Abstract
Non-native nitrogen-fixing Acacia species have been invading riparian ecosystems worldwide, potentially threatening stream communities that strongly depend on allochthonous litter. We examined the effects of the invasion of native deciduous temperate forests by Acacia species on litter decomposition and associated fungal decomposers in streams. Litter of native (Alnus glutinosa and Quercus robur) and invasive (Acacia melanoxylon) species were enclosed in fine-mesh bags and immersed in three native and three invaded streams, for 14–98 days. Litter decomposition rates, fungal biomass, and aquatic hyphomycete sporulation rates were higher in invaded than in native streams, likely due to the higher water nitrogen concentration found in invaded streams. Alnus glutinosa litter had higher aquatic hyphomycete sporulation rates and species richness, and higher decomposition rates, probably because they were soft and nitrogen rich. Quercus robur litter also had high aquatic hyphomycete sporulation rates but lower decomposition rates than Al. glutinosa, probably due to high polyphenol concentration and carbon:nitrogen ratio. Acacia melanoxylon litter had lower aquatic hyphomycete sporulation rates and species richness, and lower decomposition rates, most likely because it was very tough. Thus, litter decomposition rates varied in the order: Al. glutinosa > Q. robur > Ac. melanoxylon. The aquatic hyphomycete community structure strongly differed between native and invaded streams, and among litter species, suggesting that microbes were sensitive to water nitrogen concentration and litter characteristics. Overall, increases in water nitrogen concentration and alterations in litter characteristics promoted by the invasion of native riparian forests by Acacia species may affect the activity and community structure of microbial decomposers, and instream litter decomposition, thus altering the functioning of stream ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological invasions are an ongoing and increasing problem worldwide, posing a major threat to the services provided by ecosystems to human societies [1,2,3]. Freshwater ecosystems, for instance, have been severely degraded due to the invasion of native riparian forests by non-native plant species [4].
Australian Acacia species are considered one of the most aggressive and problematic plant invaders in several regions of the world, including southern European countries, such as Portugal, Spain, France, and Italy [5]. Acacia species create very dense and homogeneous stands of evergreen, fast-growing, nitrogen (N)-fixing trees [6], which markedly differ from native temperate decidous forests. These differences between Acacia stands and native deciduous forests are expected to alter the diversity, quality, quantity, and timing of litter fall into streams [5, 7, 8]. Therefore, stream aquatic food webs that strongly depend on litter from terrestrial origin will likely be affected, as shown in the case of other forest changes [8,9,10,11,12]. Additionally, since Acacia species can fix atmospheric N, N concentration in stream water will likely increase as shown for the invasion of riparian forests by other N-fixing species [8, 13,14,15,16,17,18].
In headwater forest streams, aquatic hyphomycetes, a polyphyletic group of fungi mainly composed by the anamorphs of Ascomycetes and Basidiomycetes, play a key role in litter decomposition, mediating the transfer of energy and nutrients from the litter into higher trophic levels [19,20,21]. Aquatic hyphomycetes community composition and structure are generally affected by litter characteristics [22, 23], while species richness can increase with increases in the diversity of tree species in the riparian vegetation [7, 24,25,26]. Also, aquatic hyphomycetes usually colonize and decompose faster soft litter species, with high nutrient concentrations (e.g., N and/or phosphorus (P)) and low concentrations of recalcitrant compounds (e.g., lignin, polyphenols) [19, 27, 28]. Thus, alterations in litter inputs characteristics, promoted by Acacia species invasion, are expected to induce changes in decomposers community structure and function, as observed in the case of other forest changes [29,30,31]. Additionally, microbial decomposer activity and consequently litter decomposition are generally stimulated by moderate increases in dissolved N concentration [22, 32,33,34,35,36]. Therefore, fundamental stream ecosystem processes, such as litter decomposition and nutrient cycling, are expected to be highly affected by the invasion of native riparian forests by non-native N-fixing Acacia species [37], as observed in the case of other forest changes [29,30,31].
The effects of the invasion of native riparian forests by Acacia species on stream ecosystems have rarely been studied. To our knowledge, only three studies addressed the effects of Acacia species invasion on litter decomposition and/or decomposer communities in streams [37,38,39]. In South Africa, field studies have shown that the invasion of the Fynbos biome (dominated by sclerophyllous species) by Acacia mearnsii De Wild. altered the abundance of macroinvertebrate functional feeding groups in streams [38], but it did not alter the density of macroinvertebrates associated with submerged litter nor litter decomposition [39]. On the other hand, a recent laboratory experiment simulating the conditions of streams flowing through native deciduous forests and streams flowing through forests invaded by Acacia species suggested that the invasion of native riparian forests by Acacia species (namely Acacia dealbata Link and Acacia melanoxylon R. Br.) can stimulate litter decomposition due to increases in water N concentration, inhibit litter decomposition due to changes in the physical and chemical characteristics of litter inputs to streams, and alter the activity and community structure of microbial decomposers [37]. This laboratory experiment was performed under controlled conditions, and therefore extrapolation of results to field conditions is limited. Thus, it is necessary to address the effects of the invasion of native deciduous riparian forests by N-fixing Acacia species on litter decomposition, and the activity of the associated microbial decomposer community, under realistic stream conditions, in order to take into account the physical, chemical, and biological characteristics of streams running through forests invaded by Acacia species.
In this study, we examined the effects of the invasion of native deciduous temperate riparian forests by non-native N-fixing Acacia species (namely Ac. dealbata and Ac. melanoxylon) on the decomposition of native (Alnus glutinosa (L.) Gaertn. and Quercus robur L.) and invasive (Ac. melanoxylon) litter species and associated fungal decomposer activity, species richness, and community structure. We predicted that: (i) water N concentration would be higher in streams flowing through forests heavily invaded by N-fixing Acacia species (invaded streams) than through native deciduous forests (native streams), as a result from the leaching of N-rich soil solutes into streams and instream decomposition of N-rich litter; (ii) fungal decomposer activity would be higher and litter decomposition would be faster in invaded than in native streams, probably because water N concentration would be higher; (iii) fungal decomposer activity would be higher and litter decomposition would be faster in litter species that are soft and have high nutritional quality and slower in litter species that are tough and have high concentrations of recalcitrant compounds; and (iv) the species richness and community structure of aquatic hyphomycetes would differ among litter species and between stream types, respectively due to differences in native and invasive litter species characteristics and water N concentrations.
Material and Methods
Study Region and Streams
This study was carried out in six small headwater forest streams located in Serra da Lousã, central Portugal (Fig. 1). Stream basins are underlined by schist bedrock, have very low human activity, and are mainly covered by deciduous forests, shrubby communities (Calluno-Ulicetea), eucalyptus and conifer plantations, and Acacia species stands (Table 1). Three streams, Maior, Cerdeira, and Candal, flow through native mixed deciduous forests mainly composed by Quercus spp. and Castanea sativa Miller (native streams), while the other three, Sotão, Fiscal, and Piedade, flow through forests that have been heavily invaded by N-fixing Ac. dealbata (dominant) and Ac. melanoxylon (sporadic) (10–43% cover across stream basins) (invaded streams) (Table 1). Additionally, the percentage of Ac. dealbata cover in the riparian vegetation of invaded streams (defined as an area 50 m wide on each stream bank and 250 m long upstream the sampling point) was very high, varying from 94 to 100% across streams (Table 1).
Litter Species and Initial Chemical Characteristics
Litter of native (Al. glutinosa and Q. robur) and invasive (Ac. melanoxylon) tree species were selected for this study. Alnus glutinosa and Q. robur are two broadleaf deciduous tree species commonly found in the riparian vegetation of streams flowing through native forests in central Portugal and were selected to represent extremes among litter characteristics of native species. Alnus glutinosa is a N-fixing (actinorhizal) species with soft leaves, high N concentration, and low concentrations of recalcitrant and secondary compounds, while Q. robur is a non-N-fixing species and has tough leaves with low N concentration and high concentration of structural and defensive compounds [40]. Acacia melanoxylon is an evergreen N-fixing (legume) species commonly found in the riparian vegetation of streams flowing through forests invaded by Acacia species. Acacia melanoxylon litter has tough phyllodes (i.e., leaf-like modified petioles) with high N concentration [37, 41].
Fresh fallen leaves (Al. glutinosa and Q. robur) or phyllodes (Ac. melanoxylon) were collected immediately after abscission in October 2016, from several trees in a small tree stand for each species located in Serra da Lousã. Trees within each stand had similar age and seemed to be exposed to similar environmental and edaphic conditions. Litter of each tree species was mixed, air-dried at room temperature, and stored in the dark until used.
Three sets of air-dried litter per tree species were oven-dried (105 °C for 48 h), ground to a fine powder (< 0.5-mm size; Retsch MM 400, Haan, Germany), and stored in the dark until nutrient analysis. The initial polyphenols [42], P [42], lignin [43], carbon, and N (Thermo Fisher Scientific Inc., CNH auto analyzer IRMS Thermo Delta V advantage with a Flash EA, 1112 series, Waltham, USA) concentrations were determined from a portion of oven-dried powder (105 °C for 48 h). Results were expressed as percentage of dry mass (DM). Litter toughness was determined using a penetrometer by measuring the mass (g) necessary for a pin (0.49-mm diameter) to punch a hole through the litter (n = 10) [42]. The specific litter area (SLA) was determined for 12-mm litter discs (n = 10), after being oven-dried at 105 °C for 24 h, as the ratio of litter discs area to mass (mm2/mg).
Litter Bags and Litter Decomposition
Air-dried litter was weighed in 3-g portions (± 0.01 g), sprayed with distilled water to turn them soft, and enclosed in fine-mesh bags (0.5-mm mesh size). On the 21st of November 2016, 12 litter bags of each litter species were tied to iron bars secured in the streambed and submerged in each stream site (12 bags × 3 litter species × 6 streams = 216 bags total). After 14, 28, 63, and 98 days of incubation, 3 replicate litter bags from each litter species were retrieved from the streams, enclosed individually in zip lock bags, and transported in a cold box to the laboratory. In the laboratory, litter was rinsed with distilled water over a 0.5-mm mesh sieve to remove sediments, and two sets of five litter discs (12-mm diameter) were cut with a cork borer from individual litter and used to determine fungal biomass and aquatic hyphomycetes sporulation rates and species richness. The remaining litter material was oven-dried (105 °C for 48 h), weighed (± 0.1 mg) to determine DM remaining, and ground to a fine powder (Retsch MM 400, Haan, Germany). A subsample of litter powder was oven-dried (105 °C for 24 h), weighed (± 0.1 mg) to determine DM, ignited (550 °C for 4 h), reweighed (± 0.1 mg) to determine ash mass, and used to estimate ash-free dry mass (AFDM). Dry mass and AFDM of subsamples were used to estimate the AFDM remaining of the sample, considering the mass of the discs extracted for microbial determinations, and results were expressed as percentage of initial AFDM. An additional set of five litter bags of each litter species was prepared as described for the other samples and used to determine the initial air-dry mass to initial AFDM conversion factor.
Water Physical and Chemical Characteristics
During the study period (98 days), stream water temperature was continuously measured using submerged data loggers (Hobo Pendant UA-001-08, Onset Computer Corp., MA, USA). In the beginning of the experiment and on each sampling date (n = 5), conductivity, pH, and dissolved O2 concentration were measured in situ with field probes (WTW, Weilheim, Germany). On the same occasions, stream water was collected, filtered through glass microfiber filters (47-mm diameter, 0.7-μm pore size; Whatman GF/F, GE Healthcare UK Limited, Little Chalfont, UK) into acid washed plastic bottles, and transported in a cool box to the laboratory. A portion of the filtered water (~ 50 mL) was stored at − 20 °C and used to determine nitrites, nitrates, and ammonium concentrations by the colorimetric method (AA3 Bran + Luebbe autoanalyzer; SEAL Analytical, Norderstedt, Germany).
Fungal Biomass
One set of five litter discs was frozen at − 20 °C until used to determine ergosterol concentrations as a surrogate for fungal biomass [42]. Litter discs were lyophilized overnight, weighed (± 0.1 mg) to determine discs DM (converted into AFDM using the ash fraction of discs used to induce sporulation; see below), and placed in tightly closed tubes with 10 mL of alkaline methanol (8 g KOH/L). Lipids were extracted from litter discs by heating the tubes at 80 °C in a water bath, during 30 min. Lipids were purified by solid-phase extraction (Waters Sep-Pak © Vac RC tC18 cartridges; Waters Corp., Milford, MA, USA), and ergosterol was eluted in isopropanol. Ergosterol was quantified at 282 nm by high-performance liquid chromatography (HPLC, Dionex DX-120, Sunnyvale, CA, USA) using a Thermo Scientific Syncronis C18 column (Thermo, Waltham, MA, USA). The system ran continuously with HPLC-grade methanol flowing at 1.4 mL/min (33 °C) [42]. Ergosterol was converted into fungal biomass assuming a 5.5 μg ergosterol/mg fungal DM [44], and results were expressed as mg fungal biomass/g litter AFDM.
Sporulation by Aquatic Hyphomycetes
Conidial production by aquatic hyphomycetes was induced in the laboratory by aeration of a set of five litter discs placed into 100-mL Erlenmeyer flasks containing 25 mL of filtered water from the stream of origin [42]. Flasks were incubated for 48 h at 18 °C, under a 12 h light and 12 h dark regime. Then, conidial suspensions were transferred into 50-mL Falcon tubes, preserved with 2 mL of formaldehyde (37%), and stored in the dark until processed. Litter discs were oven-dried (105 °C for 48 h), weighed, ignited (500 °C for 4 h), and reweighed to allow determination of litter discs AFDM.
When preparing the samples for conidia identification and counting, conidial suspensions were gently shaken, mixed with 100 μL of 0.5% Triton X-100, and appropriate aliquots of the suspensions were filtered through cellulose nitrate filters (25-mm diameter, 5-μm pore size; Sartorius Stedim Biotech GmbH, Goettingen, Germany). Filters were stained with 0.05% trypan blue in 60% lactic acid, and conidia were identified and counted under a microscope at 200 × magnification (Leica, DM1000, Wetzlar, Germany) [42]. For each sample, at least 200 spores were counted, when possible, and identified to species level using an identification key to the common temperate species of aquatic hyphomycetes [42]. In some samples, it was not possible to identify 200 spores because conidia production was extremely low (e.g., on Ac. melanoxylon litter after 14 days of stream immersion). Sporulation rates of each aquatic hyphomycete species and sample were calculated using the formula: (((G × E / F) × C / D) × 24 / B) / A, were A is the discs AFDM (mg), B is the incubation time (h), C is the total suspension volume (mL), D is the suspension volume filtered (mL), E is the total number of fields in the filter, F is the number of fields surveyed, and G is the number of conidia counted. Total sporulation rates and sporulation rates of each aquatic hyphomycete species were expressed as the number of conidia released/mg litter AFDM/day and aquatic hyphomycetes species richness as the number of species/sample.
Statistical Analysis
Data normality was checked with the Shapiro-Wilk test (or D’Agostino & Pearson test in the case of polyphenol concentration and C:N ratio), and the homogeneity of variances was checked with Levene’s test. Data was transformed when necessary to meet the assumptions of normality and homoscedasticity of analysis of variance (ANOVA) [45].
Initial litter characteristics (asin(sqrt(x)) and log(x) transformed for P and toughness, respectively) were compared among litter species by one-way ANOVAs, followed by Tukey’s honest significant difference (HSD) tests, when significant differences were detected [45]. Stream water characteristics (Box-Cox transformed for pH, and NO2–-N, NO3–-N, NH4+-N, and dissolved inorganic nitrogen (DIN) concentrations) were compared between stream types and among sampling dates by two-way ANOVAs [45].
Litter decomposition rates (k, /d) were calculated by linear regression of fraction AFDM remaining (ln(x) transformed) over time (t, days) (which assumes a negative exponential decay): ln(fraction AFDM) = − k × t. To account for temperature differences among streams, litter decomposition rates were calculated in degree days (k, /dd), by replacing time with the sum of the accumulated mean daily temperature by the sampling day.
The mean values of litter decomposition, fungal biomass, and aquatic hyphomycete sporulation rates and species richness, for each litter species per stream and sampling date (all Box-Cox transformed), were compared between stream types and among litter species and time by three-way ANOVAs, followed by Tukey’s HSD tests, when significant differences were detected [45]. In the analysis, each stream within each stream type was considered a replicate (i.e., Maior, Cerdeira, and Candal were the replicate streams for the native stream type, and Sotão, Fiscal, and Piedade were the replicate streams for the invaded stream type) for each litter species (3 litter species × 3 replicate streams × 2 stream types × 4 sampling dates).
The aquatic hyphomycete community structure, based on the mean values of conidia production by each aquatic hyphomycete species for each litter species per stream and sampling date, was assessed by nonmetric multidimensional scaling (NMDS) ordination and unweighted pair group method with arithmetic mean (UPGMA) cluster analysis. Aquatic hyphomycete communities were compared among litter species and time, and between stream types by permutational multivariate analysis of variance (PERMANOVA), followed by pair-wise comparison tests [46]. Since PERMANOVA is sensitive to differences in dispersion within groups, a PERMDISP analysis was performed to test the homogeneity of the multivariate dispersions [46]. Similarity of percentage analysis (SIMPER) was also performed to identify the aquatic hyphomycete species that most contributed to the dissimilarities among litter species and between stream types. Samples of Ac. melanoxylon litter from day 14 were not considered in the analyses because aquatic hyphomycete sporulation rates were extremely low (0 in some cases) and thus affected the ordination of the remaining samples in the analysis. Prior to all analyses, data was transformed (log(x + 1)) and converted into a Bray-Curtis similarity matrix. Multivariate analysis (NMDS, cluster, PERMANOVA, PERMDISP, and SIMPER) were performed on PRIMER 6 (v6.1.16) & PERMANOVA+ (v1.0.6; Primer-E Ltd, Plymouth, UK) software package (Primer-E Ltd, Plymouth, UK). The other statistical analyses were performed on STATISTICA 8.0 for Windows (StatSoft, Inc., Tulsa, OK, USA).
Results
Stream Water Characteristics
During the study period, stream water was cold, circumneutral, well oxygenated, had low conductivity and low nutrient concentrations (Table 2). Stream water conductivity, and NO3–-N and DIN concentrations were higher in invaded than in native streams (two-way ANOVAs, p < 0.001; Table S1). For water temperature, pH, and dissolved O2, NO2–-N, and NH4+-N concentrations, no significant differences were found between stream types (two-way ANOVAs, p ≥ 0.060; Table S1).
Initial Litter Characteristics
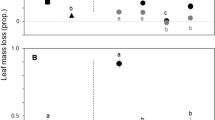
Alnus glutinosa litter had the highest N concentration and SLA, intermediate P concentration, and the lowest C:N ratio and toughness (Tukey’s HSD test, p ≤ 0.014) (Fig. 2). Quercus robur litter had the highest polyphenols and P concentrations and C:N ratio, and the lowest N concentration (Tukey’s HSD test, p < 0.001) (Fig. 2). Acacia melanoxylon litter had the lowest P concentration and SLA, intermediate N concentration and C:N ratio, and the highest toughness (Tukey’s HSD test, p ≤ 0.004) (Fig. 2). Litter species did not significantly differ in lignin and carbon concentrations (one-way ANOVAs, p = 0.147 and p = 0.145, respectively; Table S2) (Fig. 2).
Initial litter polyphenols (a), phosphorus (b), nitrogen (c), lignin (d) and carbon (e) concentrations, C:N (carbon:nitrogen) ratio (f), toughness (g), and SLA (specific litter area) (h) of the three species, Alnus glutinosa (Ag), Quercus robur (Qr), and Acacia melanoxylon (Am), used in the litter decomposition experiment. Values are means ± SE. Species with different letters are significantly different (one-way ANOVA followed by Tukey’s HSD test, p < 0.05)
Litter Decomposition
Litter mass remaining decreased exponentially over the incubation time (Fig. 3). After 98 days, AFDM remaining in Al. glutinosa ranged 27–39% in native and 23–34% in invaded streams (Fig. 3a, d), in Q. robur it ranged 49–62% in native and 45–59% in invaded streams (Fig. 3b, e), and in Ac. melanoxylon it ranged 65–69% in native and 58–65% in invaded streams (Fig. 3c, f). This translated into mean (across streams) litter decomposition rates of 0.0014 and 0.0015 /dd for Al. glutinosa litter, 0.0007 and 0.0008 /dd for Q. robur, and 0.0005 and 0.0006 /dd for Ac. melanoxylon in native and invaded streams, respectively (Table 3). Litter decomposition rates significantly differed among litter species and between stream types (three-way ANOVA, p ≤ 0.006; Table S3). Litter decomposition rates were in the order: Al. glutinosa > Q. robur > Ac. melanoxylon (Tukey’s HSD test, p < 0.001) and were significantly higher in invaded than in native streams (p ≤ 0.006) (Table 3, Table S3). No significant interaction was found between litter species and stream type (three-way ANOVA, p = 0.991; Table S3).
Fungal Biomass
Fungal biomass in Al. glutinosa and Q. robur litter generally increased until a peak was attained and then decreased, while in Ac. melanoxylon litter it increased throughout the incubation period (Fig. 4). Maximum fungal biomass in Al. glutinosa litter ranged 60–70 mg/g AFDM in native and 76–150 mg/g AFDM in invaded streams (Fig. 4a, d), in Q. robur it ranged 137–164 mg/g AFDM in native and 94–186 mg/g AFDM in invaded streams (Fig. 4b, e), and in Ac. melanoxylon it ranged 77–95 mg/g AFDM in native and 147–159 mg/g AFDM in invaded streams (Fig. 4c, f). Fungal biomass was significantly affected by litter species, stream type, and by the interaction between both factors (three-way ANOVA, p ≤ 0.019; Table S3). In native streams, fungal biomass was higher in Q. robur than in Al. glutinosa and Ac. melanoxylon litter (Tukey’s HSD test, p < 0.001), with no significant differences between the two latter species (p = 0.999). In invaded streams, fungal biomass was lower in Al. glutinosa than in Q. robur litter (Tukey’s HSD test, p = 0.037), with no significant differences between Al. glutinosa and Ac. melanoxylon (Tukey’s HSD test, p = 0.905) and between Q. robur and Ac. melanoxylon (p = 0.327). Also, fungal biomass in Ac. melanoxylon litter was higher in invaded than in native streams (Tukey’s HSD test, p = 0.007), while in Al. glutinosa and Q. robur litter fungal biomass did not differ between stream types (Tukey’s HSD test, p = 0.156 and p = 0.360, respectively).
Aquatic Hyphomycetes Sporulation Rates and Species Richness
Dynamics of sporulation rates by aquatic hyphomycetes were similar to those of fungal biomass. Sporulation rates attained a peak during the decomposition of Al. glutinosa and Q. robur litter, while it increased throughout the incubation period in Ac. melanoxylon litter (Fig. 5). Maximum sporulation rates in Al. glutinosa litter ranged 1125–2504 conidia/mg AFDM/d in native and 1828–2761 conidia/mg AFDM/d in invaded streams (Fig. 5a, d), in Q. robur it ranged 574–2117 conidia/mg AFDM/d in native and 2512–3324 conidia/mg AFDM/d in invaded streams (Fig. 5b, e), and in Ac. melanoxylon it ranged 408–896 conidia/mg AFDM/d in native and 539–808 conidia/mg AFDM/d in invaded streams (Fig. 5c, f). Aquatic hyphomycetes sporulation rates significantly differed among litter species and between stream types (three-way ANOVA, p ≤ 0.003; Table S3). Aquatic hyphomycetes sporulation rates were lower in Ac. melanoxylon than in Al. glutinosa and Q. robur litter (Tukey’s HSD test, p < 0.001), with no significant differences between Al. glutinosa and Q. robur (Tukey’s HSD test, p = 0.973). Also, aquatic hyphomycetes sporulation rates were higher in invaded than in native streams (three-way ANOVA, p = 0.003; Table S3). No significant interaction was found between litter species and stream type (three-way ANOVA, p = 0.453; Table S3).
Aquatic hyphomycete species richness generally increased sharply over the first 3 weeks of litter immersion and then roughly stabilized (Fig. 6). Maximum species richness in Al. glutinosa litter ranged 12–13 species in native and 10–13 in invaded streams (Fig. 6a, d), in Q. robur it ranged 9–13 species in native and 8–15 species in invaded streams (Fig. 6b, e), while in Ac. melanoxylon it ranged 8–9 species in native and 7–9 species in invaded streams (Fig. 6c, f). Aquatic hyphomycetes species richness significantly differed among litter species (three-way ANOVA, p < 0.001; Table S3), with higher values for Al. glutinosa and Q. robur than for Ac. melanoxylon litter (Tukey’s HSD test, p < 0.001), with no significant difference between the two former species (p = 0.310). No significant differences were found for stream type or the interaction between litter species and stream type (three-way ANOVA, p = 0.584 and p = 0.911, respectively; Table S3).
Aquatic hyphomycetes (AH) sporulation rates associated with Alnus glutinosa (a, d), Quercus robur (b, e), and Acacia melanoxylon (c, f) litter incubated in three native and three invaded streams in Serra da Lousã (central Portugal), over 98 days. Values are means ± SE (in some cases, SE bars are too small to be seen)
Aquatic hyphomycete (AH) species richness associated with Alnus glutinosa (a, d), Quercus robur (b, e), and Acacia melanoxylon (c, f) litter incubated in three native and three invaded streams in Serra da Lousã (central Portugal), over 98 days. Values are means ± SE (in some cases, SE bars are too small to be seen)
Community Structure of Aquatic Hyphomycetes
During the decomposition experiment, a total of 26 aquatic hyphomycete species were recorded across treatments (Table S4). Aquatic hyphomycetes species richness in Al. glutinosa litter ranged 19–20 species in native streams (24 species total) and 14–18 species in invaded streams (20 species total), in Q. robur it ranged 18–19 species in native streams and 11–23 in invaded streams (23 species total in both stream types), and in Ac. melanoxylon it ranged 10–17 in native streams (19 species total) and 10–15 in invaded streams (15 species total) (Table S4). The community structure of aquatic hyphomycetes significantly differed among all litter species (pair-wise comparison tests, p < 0.001) and between stream types (three-way multivariate PERMANOVA, p < 0.001; Table S5) (Fig. 7). The dissimilarity of aquatic hyphomycete communities between Al. glutinosa and Q. robur litter was, however, mild (two-way SIMPER analysis, average dissimilarity = 40%; Table S6). Stronger dissimilarities in aquatic hyphomycete communities were found between Al. glutinosa and Ac. melanoxylon litter (average dissimilarity = 53%; Table S7) and between Q. robur and Ac. melanoxylon litter (average dissimilarity = 50%; Table S8), with Tetrachaetum elegans Ingold and Articulospora tetracladia Ingold contributing the most to the separation between litter species (Fig. 8). Dissimilarities in aquatic hyphomycete communities between native and invaded streams were also mild (two-way SIMPER analysis, average dissimilarity = 42%; Table S9). Accordingly with the previous results, the cluster analysis showed that aquatic hyphomycetes communities were primarily separated by litter type, with communities on Al. glutinosa and Q. robur litter (native species) clearly differing from those on Ac. melanoxylon (invasive species), and to a lesser extent by litter species and stream type (Fig. 9).
Aquatic hyphomycetes (AH) relative contribution (based on conidial production) associated with Alnus glutinosa (a), Quercus robur (b), and Acacia melanoxylon (c) litter incubated in three native and three invaded streams in Serra da Lousã (central Portugal), over 98 days. Only species with > 8% of relative contribution in at least one treatment are represented
Discussion
The invasion of native deciduous riparian forests by non-native N-fixing Acacia species is expected to alter the intrinsic quality of litter inputs to streams and alter water N concentration, thereby affecting the functioning and structure of stream ecosystems [8, 37]. In this study, litter decomposition rates in fine-mesh bags and associated fungal decomposer activity and community structure differed between native and invaded streams, probably due to differences in water N concentrations, and among litter species, probably due to differences in the litter physical and chemical characteristics.
Litter Decomposition Was Slightly Faster in Invaded than in Native Streams
In this study, litter decomposition rates in fine-mesh bags were slightly, but significantly, higher in invaded than in native streams, likely due to the higher water N concentration in the former streams. This agrees with the findings from a previous laboratory experiment where we simulated the conditions of streams flowing through native deciduous forests and streams flowing through forests invaded by Acacia species [37]. As observed before, the small magnitude of the effect can most likely be attributed to water N concentrations being still in the oligotrophic range, despite the 5× higher NO3–-N and 2× higher DIN concentration in invaded than in native streams [34]. In invaded streams basins, agricultural activity was absent, urban and industrial settlements were residual, and Acacia species stands occupied more than 10% of the basin area and covered more than 94% of the riparian zone of each stream site (Table 1); thus, the higher water N concentration was likely a result of the invasion of riparian forests by N-fixing Acacia species. Indeed, it has been shown previously that increases in land cover by N-fixing species can increase N concentration in streams through two main non-exclusive pathways: (i) the mineralization of litter N by aquatic microbes during the decomposition of the N-rich litter inputs provided to streams by the surrounding riparian vegetation, and (ii) the surface and groundwater runoff of N-rich leachates (from litter decomposition in the soil) and N-rich root exudates from the surrounding riparian areas [13,14,15,16,17,18].
Increases in water N concentration, especially when background N concentration is low, generally accelerate decomposition rates [32,33,34,35, 37], due to a stimulation of microbial decomposer activity on litter [e.g., 22, 32, 36]. In this study, fungal biomass and aquatic hyphomycete sporulation rates were generally higher in invaded than in native streams, suggesting that microbial growth and reproduction were likely stimulated by the higher water N concentrations in invaded streams. This agrees with previous studies showing that even small increments in water N concentration can stimulate microbial decomposer activity on litter [22, 37, 47,48,49]. Nevertheless, we could hypothesize that in invaded streams, litter decomposition rates may increase further if water N concentration continues to increase as the invasion of native forests by Acacia species progresses through the years, and the area covered by Acacia species increases in the invaded stream basins [8].
In this study, water conductivity was also higher in invaded than in native streams, suggesting that ions other than NO3–-N could also be available in slightly higher concentrations in the former stream type, which could also affect the activity of microbial decomposers on litter decomposition. For instance, increases in water P-PO4– concentration have been shown to stimulate microbial activity on litter and thus accelerate litter decomposition in streams [36, 47]. However, previous studies comparing streams flowing through non-invaded forests and streams flowing through forests invaded by other N-fixing species found no significant differences in P concentrations between stream types [16, 50, 51], while others found a negative correlation between nitrate and phosphate concentrations [18]. Thus, although we did not measure P concentration in water, it could be anticipated that its concentration probably does not differ substantially between stream types, and the slightly higher litter decomposition rates observed in invaded streams occurred most likely due to higher water N concentration.
Water temperature, although not significantly different between stream types, was also slightly higher in invaded than in native streams, which could have contributed to the stimulation of microbial activity. Indeed, increased water temperature has been reported before to stimulate microbial activity on litter decomposition [49, 52, 53]. However, the difference in mean temperature between stream types was < 1 °C, which was likely not enough to induce a significant stimulation of microbial activity. In fact, a whole-stream manipulative experiment carried out at Candal stream (native stream), where water temperature was increase by ~ 3 °C above ambient temperature over ~ 1 year, showed no noticeable effects of warming on decomposer activity [54, 55]. Also, since litter decomposition rates were normalized by water temperature and expressed in degree days, the observed differences in decomposition rates between stream types cannot be directly associated with differences in water temperature [36].
We should also take into consideration that, even though this study focused mostly in assessing the effects of Acacia species invasion on the activity of litter associated aquatic hyphomycetes, other microorganisms (e.g., bacteria, yeasts, zoosporic fungi, meiofauna) and small macroinvertebrates that could pass through the 0.5-mm mesh and enter into the litter bags may also have contributed to litter decomposition, in both stream types. For instance, zoosporic fungi have been associated with early stages of litter decomposition [56], while bacteria generally contributed more actively to litter decomposition at later stages, when litter has been partially decomposed by aquatic hyphomycetes [57, 58]. Meiofauna and small-sized macroinvertebrates have also been found to participate on litter decomposition [59]. Nevertheless, aquatic hyphomycetes have been widely recognized as the primary decomposers of litter in streams, especially during the early stages of the process [19, 32, 58]. Indeed, a recent study comparing the contributions of biotic communities (microbes, meiofauna, and macrofauna) to litter decomposition found that microbes, especially aquatic hyphomycetes, were the primary litter decomposers [59]. Thus, we may assume that, in this study, litter decomposition in fine-mesh bags was primarily carried out by aquatic hyphomycetes.
Litter Decomposition Was Slower for Acacia melanoxylon than for Native Species
Litter decomposition rates in fine-mesh bags differed among litter species in the order: Ac. melanoxylon < Q. robur < Al. glutinosa, as observed in a recent laboratory study [37]. Differences in decomposition rates among species were expected since they differed in litter physical and chemical characteristics. Previous studies have shown that microbes generally colonize and decompose faster soft litter with high nutrient concentrations and low concentrations of structural (e.g., lignin) and secondary recalcitrant compounds (e.g., polyphenols) [60,61,62,63,64]. The microbial colonization and decomposition of Al. glutinosa and Q. robur litter have been well studied as these species represent extremes in litter characteristics among the most common native species present in the riparian vegetation of European streams. As observed before, Al. glutinosa litter was soft and had high N, and low polyphenol concentrations and low C:N ratio, and consequently decomposed faster than Q. robur litter, which was tough and had low N, and high polyphenol concentrations and high C:N ratio [10, 22, 40, 47, 65, 66]. Decomposition rates for Ac. melanoxylon litter were the slowest, which agrees with other multi-species studies where Ac. melanoxylon generally was the slowest decomposing species [37, 41, 67,68,69]. The lower decomposition rates of Ac. melanoxylon litter have been often attributed to its high toughness [37, 41]. Acacia melanoxylon phyllodes have a very thick and tough cuticle, with veins placed very closely and parallel to each other, which makes this litter species a very hard substrate for microbial colonization and activity [37, 41]. Indeed, litter toughness has been shown before to be an important factor controlling decomposer activity and litter decomposition in streams [37, 41, 64, 70]. Nevertheless, it was interesting to note that, even though Ac. melanoxylon litter had the lowest aquatic hyphomycete sporulation rate, its fungal biomass was as high as that in Al. glutinosa, suggesting an efficient microbial colonization. High fungal biomass associated with Ac. melanoxylon litter was also found in insular streams [41]. The accumulation of high biomass in Ac. melanoxylon likely occurs because its high lignin concentration and toughness ensure high substrate stability, thus allowing the accumulation of fungal biomass for a longer time; it is common to find higher fungal biomass in more recalcitrant litter species [41, 71]. On the contrary, aquatic hyphomycete sporulation rates were lower in Ac. melanoxylon than in Al. glutinosa and Q. robur litter. It has been often found that reproductive activity by aquatic hyphomycetes can be more sensitive to changes in substrate quality than fungal growth [22, 72]. This suggests that, although microbial decomposers can fully colonize Ac. melanoxylon litter, their ability to reproduce is impaired, which may result in decreases in the inoculum potential (i.e., number of conidia in transport) in streams receiving high litter inputs of Ac. melanoxylon stands.
The slow decomposition of Ac. melanoxylon litter may not be, however, extrapolated to other Acacia species since they can differ in litter characteristics. For instance, Ac. dealbata has bipinnate leaves composed by a high number of very small leaflets (< 1 mm wide) that can increase the leaf area:volume ratio and facilitate microbial colonization, while the small area of leaflets may limit fungal mycelial growth. Although Ac. dealbata was the dominant species in the Acacia stands and in the riparian vegetation of invaded streams, it was not used in this study for technical reasons. The small leaflets detach very easily from the leaves and can escape from fine-mesh bags (personal observation), which would lead to an overestimation of litter decomposition rates. Although finer mesh bags could have been used, these are more easily clogged by fine sediments and biofilm development, which would limit water circulation inside the bags and jeopardize the supply of oxygen and nutrients to microbes, leading to an underestimation of microbial activity and litter decomposition. Indeed, in laboratory microcosms simulating stream conditions, Ac. dealbata and Ac. melanoxylon litter decomposed at similar low rates, despite higher microbial activity found in the former species [37].
We must also take into consideration that, in both stream types, the higher microbial activity on the native Al. glutinosa and Q. robur litter, and their faster decomposition, compared to the invasive Ac. melanoxylon, may have resulted from home-field advantage as a result from microbial decomposer communities being more specialized in degrading native home-derived high-quality litter (native species) than the non-native recalcitrant litter. For instance, a previous study addressing the effects of home-field advantage on litter decomposition and associated microbial decomposers found that stream microbes processed more rapidly conifer litter from the home region than ‘foreign’ broadleaf litter [73]. It is possible that, with time, microbial communities in invaded streams will adapt to the recalcitrant Ac. melanoxylon litter and decomposition rates may slightly increase, although litter recalcitrance will pose a challenge.
Aquatic Hyphomycete Community Structure Differed Between Native and Invaded Streams and Among Litter Species
The aquatic hyphomycete community structure based on conidia production differed between stream types, thus confirming the preliminary results from a previous laboratory experiment [37]. The observed differences between stream types were not surprising because several aquatic hyphomycete species are known to be sensitive to differences in nutrient concentrations (e.g., N) in stream water [32, 36, 37, 74]. For instance, Anguillospora filiformis Greath. and T. elegans decreased their contribution to the community composition from native to invaded streams, suggesting that both species may be poor competitors in nutrient-enriched conditions [32, 36]. On the other hand, Hydrocina chaetocladia Scheuer contributed more to the community composition of invaded streams, suggesting that it is favored by increases in nutrient availability, thus increasing conidia production [36]. Nevertheless, aquatic hyphomycete species richness was very similar in both stream types: 25 and 23 species were recorded in native and invaded streams, respectively, which was near the total number of species recorded during the study period (26 species). This was not expected since aquatic hyphomycete species richness often increases with moderate water nutrient enrichment [22, 32, 75, 76]. Additionally, aquatic hyphomycete species richness can also positively respond to changes in resource species diversity [7, 24,25,26]; it is easy to assume that species diversity of benthic litter standing stocks is lower in invaded than in native streams as a result from decreases in riparian species diversity. Therefore, it is possible that the increase in nutrient concentrations in stream water and the decrease in resource species richness counteracted each other, leading to no strong differences in aquatic hyphomycete species richness between stream types.
The aquatic hyphomycete species richness and community structure differed among litter species, also confirming the results from a previous laboratory study [37]. Aquatic hyphomycete species richness was highest for native Al. glutinosa (25 species) and Q. robur (24 species) litter and lowest for the invasive Ac. melanoxylon (19 species). This was expected since microbial decomposers are sensitive to the physical and chemical characteristics of resources [22, 27, 28, 75], probably due to differences in enzymatic capabilities [28, 77, 78] and stoichiometric requirements [79]. Interestingly, the relative contribution of the dominant aquatic hyphomycete species to the community also differed among litter species: A. tetracladia and T. elegans decreased their contribution from high quality to low quality litter species (Al. glutinosa > Q. robur > Ac. melanoxylon), while H. chaetocladia increased. Articulospora tetracladia has been shown to be more abundant in Al. glutinosa litter than in more recalcitrant resources (e.g., Eucalyptus globulus Labill., Platanus sp.) [75], while T. elegans can be more abundant in Al. glutinosa than in Q. robur [62]. On the other hand, H. chaetocladia has been shown to be more abundant in Q. robur than in Al. glutinosa [22, 71, 75]. Nevertheless, the aquatic hyphomycetes community structure on both native species differed less between them than when compared with that on Ac. melanoxylon. This suggests that the replacement of native forests with a high diversity of trees species by a homogeneous stand of Acacia species will most likely alter the community structure of microbial decomposers in streams due to a decrease in the diversity of resources, as observed in the case of other forest replacements [25, 29, 31]. Additionally, since the characteristics of Ac. melanoxylon litter are very dissimilar from those of native species, their effects on stream ecosystems will be much stronger [80].
Conclusion
Overall, our results suggest that the invasion of native deciduous riparian forests by N-fixing Acacia species will affect litter decomposition rates and the activity, species richness, and community structure of microbial decomposers in streams. Decomposition rates of given litter species will be faster in invaded than native streams, probably due to an increase in the N concentration in water, which will stimulate the activity of microbial decomposers on litter. Additionally, the replacement of native forests with high tree diversity by plant communities dominated by Acacia species will reduce the diversity and quality of resources entering streams, thus decreasing the richness and activity of aquatic microbial decomposers, and consequently litter decomposition rates at the stream level. Since the reduction of litter decomposition rates due to changes in litter characteristics (i.e., replacement of fast decomposing native species by slow decomposition Ac. melanoxylon litter) is more pronounced than the stimulation of litter decomposition due to increases in dissolved N concentration (invaded vs. native streams), the overall litter decomposition potential of invaded streams will likely decrease, consequently affecting nutrient cycling and aquatic food webs. Changes in the structure and functioning of detrital food webs may have consequences for the services provided by stream ecosystems to the human society (e.g., good water quality, secondary production). Therefore, the protection of native riparian areas and/or the recovery of the native vegetation in invaded riparian areas should be a priority to ensure that streams receive a high diversity of litter from different tree species to sustain aquatic food webs, an issue that must receive immediate attention considering the high susceptibility of such habitats to invasion [81].
References
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499. https://doi.org/10.1126/science.277.5325.494
Vilà M, Hulme P (2017) Impact of biological invasions on ecosystem services. Springer International Publishing, Switzerland
Castro-Díez P, Vaz AS, Silva JS, van Loo M, Alonso Á, Aponte C, Bayón Á, Bellingham PJ, Chiuffo MC, DiManno N, Julian K, Kandert S, La Porta N, Marchante H, Maule HG, Mayfield MM, Metcalfe D, Monteverdi MC, Núñez MA, Ostertag R, Parker IM, Peltzer DA, Potgieter LJ, Raymundo M, Rayome D, Reisman-Berman O, Richardson DM, Roos RE, Saldaña A, Shackleton RT, Torres A, Trudgen M, Urban J, Vicente JR, Vilà M, Ylioja T, Zenni RD, Godoy O (2019) Global effects of non-native tree species on multiple ecosystem services. Biol Rev 94:1477–1501. https://doi.org/10.1111/brv.12511
Castro-Díez P, Alonso Á (2017) Effects of non-native riparian plants in riparian and fluvial ecosystems: a review for the Iberian Peninsula. Limnetica 36:525–541. https://doi.org/10.23818/limn.36.19
Lorenzo P, González L, Reigosa MJ (2010) The genus Acacia as invader: the characteristic case of Acacia dealbata Link in Europe. Ann For Sci 67:101. https://doi.org/10.1051/forest/2009082
Souza-Alonso P, Rodríguez J, González L, Lorenzo P (2017) Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann For Sci 74:55. https://doi.org/10.1007/s13595-017-0651-0
Ferreira V, Castela J, Rosa P, Tonin AM, Boyero L, Graça MAS (2016) Aquatic hyphomycetes, benthic macroinvertebrates and leaf litter decomposition in streams naturally differing in riparian vegetation. Aquat Ecol 50:711–725. https://doi.org/10.1007/s10452-016-9588-x
Ferreira V, Figueiredo A, Graça MAS, Marchante E, Pereira A (2021) Invasion of temperate deciduous broadleaf forests by N-fixing tree species—consequences for stream ecosystems. Biol Rev. https://doi.org/10.1111/brv.12682
Graça MAS, Pozo J, Canhoto C, Elosegi A (2002) Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. ScientificWorldJournal 2:1173–1185. https://doi.org/10.1100/tsw.2002.193
Hladyz S, Åbjörnsson K, Giller PS, Woodward G (2011) Impacts of an aggressive riparian invader on community structure and ecosystem functioning in stream food webs. J Appl Ecol 48:443–452. https://doi.org/10.1111/j.1365-2664.2010.01924.x
Martínez A, Larrañaga A, Pérez J, Descals E, Basaguren A, Pozo J (2013) Effects of pine plantations on structural and functional attributes of forested streams. For Ecol Manage 310:147–155. https://doi.org/10.1016/j.foreco.2013.08.024
Ferreira V, Koricheva J, Pozo J, Graça MAS (2016) A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For Ecol Manage 364:27–38. https://doi.org/10.1016/j.foreco.2016.01.002
Compton JE, Church MR, Larned ST, Hogsett WE (2003) Nitrogen export from forested watersheds in the Oregon Coast Range: the role of N2-fixing Red Alder. Ecosystems 6:773–785. https://doi.org/10.1007/s10021-002-0207-4
Goldstein CL, Williard KWJ, Schoonover JE (2009) Impact of an invasive exotic species on stream nitrogen levels in Southern Illinois. J Am Water Resour Assoc 45:664–672. https://doi.org/10.1111/j.1752-1688.2009.00314.x
Shaftel RS, King RS, Back JA (2012) Alder cover drives nitrogen availability in Kenai lowland headwater streams, Alaska. Biogeochemistry 107:135–148. https://doi.org/10.1007/s10533-010-9541-3
Wiegner TN, Hughes F, Shizuma LM, Bishaw DK, Manuel ME (2013) Impacts of an invasive N2-fixing tree on Hawaiian stream water quality. Biotropica 45:409–418. https://doi.org/10.1111/btp.12024
Singh G, Mejía NMM, Williard KWJ, Schoonover JE, Groninger JW (2019) Watershed vulnerability to invasive N2-fixing autumn olive and consequences for stream nitrogen concentrations. J Environ Qual 48:614–623. https://doi.org/10.2134/jeq2018.09.0343
Stewart SD, Young MB, Harding JS, Horton TW (2019) Invasive nitrogen-fixing plant amplifies terrestrial-aquatic nutrient flow and alters ecosystem function. Ecosystems 22:587–601. https://doi.org/10.1007/s10021-018-0289-2
Hieber M, Gessner MO (2002) Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026–1038. https://doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2
Baldy V, Gobert V, Guerold F, Chauvet E, Lambrigot D, Charcosset J-Y (2007) Leaf litter breakdown budgets in streams of various trophic status: effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshw Biol 52:1322–1335. https://doi.org/10.1111/j.1365-2427.2007.01768.x
Gulis V, Su R, Kuehn KA (2019) Fungal decomposers in freshwater environments. In: Hurst CJ (ed) The structure and function of aquatic microbial communities. Advances in Environmental Microbiology. Springer International Publishing, Switzerland, pp 121–155
Ferreira V, Gulis V, Graça MAS (2006) Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149:718–729. https://doi.org/10.1007/s00442-006-0478-0
Ferreira V, Graça MAS (2016) Effects of whole-stream nitrogen enrichment and litter species mixing on litter decomposition and associated fungi. Limnologica 58:69–77. https://doi.org/10.1016/j.limno.2016.03.002
Rajashekhar M, Kaveriappa KM (2003) Diversity of aquatic hyphomycetes in the aquatic ecosystems of the Western Ghats of India. Hydrobiologia 501:167–177. https://doi.org/10.1023/A:1026239917232
Lecerf A, Dobson M, Dang CK, Chauvet E (2005) Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 146:432–442. https://doi.org/10.1007/s00442-005-0212-3
Laitung B, Chauvet E (2005) Vegetation diversity increases species richness of leaf-decaying fungal communities in woodland streams. Arch Hydrobiol 164:217–235. https://doi.org/10.1127/0003-9136/2005/0164-0217
Canhoto C, Graça MAS (1996) Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333:79–85. https://doi.org/10.1007/BF00017570
Gulis V (2001) Are there any substrate preferences in aquatic hyphomycetes? Mycol Res 105:1088–1093. https://doi.org/10.1016/S0953-7562(08)61971-1
Bärlocher F, Graça MAS (2002) Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw Biol 47:1123–1135. https://doi.org/10.1046/j.1365-2427.2002.00836.x
Menéndez M, Descals E, Riera T, Moya O (2013) Do non-native Platanus hybrida riparian plantations affect leaf litter decomposition in streams? Hydrobiologia 716:5–20. https://doi.org/10.1007/s10750-013-1539-0
Ferreira V, Faustino H, Raposeiro PM, Gonçalves V (2017) Replacement of native forests by conifer plantations affects fungal decomposer community structure but not litter decomposition in Atlantic island streams. For Ecol Manage 389:323–330. https://doi.org/10.1016/j.foreco.2017.01.004
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol 48:123–134. https://doi.org/10.1046/j.1365-2427.2003.00985.x
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graça MAS (2015) A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol Rev 90:669–688. https://doi.org/10.1111/brv.12125
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A, Malmqvist B, McKie BG, Tiegs SD, Cariss H, Dobson M, Elosegi A, Ferreira V, Graça MAS, Fleituch T, Lacoursière JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Vadineanu A, Vought LB-M, Chauvet E (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. https://doi.org/10.1126/science.1219534
Rosemond AD, Benstead JP, Bumpers PM, Gulis V, Kominoski JS, Manning DWP, Suberkropp K, Wallace JB (2015) Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science 347:1142–1145. https://doi.org/10.1126/science.aaa1958
Pereira A, Trabulo J, Fernandes I, Pascoal C, Cássio F, Duarte S (2017) Spring stimulates leaf decomposition in moderately eutrophic streams. Aquat Sci 79:197–207. https://doi.org/10.1007/s00027-016-0490-3
Pereira A, Ferreira V (2021) Invasion of native riparian forests by Acacia species affects in-stream litter decomposition and associated microbial decomposers. Microb Ecol 81:14–25. https://doi.org/10.1007/s00248-020-01552-3
Lowe SR, Woodford DJ, Impson DN, Day JA (2008) The impact of invasive fish and invasive riparian plants on the invertebrate fauna of the Rondegat River, Cape Floristic Region, South Africa. African J Aquat Sci 33:51–62. https://doi.org/10.2989/AJAS.2007.33.1.6.390
Railoun MZ (2018) Impacts of the invasive tree Acacia mearnsii on riparian and instream aquatic environments in the Cape Floristic Region, South Africa. Dissertation, Stellenbosch University
Graça MAS, Poquet JM (2014) Do climate and soil influence phenotypic variability in leaf litter, microbial decomposition and shredder consumption? Oecologia 174:1021–1032. https://doi.org/10.1007/s00442-013-2825-2
Ferreira V, Raposeiro PM, Pereira A, Cruz AM, Costa AC, Graça MAS, Gonçalves V (2016) Leaf litter decomposition in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions. Freshw Biol 61:783–799. https://doi.org/10.1111/fwb.12749
Bärlocher F, Gessner MO, Graça MAS (2020) Methods to study litter decomposition. A practical guide. Springer, Dordrecht
Goering HK, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications). Agricultural Research Service, Washington DC
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Gulis V, Ferreira V, Graça MAS (2006) Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshw Biol 51:1655–1669. https://doi.org/10.1111/j.1365-2427.2006.01615.x
Huryn AD, Butz Huryn VM, Arbuckle CJ, Tsomides L (2002) Catchment land-use, macroinvertebrates and detritus processing in headwater streams: taxonomic richness versus function. Freshw Biol 47:401–415. https://doi.org/10.1046/j.1365-2427.2002.00812.x
Fernandes I, Seena S, Pascoal C, Cássio F (2014) Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshw Biol 59:2390–2399. https://doi.org/10.1111/fwb.12445
Atwood TB, Wiegner TN, Turner P, MacKenzie RA (2010) Potential effects of an invasive nitrogen-fixing tree on a Hawaiian stream food web. Pacific Sci 64:367–379. https://doi.org/10.2984/64.3.367
Mineau MM, Baxter CV, Marcarelli AM (2011) A non-native riparian tree (Elaeagnus angustifolia) changes nutrient dynamics in streams. Ecosystems 14:353–365. https://doi.org/10.1007/s10021-011-9415-0
Ferreira V, Chauvet E (2011) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Chang Biol 17:551–564. https://doi.org/10.1111/j.1365-2486.2010.02185.x
Manning DWP, Rosemond AD, Gulis V, Benstead JP, Kominoski JS (2018) Nutrients and temperature additively increase stream microbial respiration. Glob Chang Biol 24:e233–e247. https://doi.org/10.1111/gcb.13906
Ferreira V, Chauvet E, Canhoto C (2015) Effects of experimental warming, litter species, and presence of macroinvertebrates on litter decomposition and associated decomposers in a temperate mountain stream. Can J Fish Aquat Sci 72:206–216. https://doi.org/10.1139/cjfas-2014-0119
Ferreira V, Canhoto C (2015) Future increase in temperature may stimulate litter decomposition in temperate mountain streams: evidence from a stream manipulation experiment. Freshw Biol 60:881–892. https://doi.org/10.1111/fwb.12539
Bärlocher F, Stewart M, Ryder D (2012) Processing of Eucalyptus viminalis leaves in Australian streams—importance of aquatic hyphomycetes and zoosporic fungi. Fundam Appl Limnol 179:305–319. https://doi.org/10.1127/1863-9135/2012/0229
Baldy V, Gessner MO, Chauvet E (1995) Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 74:93–102. https://doi.org/10.2307/3545678
Marks JC (2019) Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evol Syst 50:547–568. https://doi.org/10.1146/annurev-ecolsys-110218-024755
Wang F, Lin D, Li W, Dou P, Han L, Huang M, Qian S, Yao J (2020) Meiofauna promotes litter decomposition in stream ecosystems depending on leaf species. Ecol Evol 10:9257–9270. https://doi.org/10.1002/ece3.6610
Gessner MO, Chauvet E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–1817. https://doi.org/10.2307/1939639
Lecerf A, Chauvet E (2008) Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl Ecol 9:598–605. https://doi.org/10.1016/j.baae.2007.11.003
Fernandes I, Pascoal C, Guimarães H, Pinto R, Sousa I, Cássio F (2012) Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshw Biol 57:2306–2317. https://doi.org/10.1111/fwb.12004
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Li AOY, Ng LCY, Dudgeon D (2009) Effects of leaf toughness and nitrogen content on litter breakdown and macroinvertebrates in a tropical stream. Aquat Sci 71:80–93. https://doi.org/10.1007/s00027-008-8117-y
Ferreira V, Encalada AC, Graça MAS (2012) Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshw Sci 31:945–962. https://doi.org/10.1899/11-062.1
Pereira A, Geraldes P, Lima-Fernandes E, Fernandes I, Cássio F, Pascoal C (2016) Structural and functional measures of leaf-associated invertebrates and fungi as predictors of stream eutrophication. Ecol Indic 69:648–656. https://doi.org/10.1016/j.ecolind.2016.05.017
Campbell IANC, James KIMR, Hart BT, Devereaux A (1992) Allochthonous coarse particulate organic material in forest and pasture reaches of two south-eastern Australian streams. Freshw Biol 27:353–365. https://doi.org/10.1111/j.1365-2427.1992.tb00545.x
Raposeiro PM, Martins GM, Moniz I, Cunha A, Costa AC, Gonçalves V (2014) Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates vs. microbial decomposition of native vs. exotic plant species. Limnologica 45:80–87. https://doi.org/10.1016/j.limno.2013.10.006
Raposeiro PM, Ferreira V, Gea G, Gonçalves V (2018) Contribution of aquatic shredders to leaf litter decomposition in Atlantic island streams depends on shredder density and litter quality. Mar Freshw Res 69:1432–1439. https://doi.org/10.1071/MF18020
Graça MAS, Cressa C (2010) Leaf quality of some tropical and temperate tree species as food resource for stream shredders. Int Rev Hydrobiol 95:27–41. https://doi.org/10.1002/iroh.200911173
Ferreira V, Elosegi A, Gulis V, Pozo J, Graça MAS (2006) Eucalyptus plantations affect fungal communities associated with leaf-litter decomposition in Iberian streams. Arch Hydrobiol 166:467–490. https://doi.org/10.1127/0003-9136/2006/0166-0467
Arroita M, Aristi I, Flores L, Larrañaga A, Díez J, Mora J, Romaní AM, Elosegi A (2012) The use of wooden sticks to assess stream ecosystem functioning: comparison with leaf breakdown rates. Sci Total Environ 440:115–122. https://doi.org/10.1016/j.scitotenv.2012.07.090
Yeung ACY, Kreutzweiser DP, Richardson JS (2019) Stronger effects of litter origin on the processing of conifer than broadleaf leaves: a test of home-field advantage of stream litter breakdown. Freshw Biol 64:1755–1768. https://doi.org/10.1111/fwb.13367
Artigas J, Romaní AM, Sabater S (2008) Effect of nutrients on the sporulation and diversity of aquatic hyphomycetes on submerged substrata in a Mediterranean stream. Aquat Bot 88:32–38. https://doi.org/10.1016/j.aquabot.2007.08.005
Geraldes P (2011) Fungal communities and functional measures as indicators of stream ecosystem health. Dissertation, Minho University
Noel L, Bärlocher F, Culp JM, Seena S (2016) Nutrient enrichment and flow regulation impair structure and function of a large river as revealed by aquatic hyphomycete species richness, biomass, and decomposition rates. Freshw Sci 35:1148–1163. https://doi.org/10.1086/689180
Arsuffi TL, Suberkropp K (1984) Leaf processing capabilities of aquatic hyphomycetes—interspecific differences and influence on shredder feeding preferences. Oikos 42:144–154. https://doi.org/10.2307/3544786
Arsuffi TL, Suberkropp K (1988) Effects of fungal mycelia and enzymatically degraded leaves on feeding and performance of caddisfly (Trichoptera) larvae. J North Am Benthol Soc 7:205–211. https://doi.org/10.2307/1467420
Brosed M, Jabiol J, Gessner MO (2017) Nutrient stoichiometry of aquatic hyphomycetes: interstrain variation and ergosterol conversion factors. Fungal Ecol 29:96–102. https://doi.org/10.1016/j.funeco.2017.04.008
Kominoski JS, Shah JJF, Canhoto C, Fischer DG, Giling DP, González E, Griffiths NA, Larrañaga A, LeRoy CJ, Mineau MM, McElarney YR, Shirley SM, Swan CM, Tiegs SD (2013) Forecasting functional implications of global changes in riparian plant communities. Front Ecol Environ 11:423–432. https://doi.org/10.1890/120056
Figueiredo A (2016) The contribution of network driving factors to explain invasion patterns: the role of water on Madeira Island. Water Territories, 2nd International Congress of CEGOT, 7-9 March, Faculdade De Letras Da Universidade De Coimbra, Coimbra, Portugal: 109-118
Acknowledgements
We thank Olímpia Sobral (CFE, University of Coimbra) for all the help in the field and the laboratory, and Teresa Gonçalves and Chantal Fernandes (CNC, University of Coimbra) for the use of the lyophilizer. Water nutrient analyses were ordered to Centro de Apoio Científico-Tecnolóxico á Investigación (CACTI, University of Vigo, Spain). Ergosterol analyses were ordered to Instituto do Ambiente Tecnologia e Vida (IATV, University of Coimbra, Portugal).
Availability of Data and Materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Funding
This study was financed by the Portuguese Foundation for Science and Technology (FCT) through the exploratory project IF/00129/2014 granted to Verónica Ferreira and through the strategic project UIDP/04292/2020 granted to MARE. Ana Pereira received a doctoral fellowship from FCT (SFRH/BD/118069/2016), financed by the European Social Fund (FSE) from the European Union (UE), through the Programa Operacional Regional Centro (CENTRO 2020) and by National Funds from the Orçamento de Estado, through Ministério da Ciência, Tecnologia e Ensino Superior (MCTES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
ESM 1
(PDF 361 kb)
Rights and permissions
About this article
Cite this article
Pereira, A., Figueiredo, A. & Ferreira, V. Invasive Acacia Tree Species Affect Instream Litter Decomposition Through Changes in Water Nitrogen Concentration and Litter Characteristics. Microb Ecol 82, 257–273 (2021). https://doi.org/10.1007/s00248-021-01749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01749-0