDNA of A. fumigatus strain B5233 which is available in the form of phagemids isolated from a cDNA library and cloned into the cloning vector pGEM-T. For gene expression the coding sequence of Afua_3g12930 was released from pGEM-T and cloned into the vector pQE60 resulting in the expression plasmid pLW40 (Kremer et al. 2007).
Escherichia coli XL1 Blue MRF’ cells harbouring pLW40 were induced by 0.8 mM of isopropyl-beta-d-thiogalactopyranoside at 37°C. His6-7-DMATS was purified with Ni-NTA agarose to apparent homogeneity as judged by sodium dodecyl sulfate polyacrylamide gel electrophoresis and a protein yield of 5 mg of purified His6-tagged 7-DMATS per litre of culture was obtained (Kremer et al. 2007). The observed molecular weight was 50 kDa and corresponded well to the calculated value of 54 kDa for His6-7-DMATS.
Determination of molecular weight of native His6-7-DMATS
The molecular weight of native His6-7-DMATS was determined by gel filtration on a HiLoad 16/60 Superdex 200 column (GE Health Care, Freiburg, Germany) which had been equilibrated with 50 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl. The column was calibrated with dextran blue 2000 (2,000 kDa), conalbumin (75 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), ribonuclease A (13.7 kDa) and aprotinin (6.5 kDa) (GE Health Care, Freiburg, Germany). The proteins were eluted with 50 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl. The molecular weight of native His6-7-DMATS was determined as 65 kDa. This proved that native 7-DMATS acts as a monomer.
Stability of the overproduced and purified His6-7-DMATS
The purified His6-7-DMATS was relatively stable under the conditions used for enzyme assays. More than 80% of original activity was recovered after preincubation at 37°C in the absence of the substrates for 16 h.
Conditions for enzymatic reactions
All of the enzyme assays contained 50 mM Tris-HCl (pH 7.5) and 10 mM CaCl2. They differed from each other by incubation volumes, amounts of 7-DMATS and incubation times. The reaction mixtures of the standard assay for determination of the substrate specificity (100 μl) contained 1 mM tryptophan or derivatives, 1 mM DMAPP and 0.6 μM of purified His6-7-DMATS. After incubation for 1 h at 37°C, the reaction was stopped with 10 μl of trichloroacetic acid (1.5 M). After removal of the protein by centrifugation (15,000×g, 10 min, 4°C), the enzymatic products were analysed on a high performance liquid chromatography (HPLC) system described below. Two independent assays were carried out routinely for quantification. The assays for isolation of the enzymatic products for structural elucidation (5 ml) contained 1 mM tryptophan or derivatives, 2 mM DMAPP and 1.6 μM of purified His6-7-DMATS and were incubated for 16 h. The reaction mixtures were concentrated on a rotation evaporator at 30°C to a volume of 700 μl before injection. The assays for chemoenzymatic synthesis (100 μl) contained 1 mM tryptophan or derivatives, 2 mM DMAPP and 50 μM of purified His6-7-DMATS and were incubated for 16 h.
HPLC analysis and determination of the conversion rate
Reaction mixtures of 7-DMATS were analysed on an Agilent HPLC Series 1100 by using an Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm, Agilent) at a flow rate of 1 ml min−1. Water (solvent A) and acetonitrile (solvent B), each containing 0.5% (v/v) trifluoroacetic acid, were used as solvents. A gradient was run from 15% to 70% B in 15 min. After washing with 100% solvent B for 5 min, the column was equilibrated with 15% solvent B for 5 min. The substances were detected with a photo diode array detector.
In general, the conversion rate was defined as the decrease of the peak area of the substrate in the HPLC chromatogram at 277 nm. For this purpose, the peak area of the substrate was calculated from data obtained by injection of 10 nmol instead of 100 nmol substrate used in the enzyme assays, to ensure the linearity of the analysis. For substrates with low conversion rates, e.g. 2% or lower, the ratio of the peak areas of product to substrate was used.
Spectroscopic analysis
The isolated products (50–200 μg) were analyzed by 1H-nuclear magnetic resonance (NMR) spectroscopy, homonuclear correlation spectroscopy (H-H-COSY) as well as by positive and negative electrospray ionization (ESI) mass spectrometry with a ThermoFinnigan TSQ Quantum. The mass spectrometer was coupled with an Agilent HPLC series 1100 equipped with a RP18-column (2 × 250 mm, 5 μm). For separation, the column was run with 10% solvent B (methanol) in solvent A (water), each containing 0.1% of formic acid for 5 min, followed by a gradient from 10% to 100% B over 30 min. After washing with 100% B, the column was equilibrated with 10% B for 10 min. The flow rate was at 0.2 ml min−1.
NMR spectra were taken on an Avance DRX 500 spectrometer (Bruker) using 0.1 M DCl as solvent. The δ values are given in ppm and coupling constants in Hz. The solvent signal at 4.81 ppm was used as reference. NMR and MS data are given in Tables 3, 4 and 5, respectively.
Results
7-DMATS accepted, with an exception of 7-methyltryptophan, all of the tested simple indole derivatives as substrates
In the course of our investigations on prenyltransferases, we have identified a putative prenyltransferase gene Afua_3g12930 on chromosome 3 of the pathogenic fungus A. fumigatus Af293. The gene Afua_3g12930, which encodes for a 7-dimethylallyltryptophan synthase 7-DMATS (= EAL92290), belongs to a putative biosynthetic gene cluster consisting probably of eight putative genes: two putative transcription factor genes Afua_3g12940 and Afua_3g12890, one putative transporter gene (Afua_3g12900), one gene with unknown function (Afua_3g12950) and four biosynthetic genes, i.e. the non-ribosomal peptide synthetase gene Afua_3g12920, the prenyltransferase gene Afua_3g12930 described in this study, Afua_3g12960 a gene coding for a putative cytochrome P450 enzyme and a putative methyltransferase gene Afua_3g12910. Homologous clusters were also found in the genome sequences of Aspergillus terreus NIH2624 and Neosartorya fischeri NRRL181 (Kremer et al. 2007). The end product of this cluster is unknown (Cramer Jr. et al. 2006; Nierman et al. 2005). Afua_3g12920 is not or very low expressed in its native host (Cramer Jr. et al. 2006) and no data are available on the expression of Afua_3g12930. Our previous study showed that 7-DMATS catalyses the prenylation of tryptophan at position C7 (Kremer et al. 2007). To extend our knowledge on the substrate specificity, 24 simple indole derivatives were incubated with 7-DMATS under the standard assay condition (Materials and methods). With an exception of 7-methyltryptophan, product formation could be observed for all of the substrates in HPLC chromatograms (data not shown). Liquid chromatography–mass spectrometry (LC–MS) analysis of the reaction mixtures confirmed the presence of the monoprenylated derivatives. For better comparison with FgaPT2 (Steffan et al. 2007), the tested compounds are grouped in two categories (Tables 1 and 2).
The members of group I are tryptophan derivatives with modifications at the side chain, i.e. indole derivatives with different substitutions at position C3. All of the 14 compounds of this group tested in this study were accepted by 7-DMATS, with conversion ratios of about 4% to 89% of that of l-tryptophan. These results showed that 7-DMATS has a somehow broader substrate specificity than FgaPT2, which accepted 11 of 14 substances from this group (Steffan et al. 2007). Similar to results obtained from FgaPT2 (Steffan et al. 2007), the configuration at C11 is also important for the enzymatic activity of 7-DMATS. A relative yield of 11.8% of l-tryptophan, comparable to that of FgaPT2, could be detected with d-tryptophan (Table 1). This value is slightly lower than that determined in the previous study of 15.5% (Kremer et al. 2007), which is probably caused by the different conditions, e.g. different substrate concentrations, incubation times and amounts of the purified enzyme. Some of the tested substances were commercially available only as mixtures of d- and l-enantiomers. If a similar behaviour of the enzyme towards all of the tested substances is assumed (stereochemistry at the position C11), it can be expected that the l-form was accepted better than its d-form. However, an exact conversion ratio can not be predicted or detected in our experiments.
Modification of the side chain of tryptophan resulted in decreasing of relative enzymatic activity. E.g. deamination of tryptophan to indole-3-propionic acid resulted in a significantly reduced relative activity of 8.5% (Table 1), clearly lower than that observed with FgaPT2 with a relative activity of 32.2% of that of l-tryptophan. Interestingly, comparable activity to that of indole-3-propionic acid was observed for indole-3-acetic acid and indole-3-butyric acid at 10.8% and 9.1% of that of l-tryptophan, respectively. It seems that slight change of the chain length did not play an important role for an acceptance by 7-DMATS. This finding is in contrast to the results obtained from FgaPT2, for which a complete loss of enzymatic activity was observed after shortening the side chain to indole-3-acetic acid or extending to indole-3-butyric acid (Steffan et al. 2007). Additional modifications at C11 of indole-3-propionic acid by hydroxylation to indole-3-lactic acid and by hydroxylation and oxidation to indole-3-pyruvic acid reduced the enzyme activity to about half of that of indole-3-propionic acid (Table 1).
Decarboxylation of l-tryptophan to tryptamin, methylation to l-tryptophan methyl ester or acetylation of the 11-amino moiety to N-acetyltryptophan resulted in decreased, but detectable and comparable enzyme activities of about 7% of that of l-tryptophan (Table 1). Hydroxymation of tryptophan resulted in much stronger reduction of enzyme activity (Table 1).
Significant enzyme activity of 82.2% and 28.2% of that of l-tryptophan, respectively, could be detected with l-abrine and l-ß-homotryptophan as substrates. This finding is in consistence with their high conversion rates by FgaPT2 (Steffan et al. 2007). Interestingly, 11-methyl-dl-tryptophan, which was accepted by FgaPT2 with a relative activity of only 1.6% of that of l-tryptophan, was very well accepted by 7-DMATS, with a relative activity of 19.1% of that of l-tryptophan. As conclusion, the retention of the free carboxyl and amino group of tryptophan is also important for the enzyme activity of 7-DMATS. Methylation at the amino group or at C11 as well as insertion of a carbon atom between amino and carboxyl groups showed lower effect on the enzyme activity than the decarboxylation, deamination, esteration, hydroxymation or acetylation of tryptophan.
The members of group II (Table 2) consist of tryptophan and its 10 derivatives with modifications at the indole ring. With an exception of 7-methyltryptophan, all of the tested compounds were accepted by 7-DMATS (Table 2). Methylation at the indole ring resulted in reduced, but still significant product formation. In comparison to that of l-tryptophan, relative yields of 35.8%, 89.4%, 74.8% and 19.8% were observed for 1-, 4-, 5-, and 6-methyltryptophan (Table 2), respectively, which is significantly higher than those of FgaPT2 in the range of 8.1% to 21% of l-tryptophan. As observed for FgaPT2, the efficiency of the prenylation reduced progressively with the decreased distance of the methyl group to the prenylation position (Steffan et al. 2007). The relative yields of the racemic mixtures of 4-methyltryptophan and 5-methyltryptophan were higher than 55.9%, which could be calculated by a complete conversion of the l-forms and 11.8% of the l-forms as determined with d-tryptophan. This indicates that methylation at the indole ring could decrease the preference of the enzyme for l- over d-configured substrates. As expected for a 7-dimethylallyltryptophan synthase, no product could be detected with 7-methyl-dl-tryptophan, which confirmed the regioselectivity of the enzymatic reaction. Similar relative activities towards 5-hydroxy-l-tryptophan were observed with 7-DMATS and FgaPT2 at 26.7% and 21.0% of l-tryptophan, respectively. Similar to FgaPT2, methylation of this hydroxy group resulted in reduction of the enzymatic activity. 13.3% of that of l-tryptophan was observed for 5-methoxy-dl-tryptophan with 7-DMATS (Table 2). In contrast to the results obtained by using FgaPT2, halogenation at the indole ring resulted in reduced, but still significant enzyme activity of 7-DMATS. With 5-bromo-, 5-fluoro- and 6-fluoro-tryptophan, which were not accepted by FgaPT2 (Steffan et al. 2007), relative activities of 7.2%, 10.7% and 33.7% of that of l-tryptophan were detected.
7-DMATS catalyses the prenylation at position C7
Our previous results proved unequivocally the prenylation of tryptophan at position C7 (Kremer et al. 2007). To confirm the prenylation position, enzymatic products of 10 additional simple indole derivatives were isolated on a preparative scale and subjected to NMR and MS analysis. If necessary, the correlation of the protons was proven by H-H-COSY spectra. Comparison of the 1H-NMR spectra of the isolated products (Tables 3 and 4) with the respective substrates revealed the disappearance of signals for one aromatic proton at position C4 or C7 and the presence of signals for a dimethylallyl moiety at 5.2–5.5 (t, H-2′), 3.5–3.6 (d, H2-1′) and 1.7–1.9 ppm (H-4′ and H-5′), respectively. This indicated that the prenylation has taken place at position C4 or C7 of the indole moieties. Comparison of the spectra of the isolated compounds with those of FgaPT2 showed that the products of 7-DMATS differed clearly from those of the 4-prenylated derivatives obtained by using FgaPT2 (Steffan et al. 2007). Therefore, the structures of 7-DMA-β-homotryptophan, 7-DMA-abrine, N-acetyl-7-DMAT, 11-methyl-7-DMAT, 1-methyl-7-DMAT, 4-methyl-7-DMAT, 5-methyl-7-DMAT, 6-methyl-7-DMAT, 5-bromo-7-DMAT and 6-fluoro-7-DMAT could be identified unequivocally as prenylated derivatives of the respective substrates and the prenylation has taken place at position C7 of the indole rings (Tables 1 and 2). These results were also confirmed by LC–MS analysis (Table 5).
7-DMATS as a tool for chemoenzymatic synthesis
To test the potential of 7-DMATS for chemoenzymatic synthesis, eight selected substrates were incubated over night to determine the conversion rates. HPLC-chromatograms of the incubation mixtures are illustrated in Fig. 1.
Under the condition described in the “Materials and methods”, l-tryptophan, l-abrine and ß-homotryptophan were converted almost completely to their prenylated derivatives. Very high conversion yields from 83.7% to 95.0% were observed with 4-methyl-dl-tryptophan, 5-methyl-dl-tryptophan and 11-methyl-dl-tryptophan. Somehow lower, but still significant conversion yields of 64.6 and 55.9% were determined with 6-methyl-dl-tryptophan and 6-fluoro-dl-tryptophan, respectively. These results demonstrated clearly that the soluble prenyltransferase 7-DMATS prenylated the selected substrates with a high efficiency and could be used as an effective tool for the prenylation of indole derivatives at position C7 of the indole rings.
Discussion
Information on substrate specificity is a prerequisite for successful and efficient chemoenzymatic synthesis, which became in the last years a new strategy for drug discovery and development. In comparison to chemical synthesis, chemoenzymatic synthesis is performed under mild conditions, e.g. at low incubation temperature and in aqueous solutions with nearly neutral pH values. Therefore, undesired reactions and rearrangements could be usually avoided. As shown in this study, it is not necessary to protect functional groups of the substrates for chemoenzymatic synthesis, which is usually essential for chemical synthesis. Furthermore, different protecting and deprotecting strategies must be often developed for similar reactants in the chemical synthesis. An additional aspect is the high regio- and stereoselectivity of chemoenzymatic synthesis, which is often difficult to be realized in the chemical synthesis.
The results described in this study showed the broad substrate specificity of a dimethylallyltryptophan synthase 7-DMATS from A. fumigatus. With the exception of 7-methyltryptophan, all of the 24 tested indole derivatives were accepted by this enzyme. These substrates also included substances such as halogenated tryptophan derivatives, which were not accepted by another dimethylallyltryptophan synthase FgaPT2 from the same fungus (Steffan et al. 2007), indicating a more flexible substrate specificity of 7-DMATS than that of FgaPT2. By comparison of relative activities of the substrates (Tables 1 and 2), it is obvious that the modifications at the indole ring showed lower effect on the enzyme activity of 7-DMATS than the modifications at the side chain of tryptophan.
The non-acceptance of 7-methyltryptophan by 7-DMATS indicated the prenylation position at C7 of the indole moiety (Kremer et al. 2007) and the high regioselectivity of the enzyme towards simple tryptophan derivatives, which could be proven by isolation and structural elucidation of 11 enzymatic products. NMR and MS analysis (Tables 3, 4 and 5) revealed unequivocally the prenylation of these compounds at position C7 of the indole rings.
The results obtained with 7-DMATS are in consistence with those observed with other indole prenyltransferases from A. fumigatus (Grundmann and Li 2005; Ruan et al. 2008; Steffan et al. 2007; Yin et al. 2007). All of these enzymes accepted only DMAPP as prenyl donor, but showed high flexibility towards their aromatic substrates and catalysed regiospecific prenylations. E.g., both FtmPT1 and CdpNPT accepted the same tryptophan-containing cyclic dipeptides as substrates, but catalysed prenyl transfer reactions at different positions of the indole moiety, i.e. FtmPT1 at C2 and CdpNPT at N1 (Grundmann and Li 2005; Ruan et al. 2008; Yin et al. 2007). Similar phenomenon was observed with FgaPT2 and 7-DMATS, which catalyse the prenylations of tryptophan at position C4 and C7, respectively. In summary, products with different prenylation patterns could be obtained from one indole derivative by using different prenyltransferases. This makes the enzymes in this group especially attractive for biotechnological production of active compounds.
Our study also exemplifies that the indole prenyltransferases could be conveniently used for production of different structures. Overnight incubation of eight substances with 7-DMATS showed that the conversion ratios were in the range of 55.9% to 99.7%. More importantly, these enzymes are soluble proteins and could be actively overproduced in E. coli. The development of soluble recombinant enzymes in a convenient system is one of the best solutions in terms of ease of manipulation, efficiency of catalysis and overall cost of the biotransformation. Such approaches offer the potential to significantly increase the structural diversity in drug discovery programmes.
References
Abe K, Gomi K, Hasegawa F, Machida M (2006) Impact of Aspergillus oryzae genomics on industrial production of metabolites. Mycopathologia 162:143–153
Blixt O, Razi N (2006) Chemoenzymatic synthesis of glycan libraries. Methods Enzymol 415:137–153
Botta B, Monache GD, Menendez P, Boffi A (2005a) Novel prenyltransferase enzymes as a tool for flavonoid prenylation. Trends Pharmacol Sci 26:606–608
Botta B, Vitali A, Menendez P, Misiti D, Delle MG (2005b) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12:717–739
Buskas T, Ingale S, Boons GJ (2006) Glycopeptides as versatile tools for glycobiology. Glycobiology 16:113R–136R
Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432:829–837
Cramer RA Jr., Stajich JE, Yamanaka Y, Dietrich FS, Steinbach WJ, Perfect JR (2006) Phylogenomic analysis of non-ribosomal peptide synthetases in the genus Aspergillus. Gene 383:24–32
Floss HG (2006) Combinatorial biosynthesis—potential and problems. J Biotechnol 124:242–257
Galm U, Dessoy MA, Schmidt J, Wessjohann LA, Heide L (2004) In vitro and in vivo production of new aminocoumarins by a combined biochemical, genetic and synthetic approach. Chem Biol 11:P173–183
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Grunewald J, Marahiel MA (2006) Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides. Microbiol Mol Biol Rev 70:121–146
Haagen Y, Unsold I, Westrich L, Gust B, Richard SB, Noel JP, Heide L (2007) A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates. FEBS Lett 581:2889–2893
Jones MG (2007) The first filamentous fungal genome sequences: Aspergillus leads the way for essential everyday resources or dusty museum specimens? Microbiology 153:1–6
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol 3:937–947
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435:983–987
Langenhan JM, Griffith BR, Thorson JS (2005) Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification. J Nat Prod 68:1696–1711
Li S-M, Unsöld IA (2006) Post genome research on the biosynthesis of ergot alkaloids. Planta Med 72:1117–1120
Lysek N, Rachor E, Lindel T (2002) Isolation and structure elucidation of deformylflustrabromine from the North Sea bryozoan Flustra foliacea. Z Naturforsch [C] 57:1056–1061
Newman DJ, Cragg GM (2007) Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod 70:461–477
Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C et al (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156
Pojer F, Wemakor E, Kammerer B, Chen H, Walsh CT, Li S-M, Heide L (2003) CloQ, a prenyltransferase involved in clorobiocin biosynthesis. Proc Natl Acad Sci USA 100:2316–2321
Raskin DM, Seshadri R, Pukatzki SU, Mekalanos JJ (2006) Bacterial genomics and pathogen evolution. Cell 124:703–714
Ruan H-L, Yin W-B, Wu J-Z, Li S-M (2008) Reinvestigation on a cyclic dipeptide N-prenyltransferase reveals rearrangement of N1-prenylated indole derivatives. Chembiochem 9:1044–1047
Rupprath C, Schumacher T, Elling L (2005) Nucleotide deoxysugars: essential tools for the glycosylation engineering of novel bioactive compounds. Curr Med Chem 12:1637–1675
Sanz-Cervera JF, Stocking EM, Usui T, Osada H, Williams RM (2000) Synthesis and evaluation of microtubule assembly inhibition and cytotoxicity of prenylated derivatives of cyclo-L-Trp-L-Pro. Bioorg Med Chem 8:2407–2415
Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 8:1298–1307
Stocking EM, Williams RM, Sanz-Cervera JF (2000) Reverse prenyl transferases exhibit poor facial discrimination in the biosynthesis of paraherquamide A, brevianamide A, and austamide. J Am Chem Soc 122:9089–9098
Tello M, Kuzuyama T, Heide L, Noel JP, Richard SB (2008) The ABBA family of aromatic prenyltransferases: broadening natural product diversity. Cell Mol. Life Sci. in press
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Unsöld IA, Li S-M (2006) Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification and characterization of fumigaclavine C synthase FgaPT1. Chembiochem 7:158–164
Walsh T (2003) Where will new antibiotics come from? Nat Rev Microbiol 1:65–70
Weissman KJ (2007) Mutasynthesis—uniting chemistry and genetics for drug discovery. Trends Biotechnol 25:139–142
Weist S, Süssmuth RD (2005) Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics. Appl Microbiol Biotechnol 68:141–150
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids, Topics Curr. Chem. vol. 209. Springer, Berlin, pp 97–173
Woodside AB, Huang Z, Poulter CD (1988) Triammonium germanyl diphosphate. Org Synth 66:211–215
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Zhao S, Smith KS, Deveau AM, Dieckhaus CM, Johnson MA, Macdonald TL, Cook JM (2002) Biological activity of the tryprostatins and their diastereomers on human carcinoma cell lines. J Med Chem 45:1559–1562
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SPP 1152: Evolution of metabolic diversity, to S.-M. Li).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kremer, A., Li, SM. Potential of a 7-dimethylallyltryptophan synthase as a tool for production of prenylated indole derivatives. Appl Microbiol Biotechnol 79, 951–961 (2008). https://doi.org/10.1007/s00253-008-1505-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1505-3