Abstract
Shredders play a crucial role in litter decomposition in streams. However, in oceanic islands, many streams have low shredder density and richness, and microbes seem to be the main litter decomposers. Here, we evaluate the effects of shredders and aquatic hyphomycetes on litter decomposition in insular streams. Three leaf species differing in physical and chemical characteristics, Alnus glutinosa, Clethra arborea, and Cryptomeria japonica, were enclosed in bags of coarse and fine mesh to allow and avoid macroinvertebrate access to the litter, respectively, and incubated in six streams along a gradient of Limnephilus atlanticus (Trichoptera) density in São Miguel Island. In streams with higher L. atlanticus density, leaf mass loss was higher in coarse than fine mesh bags. However, no difference in litter mass loss was found between bag types in streams with no L. atlanticus, despite the presence of other shredder taxa. These results suggest that when L. atlanticus are present at relatively high densities, they significantly contribute to litter decomposition, while litter decomposition is mainly driven by microbes when L. atlanticus density is low, or they are absent. Moreover, litter decomposition depends on litter quality, with leaves with high nutrient concentration and low concentration of secondary compounds being preferred by shredders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Streams, i.e., small water courses (order ≤ 4; Strahler, 1957), represent the majority of water courses in hydrographic basins, both in number and in length (Allan & Castillo, 2007). This is especially true for volcanic insular systems, where watersheds exhibit a predominantly radial drainage pattern, as streams flow away from the central peaks, the center of volcanic activity, dropping dramatically in altitude (Hughes, 2005). These streams are thus characteristically very steep with near vertical valley walls, short, narrow, shallow, and straight, and have a turbulent, torrential, and often seasonal flow regime (Malmqvist, 2002; Hughes, 2003; Smith et al., 2003). This is the case of streams in Atlantic islands that are geologically young and truly oceanic, i.e., with no physical connection to continental landmass at any time. Specifically, in the Azores archipelago there are 763 hydrographic basins draining the 2322 km2 of land surface (DROTH & INAG, 2001; Cruz & Soares, 2018) and streams have a maximum length of 29 km (Raposeiro et al., 2013).
Many of these streams are covered by dense riparian vegetation that decreases the amount of solar energy that could be used by instream primary producers (Graça & Canhoto, 2006). Thus, allochthonous organic matter constitutes the primary source of energy and matter for aquatic food webs (Anderson & Sedell, 1979; Vannote et al., 1980; Wallace et al., 1997), and it is present generally in the form of leaf litter (Abelho, 2001).
Litter decomposition is mainly a biological process in which microbes (mostly aquatic hyphomycetes) and macroinvertebrate shredders are the main players (Cummins et al., 1973; Hieber & Gensser, 2002; Cornut et al., 2010). Microbes colonize leaf litter soon after leaf immersion, mostly after leaching of soluble secondary compounds (Canhoto & Graça, 1996). Microbes decompose litter through respiration and incorporation of carbon into reproductive structures (spores) and biomass (Hieber & Gessner, 2002; Cornut et al., 2010). Also, aquatic hyphomycetes produce exoenzymes that depolymerize pectin, xylan and cellulose from litter leading to litter softening and the release of fine particulate organic matter (Gulis & Suberkropp, 2003). Litter softening and microbial biomass accumulation increase litter palatability to shredders, which incorporate it into secondary production and promote the release of fine particles leading to further litter mass loss (Arsuffi & Suberkropp, 1989; Graça, 2001; Gulis et al., 2006; Graça & Cressa, 2010).
It is well known that macroinvertebrate shredders play an important role in leaf decomposition in continental temperate streams (Graça, 2001; Graça & Canhoto, 2006; Pozo et al., 2011). However, less is known about shredders’ contribution to litter decomposition in remote islands. Oceanic island freshwater assemblages are subject to ‘biogeographical filters’; strong physical barriers such as distance between the mainland and islands, influence dispersal and species colonization (Bilton et al., 2001; Covich, 2009) resulting in less diverse biotic assemblages in islands compared to continental systems (Hughes, 2006). In the Azores archipelago, isolation and numerous geological events and volcanic eruptions contributed to the low diversity of freshwater species (Whittaker & Fernandez-Palacios, 2007), but high level of endemism (11% of the Azorean freshwater invertebrate fauna; Raposeiro et al., 2012). In fact, only few shredder taxa have been identified in Azorean streams: Jaera nordica insulana Veuille, 1976 (isopod), Limnephilus atlanticus Nybom, 1948 (caddisfly), Dicranomyia sp., Tipula macaronesica Savchenko, 1962 and T. oleracea Linnaeus, 1758 (crane flies) (Borges et al., 2010; Raposeiro et al., 2012; Ferreira et al., 2016), and abundances are generally low (Raposeiro et al., 2013). In these, and other, oceanic island streams characterized by low shredder density or even absence, microbes seem to be the main litter decomposers (Larned, 2000; Benstead et al., 2009; Raposeiro et al., 2014; Ferreira et al., 2016). In some island streams, however, shredder density is high, and they are active players on litter decomposition (e.g., Li & Dudgeon, 2009; Longo & Blanco, 2014). In fact, several studies showed that high shredder density is a crucial driver of litter decomposition, and shredder density and decomposition rate are positively correlated (Encalada et al., 2010; Rincón & Covich, 2014; Raposeiro et al., 2018).
Litter decomposition rates also depend on litter intrinsic characteristics. Soft leaves, with high concentration of nutrients (e.g., nitrogen) and low concentration of structural (e.g., lignin) and secondary compounds (e.g., polyphenols) are generally colonized and decomposed faster by microbes and macroinvertebrate shredders than tough litter, with low concentration of nutrients (Canhoto & Graça, 1995; Ferreira et al., 2016; Raposeiro et al., 2018). Shredders are especially sensitive to the concentration of secondary and structural compounds that affect litter palatability, and therefore these characteristics may be more important determining biological litter decomposition than litter nutrient concentrations (Graça & Cressa, 2010; Claeson et al., 2013; Ferreira et al., 2016). In fact, stronger differences in litter decomposition have been found among litter species in the presence of shredders than when decomposition is only microbial-driven (Pereira et al., 1998; Hieber & Gessner, 2002; Ferreira et al., 2012; Raposeiro et al., 2018).
This study evaluates litter decomposition in relation to macroinvertebrate shredder presence and density and litter characteristics by comparing the decomposition of three litter species with distinct physical and chemical characteristics (Alnus glutinosa (L.) Gaertn., Clethra arborea Aiton, and Cryptomeria japonica D. Don.), enclosed in coarse mesh bags (that allow shredder access to the litter) and in fine mesh bags (that prevent shredder access to the litter and where decomposition is mostly microbial-driven), and incubated in streams along a gradient of Limnephilus atlanticus density (from absence to high density) in São Miguel Island (Azores archipelago). The following hypotheses were tested: (1) litter decomposition in coarse mesh bags is higher in the presence of shredders and is positively correlated with benthic shredder density, (2) litter decomposition is higher in coarse mesh than in fine mesh bags in streams with shredders but not in streams without shredders, (3) litter decomposition varies among litter species and is highest for soft litter with high nutrient concentration, and (4) there is an interaction between stream (shredder density), mesh bag type and litter species on litter decomposition with (4a) shredders playing a stronger role on the decomposition of palatable than recalcitrant litter and (4b) differences among litter species being stronger in the presence than in the absence of shredders (when considering different streams or mesh bag type). Additionally, this study evaluates aquatic hyphomycetes communities and reproductive activity associated with Alnus glutinosa (the most palatable litter species) to test the following hypotheses: (5) spore production and community structure differ among streams as a result from differences in environmental characteristics and (6) spore production and community structure differ between coarse and fine mesh bags as a result from predation by shredders and competition for leaf litter in coarse mesh bags.
Materials and methods
Study area
The study was conducted in streams in São Miguel Island, Azores archipelago. The Azores archipelago is located in the middle of the northern Atlantic Ocean, between the latitudes 36° 45′ N and 39° 43′ N and the longitudes 24° 45′ W and 31° 17′ W, about 1500 km off Portugal mainland (Santos et al., 2004). It comprises nine islands of volcanic origin with a total land surface area of 2325 km2, with São Miguel Island being the largest. The climate is temperate oceanic with a mean annual temperature of 15°C (Machado & Gonçalves, 2004). Mean annual precipitation ranges from 1500 to 3000 mm, depending on altitude, and relative humidity is high (Silva & Smith, 2004). Vegetation is composed by a larger percentage of non-indigenous (69%) than indigenous (31%) species (Silva & Smith, 2004). Native forests cover less than 10% of the total area (Borges et al., 2009) as the result of exploitation of the forest resources and clearance for agriculture and urbanization (Constância, 1963).
Streams selection
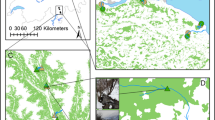
Based on extensive surveys of island streams undertaken in the context of stream bioassessment programs (Gonçalves et al., 2016, 2017) and previous studies (Raposeiro et al., 2013, 2018) and on surveys specifically done in preparation for this experiment during March 2017, six permanent streams with similar hydrology, geomorphology (low order, ~ 7 km long, < 4 m wide, ~ 0.5 m deep, substrates dominated by mixed gravel/cobbles with occasional large and submerged boulders) and riparian vegetation and distinct Limnephilus atlanticus densities (no. ind/m2) were selected at similar elevation (600–800 m a.s.l.) in Planalto dos Graminhais, northeastern São Miguel Island (Fig. S1). This area extends over more than 100 ha and is partially included in the Special Protection Zone of Pico da Vara/Ribeira do Guilherme. It has the largest spot of altitude bogs of the island, which highlights its enormous importance as a natural water reservoir (Botelho & Peñil, 2013). Streams were named from RIB1 to RIB6, where RIB1 had the lowest L. atlanticus density (absent) and RIB6 the highest.
Water variables
Water temperature was recorded hourly for the duration of the experiment (4 April to 24 May, 2017) using data loggers (Hobo Pendant UA-001-08, Onset Computer Corp., MA, U.S.A) and hourly values were averaged to produce daily means. Electrical conductivity, pH and dissolved oxygen, were recorded five times (4, 11 and 18 April, 5 and 24 May, 2017) with a multiparametric field probe (Horiba model U-52G, Horiba Instruments, U.K.). Water samples were collected, transported to the laboratory, filtered (47 mm diameter, 1.2 µm pore size; Whatman GF/C, GE Healthcare Europe GmbH, Little Chalfont, U.K.) and frozen at – 22°C until analyzed. Nutrient concentrations were determined using a Continuous Flow Analyser Skalar San++ (Skalar Analytical B.V., Breda, The Netherlands) with segmented flow analysis (SFA) according to Skalar methods M461-318 (EPA 353.2), for nitrite, nitrate and total nitrogen, M155-008R (EPA 350.1), for ammonium, and M503-555R (Standard Method 450-P I), for phosphate and total phosphorus (Skalar, 2004). Total phosphorus, nitrite and ammonium concentrations were below detection limit (502.0 µg/l, 18.3 µg/l, and 5.2 µg/l, respectively). Water analyses were done at MARINNOVA—Marine and Environmental Innovation, Technology and Services, Porto.
Litter decomposition experiment
Leaf species
Three leaf species with distinct characteristics were selected for the decomposition experiment: Alnus glutinosa, Clethra arborea, and Cryptomeria japonica. Alnus glutinosa is a deciduous broadleaf tree species that is exotic to the Azores archipelago, and in São Miguel Island is only found in the surroundings of Furnas, Azul and Verde lakes (Berger & Aptroot, 2002). Alnus glutinosa leaves are often used for large scale comparisons (Boyero et al., 2011; Ferreira et al., 2019; Seena et al., 2019) and were used here for their high palatability to shredders owing to high nutrient concentrations and softness (Friberg & Jacobsen, 1994; Graça & Cressa, 2010). It has been shown that decomposers are more responsive to litter characteristics than to litter origin, especially for high quality litter, and thus A. glutinosa foreign origin likely did not affect the results (Kennedy & El-Sabaawi, 2017; Yeung et al., 2019). Clethra arborea is an invasive perennial broadleaf tree species and C. japonica is an exotic perennial conifer tree species, but both common in the Azorean riparian vegetation and dominant at the study streams. Senescent leaves of A. glutinosa were collected after natural senescence in autumn 2015. Clethra arborea leaves were directly collected from trees on March 2015. Cryptomeria japonica freshly fallen twigs were picked from the ground on March 2017 and the 5–10 cm tips, corresponding to the last growth period, were used (Ferreira et al., 2017). Leaves and tips were transported to the laboratory, air dried at ambient conditions and stored in the dark until used.
Leaf species were characterized regarding toughness and chemical composition at MARE—Marine and Environmental Sciences Centre, University of Coimbra. Leaf toughness was determined using a penetrometer after leaves had been soaked in distilled water for 1 h and results were expressed as the force (g/mm2) needed to penetrate the leaf mesophyll with an iron rod (Graça et al., 2005). Subsamples of leaves and tips were ground to fine powder (< 1 mm), dried (105°C), weighed and used for the determination of nitrogen (N) and carbon (C) (IRMS Thermo Delta V advantage with a Flash EA—1112 series), phosphorus (P; APHA, 1995), lignin (Goering & Van Soest, 1970) and polyphenol concentration (Graça et al., 2005). Results were expressed as % DM.
Experimental set up
Air dried leaves and tips were weighted (0.01 g precision) in batches of 3.30–3.55 g and sprayed with distilled water to make them soft and less susceptible to break due to manipulation. Substrates were enclosed in fine mesh bags (0.5 mm mesh size; 10 × 12 cm), to avoid macroinvertebrates access and allow microbial decomposition only, and coarse mesh bags (5 mm mesh size with 10 mm holes; 10 × 12 cm) to allow macroinvertebrate access.
Twelve litter bags of each mesh size and species were deployed in each stream, in areas of natural organic matter accumulation (12 bags × 3 leaf species × 2 mesh sizes × 6 streams, 432 bags in total) on 4 April, 2017. After 7, 14, 31, and 50 days, three randomly selected bags for each species and mesh size were collected from each stream (3 bags × 3 leaf species × 2 mesh size × 6 streams, 108 bags per date), enclosed into plastic bags and transported cold to the laboratory. Although the experiment was performed in spring, riparian vegetation in Azorean islands is dominated by evergreen species and thus organic matter can be found in the benthos year round. Also, this is the time when late instars of aquatic insects (also Limnephilus atlanticus) are more common (pers. observ.), maximizing the chance of finding effects of shredders on litter decomposition.
Litter mass remaining
Litter remaining in bags was gently rinsed with distilled water into a 0.5-mm mesh sieve to retain small litter fragments (and macroinvertebrates in the case of coarse mesh bags; see below). Litter remaining was placed in pre-weighted aluminum pans, oven dried at 60°C for 48 h (Memmert GmbH + Co, Schwabach, Germany) and weighed to determine dry mass (DM). Substrates were ignited at 500°C for 4 h (Lenton EF 11/8B, Hope Valley, U.K.) and reweighed to determine ash mass. Ash-free dry mass (AFDM) remaining was calculated as the difference between DM and ash mass (taking into account the AFDM of the leaf discs taken to induce sporulation in the case of Alnus glutinosa; see below).
Percentage AFDM remaining was calculated as final AFDM/initial AFDM × 100, with initial AFDM being estimated by multiplying the initial air dry mass of samples by an initial air dry mass to initial AFDM conversion factor derived from an extra set of samples. For the creation of the conversion factor, three litter bags for each species and mesh size (18 bags in total) were prepared as described above, immersed in RIB2 for 10 min and taken back to the laboratory on day 0. Litter was washed with distilled water and placed in pre-weighed aluminum pans for DM and AFDM determination as described. The initial air dry mass to initial AFDM conversion factor was estimated as the ratio between initial AFDM and initial air dry mass.
Macroinvertebrates
Benthic macroinvertebrate samples were collected at each stream on three occasions (beginning, middle, and end of the litter decomposition experiment, on days 0, 14, and 50, respectively). Benthic samples were composed of six subsamples (1 m long) taken with a kicknet (0.33 m wide, 0.5 mm mesh) from the different existing microhabitats along a 50 m reach and preserved with 70% ethanol. Macroinvertebrates were identified and counted with a stereo microscope (Olympus SZ61, Tokyo, Japan). Identification was done to the lowest possible taxonomic level using identification keys (Tachet et al., 2000; Borges et al., 2010; Kriska, 2013) and organisms were assigned to functional feeding groups (Schmidt-Kloiber & Hering, 2012). Relative abundance was expressed as a percentage of each taxon relative to the total number of individuals present for each stream and taxa richness was expressed as the number of taxa per sample. Limnephilus atlanticus density was calculated as number of individuals per m2.
Additionally, macroinvertebrates from coarse mesh bags were saved and preserved in 70% ethanol for further identification and counting as described for benthic macroinvertebrates. Shredder abundance was expressed as the number of shredders per gram of leaf litter AFDM.
Aquatic hyphomycetes
Aquatic hyphomycetes are the main microbial decomposers in aquatic systems (Hieber & Gessner, 2002; Pascoal et al., 2005). Community structure and sporulation rates of aquatic hyphomycetes associated with Alnus glutinosa leaf litter were assessed after inducing sporulation under laboratory conditions (Bärlocher, 2005). Aquatic hyphomycetes were assessed only for A. glutinosa because faster colonization and higher activity was anticipated for this species due to its higher nutritional quality compared with the other two species (Ferreira et al., 2012, 2016). For each sample, one set of five leaf discs were cut using a cork borer (12 mm diameter). Sets of leaf discs were placed in Erlenmeyer flasks with 25 mL of filtered stream water (47 mm diameter, 1.2 µm pore size; Whatman GF/C, GE Healthcare Europe GmbH, Little Chalfont, U.K.) and incubated on an orbital shaker (100 r.p.m.) at 10°C for 48 h under a 8:16 h light:dark photoperiod to simulate light conditions under the shaded streams. After 48 h, suspensions were gently shaken to dislodge spores attached to the flask walls, transferred into 50 mL graduated tubes, fixed with 2 mL formalin 37% and the final volume adjusted to 35 mL with distilled water. Spore suspensions were stored in the dark until used. Leaf discs were transferred to pre-weighted aluminum cups, oven dried at 60°C for 48 h and weighed to the nearest 0.1 mg for DM determination. Dry leaf discs were ignited for at 500°C 4 h to determine ash mass and AFDM.
Spore suspensions were gently shaken and transferred into a beaker with 100 μL Triton X-100 solution (0.5%). Suspensions were homogenized with a magnetic stirrer and aliquots were filtered through cellulose nitrate filters (25 mm diameter, 5 μm pore size; SMWP, Merk Millipore Ltd. Cork, Ireland). Filters were stained with cotton blue in 60% lactic acid (0.05%) and mounted on a microscope slide. Spores were identified and counted under a microscope (Leica DM2500, Leica Microsystems CMS GmbH, Wetzlar, Germany) at × 200 magnification (Gulis et al., 2005). Sporulation rates were expressed as number of spores released per mg AFDM per day and aquatic hyphomycete species richness as number of species per sample.
Statistical analyses
Water characteristics were compared among streams using one-way analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) test when significant effects were detected in ANOVA. Initial litter chemical and physical characteristics were compared among species by one-way ANOVA followed by Tukey’s HSD test.
Although litter bags were sampled on four occasions, and litter decomposition is often expressed as decomposition rate, in this case, neither the negative exponential model nor the negative linear model successfully fitted the data in all treatments. Thus, comparisons among streams, species and mesh sizes were done based on fraction of AFDM remaining (arcsin square root transformed) by the last sampling date (day 50) using three-way ANOVA, followed by Tukey’s HSD test when significant effects were detected. Pearson correlation was used to relate percentage of AFDM remaining and density of Limnephilus atlanticus in the benthos.
Limnephilus atlanticus densities in the benthos were compared among streams using one-way ANOVA followed by Fisher’s least square difference (LSD) test. Benthic macroinvertebrates communities (log (x + 1) transformed) were compared among streams by permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis similarity matrix followed by pairwise test (Anderson, 2001; McArdle & Anderson, 2001). Total macroinvertebrate and shredder abundance associated with decomposing litter in coarse mesh bags were correlated using Pearson correlation. Since high correlation existed between total macroinvertebrates and shredder abundance in litter bags (Pearson correlation: r = 0.93, P = 0.001), only shredder abundance (no. shredder/g AFDMr, log (x + 1) transformed) was compared among streams, leaf species and time by three-way ANOVA followed by Tukey’s HSD test.
Aquatic hyphomycetes communities (based on spore production; log (x + 1) transformed) associated with decomposing litter were compared among streams, mesh sizes and time by PERMANOVA as described above. Aquatic hyphomycetes sporulation rates and species richness (log (x + 1) transformed) associated with Alnus glutinosa litter were compared among streams, mesh sizes and time by three-way ANOVA followed by Tukey’s HSD test. Only data for day 7 and day 14 were considered since not all coarse mesh bags had enough litter mass remaining to induce sporulation by day 31 and 50.
Data were checked for homoscedasticity (Bartlett’s test) and normality (Shapiro–Wilk test) before analyses and transformed when necessary. Univariate analyses were performed using STATISTICA 7 (StatSoft, Tulsa, OK, U.S.A.) and community analyses were performed using PRIMER 6 v6.1.11 & PERMANOVA + v1.0.1 (Primer-E Ltd, Plymouth, U.K.).
Results
Water variables
Stream water was 10.5–11.6°C, slightly alkaline (pH 7.7–8.4), well oxygenated (80.6–91.1% dissolved oxygen), with low conductivity (57.2–75.8 µS/cm), and moderate–high nutrient concentration (total N: 1081–1233 µg/L; PO43−: 77–228 µg/L; NO3−: 107–1127 µg/L) (Table 1). Significant differences among streams were found for conductivity, with RIB5 having significantly higher conductivity than RIB1 and RIB6; phosphate, with RIB5 having significantly higher concentration than RIB3 and RIB6; and nitrate, with RIB5 having the highest concentration, followed by RIB2, RIB1, RIB6, RIB4 and RIB3 (Table 1).
Leaf species
The three leaf species selected differed in chemical characteristics (Table 2). Alnus glutinosa leaves had the highest nutrient (both nitrogen and phosphorus) concentrations and the lowest polyphenols concentrations (Table 2). Clethra arborea leaves had the lowest carbon and lignin concentration, the highest polyphenols concentration and intermediate nutrient concentrations (Table 2). Cryptomeria japonica leaves had the highest lignin concentration, intermediate polyphenols concentration and the lowest nutrient concentrations (Table 2). Alnus glutinosa and C. arborea leaves did not differ significantly in toughness (Table 2).
Litter mass remaining
Litter mass remaining decreased gradually over time (Fig. 1), with AFDM remaining on fine mesh bags varying between 53.8 and 70.5% of initial mass for Alnus glutinosa leaves, 55.0–71.4% for Clethra arborea and 77.7–94.2% for Cryptomeria japonica, and on coarse mesh bags varying between 0.2 and 58.5% for A. glutinosa, 6.4–58.6% for C. arborea and 9.1–87.4% for C. japonica (Fig. 2).
Percentage litter mass remaining after 50 days incubation differed significantly among litter species, mesh sizes and streams (three-way ANOVA, P < 0.001; Fig. 2, Table S1). There were, however, significant interactions among factors (three-way ANOVA, P < 0.001; Table S1). Percentage of AFDM remaining was significantly lower in coarse than in fine mesh bags for the three leaf species in streams with higher shredder density (RIB4–RIB6) (Tukey’s test, P < 0.001), while no significant differences were found in RIB1–RIB3 (except for Alnus glutinosa in RIB3). Litter mass remaining was significantly higher for Cryptomeria japonica than for Clethra arborea and Alnus glutinosa in fine mesh bags (Tukey’s test, P < 0.001), with no significant difference among the two latter species (P = 0.995), while litter mass remaining differed significantly among the three leaf species in coarse mesh bags (A. glutinosa < C. arborea < C. japonica; P < 0.001).
Percentage of AFDM remaining for Cryptomeria japonica was significantly lower in RIB5 and RIB6 than in RIB1–RIB4 (Tukey’s test, P < 0.001), with significant differences within the former group (RIB5 > RIB6; P < 0.001) but not within the latter (P ≥ 0.650). Litter mass remaining in Clethra arborea bags was significantly lower in RIB4–RIB6 than in RIB1–RIB3 (Tukey’s test, P < 0.043), with no significant differences within each group (P ≥ 0.998). Litter mass remaining for Alnus glutinosa did not differ significantly between RIB1 and RIB2, between RIB3 and RIB4, and between RIB5 and RIB6 (Tukey’s test, P = 0.616, P = 1.000, and P = 0.999, respectively), but differed significantly among the three groups (first > second > third; P < 0.030).
Percentage of AFDM remaining in fine mesh bags was significantly higher on RIB1 than on RIB3 and on RIB3 than on RIB5 (Tukey’s test, P < 0.001). For coarse mesh bags, litter mass remaining was significantly higher in streams without Limnephilus atlanticus (RIB1 and RIB2) than in streams with L. atlanticus (RIB3–RIB6) (Tukey’s test, P < 0.010) (Fig. 2), with significant differences among streams in the latter group (RIB3 > RIB4 > RIB6 > RIB5; P < 0.001) (Fig. 2).
Moreover, litter mass remaining in coarse mesh bags was negatively correlated with Limnephilus atlanticus density in the benthos (Pearson correlation: Alnus glutinosa, r = – 0.94, P = 0.003; Clethra arborea, r = – 0.90, P = 0.007; Cryptomeria japonica, r = – 0.82, P = 0.024; n = 6).
Macroinvertebrates
Thirty-two macroinvertebrate taxa were found in the benthos (Table 3). Benthic communities in RIB1 and RIB2 were dominated by Orthocladiinae midges, in RIB3 by Orthocladiinae midges and the caddisfly Limnephilus atlanticus, in RIB4 by the isopod Jaera nordica insulana, and in RIB5 and RIB6 by Orthocladiinae midges and the black fly Simulium azorense Carlsson. Only four species of shredders were collected, Tipula sp. (0.2% relative abundance), Dicranomyia sp. (1.5–16.0%), J. nordica insulana (0.2–47.7%) and L. atlanticus (0.2–20.7%). Each stream had at least one shredder species, but dominance varied between Dicranomyia sp. in RIB1 and RIB2, J. nordica insulana in RIB4 and L. atlanticus in RIB3, RIB5 and RIB6 (Table 3). Moreover, benthic macroinvertebrate communities were significantly different among streams (PERMANOVA, P = 0.006, permutations 998).
There was a tendency for an increase in Limnephilus atlanticus density from RIB1, where they were absent, to RIB6 (Fig. 3). Limnephilus atlanticus density in the benthos differed significantly among streams (one-way ANOVA, P = 0.017), with higher values in RIB3–RIB6 than in RIB1 and RIB2 (Tukey’s test, P = 0.036), although no significant difference was found within either stream group (P > 0.638). Among shredders, L. atlanticus was the dominant species (99.7%), followed by Dicranomyia sp. (0.2%) and Jaera nordica insulana (0.1%). Tipula sp. was not found associated with litter.
Shredder abundance differed significantly among leaf species (three-way ANOVA, P < 0.001; Table S2) with significantly higher values on Alnus glutinosa than on Clethra arborea and Cryptomeria japonica (Tukey’s test, P < 0.001), which did not differ significantly (P = 0.905) (Fig. 4). Shredder abundance also differed significantly among streams (three-way ANOVA, P < 0.001; Table S2) with significantly higher values in RIB3–RIB6 than in RIB1 and RIB2 (Tukey’s test, P = 0.036), with no significant differences being found within either stream group (P ≥ 0.957). Litter bags from RIB6 had the highest shredder abundance (Fig. 4). Time was also a significant factor (three-way ANOVA, P = 0.030; Table S2), with shredder abundance being highest on A. glutinosa on day 7 and decreasing thereafter. All interactions were significant (three-way ANOVA, P < 0.002; Table S2). In particular, shredder abundance was similar for the three species in streams RIB1 and RIB2 (Tukey’s test, P = 1.000), while shredder abundance was significantly higher for A. glutinosa than for C. japonica and C. arborea in streams RIB3–RIB6 (Tukey’s test, P < 0.002), with no significant difference between the former two species (P ≥ 0.998).
Aquatic hyphomycetes
Twenty-five species of aquatic hyphomycetes were found associated with decomposing litter of Alnus glutinosa (Table S3). Fungal communities did not differ significantly between mesh sizes (PERMANOVA, P = 0.797; Table S4). However, they differed significantly between sampling dates (PERMANOVA, P = 0.001; Table S4) and among streams (P = 0.001). Significant interactions were only found for stream × time (PERMANOVA, P = 0.001; Table S4).
Most abundant species varied among streams, and often among bag types. However, Alatospora pulchella Marvanová, Articulospora tetracladia Ingold, Heliscus lugdunensis Saccardo & Therry, Lemonniera aquatica De Wildeman and Tetrachaetum elegans Ingold were most often the dominant or most abundant species (Fig. 5).
Species richness significantly differed among streams (three-way ANOVA, P < 0.001; Table S5), with higher values in RIB1 in coarse mesh bags and RIB5 in fine mesh bags (Fig. 6). Differences between sampling dates were also found (three-way ANOVA, P < 0.001; Table S5). Species richness increased from one species on day 7 to a maximum of 11 species in coarse mesh bags and from one species on day 7 to 13 species in fine mesh bags (Fig. 6). However, no significant differences were found between coarse and fine mesh bags (three-way ANOVA, P = 0.263; Table S5). Only interaction between stream × time was found (three-way ANOVA, P = 0.009; Table S5), with significant differences between day 7 and 14 for RIB1 (Tukey’s test, P = 0.012), RIB5 (P = 0.006) and RIB6 (P < 0.001).
Sporulation rates differed significantly among sampling dates (three-way ANOVA, P < 0.001; Table S5), increasing over the incubation period (Fig. 6), and among streams (P < 0.001). Three homogeneous groups were found among streams (Tukey’s test, P < 0.046): RIB2, RIB3, RIB4 and RIB6 (P≥0.553); RIB1, RIB2 and RIB6 (P ≥ 0.661); and RIB1, RIB5 and RIB6 (P ≥ 0.635). Sporulation rates did not differ significantly between mesh sizes (three-way ANOVA, P = 0.062; Table S5). Only interaction between stream × time was found (three-way ANOVA, P = 0.009; Table S5), with differences between day 7 and 14 being only found for RIB5 (Tukey’s test, P < 0.001) and RIB6 (P < 0.001).
Discussion
It has been widely reported that shredders play a crucial role in leaf litter decomposition, especially in continental temperate streams, but this is not always the case in oceanic island streams (Larned, 2000; Raposeiro et al., 2014; Ferreira et al., 2016). Here, we addressed the decomposition of three leaf litter species in Azorean streams with different Limnephilus atlanticus densities (absence to high density) and showed that litter decomposition was higher when L. atlanticus were present, but the effects of L. atlanticus presence strongly depended on litter quality.
Leaf litter decomposition was faster in the presence of shredders
Macroinvertebrate communities in Azorean streams are characterized by low diversity, with very few shredder taxa (Borges et al., 2010; Raposeiro et al., 2011, 2012; Ferreira et al., 2016). Shredder density is also generally low (Raposeiro et al., 2013). Previous studies in Azorean streams with low shredder density have shown that microbes are the main players in litter decomposition (Raposeiro et al., 2014; Ferreira et al., 2016). However, some Azorean streams located at higher elevation, in restricted areas of difficult access, have comparatively high densities of Limnephilus atlanticus (7–15 ind/m2). In these streams (RIB3–RIB6), litter decomposition in coarse mesh bags (that allow shredder access to the litter) was faster than in streams with lower density or from where L. atlanticus were absent (RIB1 and RIB2), which translated into a negative correlation between litter mass remaining and L. atlanticus density in the benthos. Also, litter decomposition was faster in coarse mesh bags than in fine mesh bags only for streams with higher shredders density (RIB3–RIB6). These results suggest that when shredders are present in higher densities they may have a significant role on litter decomposition, while this process is mainly driven by microbes when shredder density is low or they are absent. This agrees with a recent study that showed faster litter decomposition in coarse mesh bags in an Azorean stream where shredder density was high (5–13 L. atlanticus/m2) than in the absence of shredders (fine mesh bags) or in treatments with low L. atlanticus density (fine mesh bags with 1–3 individuals) (Raposeiro et al., 2018). Other studies have found that macroinvertebrates played a minor role on litter decomposition in some tropical island streams with low shredder density (Larned et al., 2003; Benstead et al., 2009; MacKenzie et al., 2013), while in other streams invertebrate macroconsumers contributed substantially to litter decomposition (Pringle et al., 1993; Larned et al., 2003; Wright & Covich, 2005).
Previous studies (Crowl et al., 2001; Encalada et al., 2010; Raposeiro et al., 2018) suggested that the rate of litter decomposition was not related to the overall diversity of the shredder community, but to the presence and abundance of a single species, whose feeding behavior makes it a predominant driver of this ecosystem process. Among the shredder species found in our streams, only Limnephilus atlanticus individuals were found associated with litter in coarse mesh bags, suggesting that this species acts as an important shredder in these streams as proposed before (Balibrea et al., 2017). On the contrary, Jaera nordica insulana and Dycranomyia sp. may be acting as generalist feeders in Azorean streams, which could explain why they are not contributing to litter decomposition despite being relative abundant in some streams (RIB1, RIB2 and RIB4). In fact, it has been shown before that macroinvertebrate have a certain degree of feeding plasticity depending on food resources availability and quality (Friberg & Jacobsen, 1999; Callisto et al., 2007; Carvalho & Graça, 2007).
Physical abrasion is always a factor to consider when comparing litter decomposition between streams or between coarse and fine mesh bags. However, we consider that it was not a relevant factor in our study because (i) litter bags were deployed in depositional areas where the impact of physical abrasion is much reduced, (ii) the six streams were similar in flow and current velocity, and (iii) litter mass remaining was similar between coarse and fine mesh bags for streams without Limnephilus atlanticus but distinct in streams with L. atlanticus.
The effect of shredders on litter decomposition depended on litter species
Analysis of variance detected a significant species × stream interaction, suggesting that effects of shredders on litter decomposition may depend on litter species. In fact, Alnus glutinosa seemed to be the most sensitive species with litter mass remaining in coarse mesh bags varying in the order: RIB6–RIB5 < RIB4–RIB3 < RIB–RIB1; followed by Clethra arborea with litter mass remaining varying in the order: RIB6–RIB5 < RIB4 < RIB3–RIB2–RIB1; and Cryptomeria japonica with litter mass remaining varying in the order: RIB5 < RIB6 < RIB4 < RIB3–RIB2–RIB1. In other words, the effects of shredder presence on litter decomposition in coarse mesh bags were stronger for A. glutinosa than for C. arborea and C. japonica since the density of Limnephilus atlanticus needed to affect litter decomposition increased from the former to the latter litter species. These differences in the response of litter decomposition among litter species may be due to differences in litter characteristics. Alnus glutinosa litter had the highest nutrient concentration and the lowest polyphenol concentration, making it a high quality resource, and previous studies have shown that high quality litter is generally colonized faster by the microbial community and supports higher microbial activity than more recalcitrant litter (Gessner & Chauvet, 1994; Gulis & Suberkropp, 2003; Gulis et al., 2006; Ferreira et al., 2012). The higher microbial biomass accumulation and higher activity of microbial extracellular enzymes on high quality litter generally improve its palatability to shredders (Canhoto & Graça, 2008; Bärlocher & Sridhar, 2014), often leading to faster litter decomposition of this litter (Ferreira et al., 2006; Gulis et al., 2006; Martínez et al., 2013; Raposeiro et al., 2018). Laboratory studies have shown that shredders prefer to feed on palatable litter (Canhoto & Graça, 1995; Azevedo-Pereira et al., 2006; Graça & Cressa, 2010; Balibrea et al., 2017), which justifies the interaction between litter quality and shredder presence on litter decomposition observed here. High concentration of structural and secondary compounds may affect litter palatability to shredders translating in low decomposition rates (Abelho & Graça, 1996; Graça & Cressa, 2010). It has been shown that shredders may use alternative food sources and supplement their diet with algae and macrophyte tissue even when decomposing leaves were present according to food resources quality (Canhoto & Graça, 1999; Callisto et al., 2007; Carvalho & Graça, 2007). This may explain why some shredder species, as Jaera nordica insulana and Dycranomyia sp., may act as generalist feeders in Azorean streams where the riparian vegetation is composed mainly by sclerophyll evergreen plants species that have many secondary compounds, such as essential oils, which may be toxic for invertebrates (Dias et al., 2007; Rosa et al., 2010; Balibrea et al., 2017), especially endemic species (Borges et al., 2009).
Microbial litter decomposition did not vary among streams but depended on litter species
Microbial-driven litter decomposition (in fine mesh bags) did not differ significantly among streams, despite differences in environmental characteristics and aquatic hyphomycetes community structure. Changes in decomposer community structure that are not translated into differences in litter decomposition among streams may suggest some degree of functional redundancy among decomposer communities (Bärlocher & Graça, 2002; Dang et al., 2009; Ferreira et al., 2015, 2017).
Microbial-driven litter decomposition differed, however, among litter species, being faster for Alnus glutinosa and Clethra arborea than for Cryptomeria japonica. These differences in microbial-driven litter decomposition among litter species may be due to differences in litter characteristics. Conifer species like C. japonica are considered poor quality litter for microbial decomposers due to low nutrient concentration, elevated concentration of lignin and polyphenols and high toughness (Bärlocher & Oertli, 1978; Girisha et al., 2003; Martínez et al., 2013; Ferreira et al., 2017). Alnus glutinosa with high nutrient concentration and low polyphenol concentration and C. arborea with low lignin concentration were likely more attractive to microbial decomposers resulting in faster litter decomposition. Similar results were found before in Azorean streams where higher microbial-driven decomposition rates occurred for leaf species with higher nutrient concentration and lower concentrations of structural and secondary compounds (Ferreira et al., 2016, 2017; Raposeiro et al., 2018).
Microbial decomposers activity and community composition vary among streams but are not sensitive to shredder presence
Aquatic hyphomycete community composition, species richness, and sporulation rate on Alnus glutinosa leaves differed among streams, likely reflecting differences in environmental conditions. Previous studies have found differential responses of aquatic hyphomycetes species to dissolved nutrient concentrations with some species being promoted while others are inhibited, although the total spore production is generally enhanced (Gulis & Suberkropp, 2003; Ferreira et al., 2006; Artigas et al., 2008). Species richness and spore production can also differ among streams as a result from potential differences in current velocity (Ferreira & Graça, 2006). These differences, however, did not affect litter decomposition as explained above, suggesting functional redundancy among aquatic hyphomycete communities.
Aquatic hyphomycete parameters did not differ between coarse and fine mesh bags, suggesting that the presence of invertebrates was of minor importance in structuring aquatic hyphomycetes communities and activity. Shredders seem to prefer certain fungal species over others when these are allowed to colonize leaf litter individually in laboratory trials (Arsuffi & Suberkropp, 1985, 1986; Chung & Suberkropp, 2009). However, it may be more difficult for shredders to distinguish among fungal species in environmental samples where the mycelia may be intermingled (Ferreira & Graça, 2006; Gonçalves et al., 2007; Chung & Suberkropp, 2008).
Final considerations
The results from this study allow anticipating that human-induced changes that lead to changes in shredder density and in litter quality may have important effects on litter decomposition and consequently on stream functioning and aquatic food webs. Currently, several human activities have the potential to affect instream communities and riparian composition in oceanic islands, including clearing of the native vegetation, water pollution, and introduction of exotic species. Protection areas should take into consideration the presence of Limnephilus atlanticus, which besides being an endemic species plays a key role on litter decomposition.
References
Abelho, M., 2001. From litterfall to breakdown in streams: a review. The Scientific World Journal 1: 6565–6680.
Abelho, M. & M. A. S. Graça, 1996. Effects of eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in Central Portugal. Hydrobiologia 324: 195–204.
Allan, J. D. & M. M. Castillo, 2007. Stream ecology: structure and function of running waters. Springer, Dordrecht.
Anderson, M. J., 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58: 6265–6639.
Anderson, N. H. & J. R. Sedell, 1979. Detritus processing by macroinvertebrates in stream ecosystems. Annual Review of Entomology 24: 351–377.
Apha, A., 1995. Standard methods for the examination of water and watershed. American Public Health Association, Washington DC.
Arsuffi, T. L. & K. Suberkropp, 1985. Selective feeding by stream caddisfly (Trichoptera) detritivores on leaves with fungal-colonized patches. Oikos 45: 50–58.
Arsuffi, T. L. & K. Suberkropp, 1986. Growth of two stream caddisflies (Trichoptera) on leaves colonized by different fungal species. Journal of the North American Benthological Society 5: 297–305.
Arsuffi, T. L. & K. Suberkropp, 1989. Selective feeding by shredders on leaf-colonizing stream fungi: comparison of macroinvertebrate taxa. Oecologia 79: 30–37.
Artigas, J., A. M. Romaní & S. Sabater, 2008. Effect of nutrients on the sporulation and diversity of aquatic hyphomycetes on submerged substrata in a Mediterranean stream. Aquatic Botany 88: 32–38.
Azevedo-Pereira, H. V. S., M. A. S. Graça & J. M. González, 2006. Life history of Lepidostoma hirtum in an Iberian stream and its role in organic matter processing. Hydrobiologia 559: 183–192.
Balibrea, A., V. Ferreira, V. Gonçalves & P. M. Raposeiro, 2017. Consumption, growth and survival of the endemic stream shredder Limnephilus atlanticus (Trichoptera, Limnephilidae) fed with distinct leaf species. Limnologica 64: 31–37.
Bärlocher, F., 2005. Sporulation by aquatic hyphomycetes. In Graça, M. A. S., F. Bärlocher & M. O. Gessner (eds), Methods to Study Litter Decomposition: A Practical Guide. Springer, New York: 185–188.
Bärlocher, F. & M. A. S. Graça, 2002. Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshwater Biology 47: 1123–1135.
Bärlocher, F. & J. J. Oertli, 1978. Inhibitors of aquatic hyphomycetes in dead conifer needles. Mycologia 70: 964–974.
Bärlocher, F. & K. R. Sridhar, 2014. Association of animals and fungi in leaf decomposition. In Jones, E. B. G., K. D. Hyde & K. L. Pang (eds), Freshwater Fungi. De Gruyter, Germany: 413–442.
Benstead, J. P., J. G. March, C. M. Pringle, K. C. Ewel & J. W. Short, 2009. Biodiversity and ecosystem function in species-poor communities: community structure and leaf litter breakdown in a Pacific island stream. Journal of the North American Benthological Society 28: 454–465.
Berger, F. & A. Aptroot, 2002. Further contributions to the flora of lichens and lichenicolous fungi of the Azores. Arquipélago. Life and Marine Sciences 19A: 1–12.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Borges, P. A., E. B. Azevedo, A. E. S. D. Borba, F. Dinis, R. Gabriel & E. Silva, 2009. Ilhas Oceânicas. Portugal Millenium Ecosystem Assessment. Escolar Editora, Lisboa: 463–510.
Borges, P. A., A. Costa, R. Cunha, R. Gabriel, V. Gonçalves, A. F. Martins, I. Melo, M. Parente, P. M. Raposeiro, P. Rodrigues & R. S. Santos, 2010. Listagem dos organismos terrestres e marinhos dos Açores. Princípia Editora, Lda., Cascais: 432.
Botelho, R. & L. Peñil, 2013. Requalificação Ambiental das Turfeiras do Planalto dos Graminhais pelo projeto LIFE + Laurssilva Susténtavel – Ação C3, C5, E1. Sociedade Portuguesa para o Estudo das Aves, Lisboa.
Boyero, L., R. G. Pearson, M. O. Gessner, L. A. Barmuta, V. Ferreira, M. A. S. Graça, D. Dudgeon, A. J. Boulton, M. Callisto, E. Chauvet, J. E. Helson, A. Bruder, R. J. Albariño, C. M. Yule, M. Arunachalam, J. N. Davies, R. Figueroa, A. S. Flecker, A. Ramírez, R. G. Death, T. Iwata, J. M. Mathooko, C. Mathuriau, J. F. Gonçalves, M. S. Moretti, T. Jingut, S. Lamothe, C. M’Erimba, L. Ratnarajah, M. H. Schindler, J. Castela, L. M. Buria, A. Cornejo, V. D. Villanueva & D. C. West, 2011. A global experiment suggests climate warming will not accelerate litter decomposition in streams but may reduce carbon sequestration. Ecology Letters 14: 289–294.
Callisto Jr., M., J. F. Gonçalves & M. A. S. Graça, 2007. Leaf litter as a possible food source for chironomids (Diptera) in Brazilian and Portuguese headwater streams. Revista Brasileira de Zoologia 24: 442–448.
Canhoto, C. & M. A. S. Graça, 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: Tipula lateralis. Freshwater Biology 34: 209–214.
Canhoto, C. & M. A. S. Graça, 1996. Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333: 79–85.
Canhoto, C. & M. A. S. Graça, 1999. Leaf barriers to fungal colonization and shredders (Tipula lateralis) consumption of decomposing Eucalyptus globulus. Microbial Ecology 37: 163–172.
Canhoto, C. & M. A. S. Graça, 2008. Interactions between fungi (aquatic hyphomycetes) and invertebrates. In Sridhar, K. R., F. Bärlocher & K. D. Hyde (eds), Novel Techniques and Ideas in Mycology. Fungal Diversity Research Series. University of Hong Kong, Hong Kong: 205–325.
Carvalho, E. M. & M. A. S. Graça, 2007. A laboratory study on feeding plasticity of the shredder Sericostoma vittatum Rambur (Sericostomatidae). Hydrobiologia 575: 353–359.
Chung, N. & K. Suberkropp, 2008. Influence of shredder feeding and nutrients on fungal activity and community structure in headwater streams. Fundamental and Applied Limnology/Archiv für Hydrobiologie 173: 35–46.
Chung, N. & K. Suberkropp, 2009. Effects of aquatic fungi on feeding preferences and bioenergetics of Pycnopsyche gentilis (Trichoptera: Limnephilidae). Hydrobiologia 630: 257–269.
Claeson, S. M., C. J. LeRoy, J. R. Barry & K. A. Kuehn, 2013. Impacts of invasive riparian knotweed on litter decomposition, aquatic fungi, and macroinvertebrates. Biological Invasions 16: 1531–1544.
Constância, J. M., 1963. Evolução da paisagem humanizada da Ilha de S. Miguel. Boletim do Centro de Estudos Geográficos 3: 5–60.
Cornut, J., A. Elger, D. Lambrigot, P. Marmonier & E. Chauvet, 2010. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biology 55: 2541–2556.
Covich, A. P., 2009. Freshwater ecology. In Gillespie, R. G. & D. A. Clague (eds), Encyclopedia of Islands. University of California Press, Berkeley: 343–347.
Crowl, T. A., W. H. McDowell, A. P. Covich & S. L. Johnson, 2001. Freshwater shrimp effects on detrital processing and nutrients in a tropical headwater stream. Ecology 82: 775–783.
Cruz, J. & N. Soares, 2018. Groundwater governance in the Azores Archipelago (Portugal): valuing and protecting a strategic resource in small islands. Water 10: 408.
Cummins, K. W., R. C. Petersen, F. O. Howard, J. C. Wuycheck & V. L. Holt, 1973. The utilization of leaf litter by stream detritivores. Ecology 54: 336–345.
Dang, C. K., M. Schindler, E. Chauvet & M. O. Gessner, 2009. Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90: 122–131.
Dias, E., R. B. Elias, C. Melo & C. Mendes, 2007. Biologia e ecologia das florestas das ilhas-Açores. Açores e Madeira: A Floresta das Ilhas 6: 51–80.
DROTH & INAG,2001. Plano Regional da Água. Relatório Técnico. Versão para Consulta Pública. Direcção Regional do Ordenamento do Território e dos Recursos Hídricos e Instituto da Água, Ponta Delgada.
Encalada, A. C., J. Calles, V. Ferreira, C. M. Canhoto & M. A. S. Graça, 2010. Riparian land use and the relationship between the benthos and litter decomposition in tropical montane streams. Freshwater Biology 55: 1719–1733.
Ferreira, V. & M. A. S. Graça, 2006. Do invertebrate activity and current velocity affect fungal assemblage structure in leaves? International Review of Hydrobiology 91: 1–14.
Ferreira, V., V. Gulis & M. A. S. Graça, 2006. Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149: 718–729.
Ferreira, V., A. C. Encalada & M. A. S. Graça, 2012. Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshwater Science 31: 945–962.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet & M. A. S. Graça, 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688.
Ferreira, V., P. M. Raposeiro, A. Pereira, A. M. Cruz, A. C. Costa, M. A. S. Graça & V. Gonçalves, 2016. Leaf litter decomposition in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions. Freshwater Biology 61: 783–799.
Ferreira, V., H. Faustino, P. M. Raposeiro & V. Gonçalves, 2017. Replacement of native forests by conifer plantations affects fungal decomposer community structure but not litter decomposition in Atlantic island streams. Forest Ecology and Management 389: 323–330.
Ferreira, V., L. Boyero, C. Calvo, F. Correa, R. Figueroa, J. F. Gonçalves, G. Goyenola, M. A. S. Graça, L. U. Hepp, S. Kariuki, A. Lopez-Rodriguez, N. Mazzeo, C. M’Erimba, A. Peil, J. Pozo, R. Rezende & F. Teixeira de Mello, 2019. A global assessment of the effects of eucalyptus plantations on stream ecosystem functioning. Ecosystems 22: 629–642.
Friberg, N. & D. Jacobsen, 1994. Feeding plasticity of two detritivore-shredders. Freshwater Biology 32: 133–142.
Friberg, N. & D. Jacobsen, 1999. Variation in growth of the detritivore-shredder Sericostoma personatum (Trichoptera). Freshwater Biology 42: 625–635.
Gessner, M. O. & E. Chauvet, 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75: 1807–1817.
Girisha, G. K., L. M. Condron, P. W. Clinton & M. R. Davis, 2003. Decomposition and nutrient dynamics of green and freshly fallen radiata pine (Pinus radiata) needles. Forest Ecology and Management 179: 169–181.
Goering, H. K. & P. J. Van Soest, 1970. Forage fiber analysis (apparatus, reagents, procedures and some applications). Agricultural Handbook No. 379. ARS-USDA, Washington DC, USA.
Gonçalves, A. L., A. M. Gama, V. Ferreira, M. A. S. Graça & C. Canhoto, 2007. The breakdown of blue gum (Eucalyptus globulus Labill.) bark in a Portuguese stream. Fundamental and Applied Limnology/Archiv für Hydrobiologie 168: 307–315.
Gonçalves, V., P. M. Raposeiro, H. Marques, J. Vilaverde, A. Balibrea, J. Camille-Riva, R. Luz & A. C. Costa, 2016. Monitorização das Massas de Água Interiores e de Transição da Região Hidrográfica dos Açores. Relatório Anual do 1º Ano (R5/1º Ano). CIBIO Açores, Departamento de Biologia, Universidade dos Açores, Ponta Delgada: 86.
Gonçalves, V., P. M. Raposeiro, H. Marques, J. Vilaverde, A. Balibrea, R. Luz & A. C. Costa, 2017. Monitorização das Massas de Água Interiores e de Transição da Região Hidrográfica dos Açores. Relatório da 1ª Campanha do Ano 2 (R1/Ano 2). CIBIO Açores, Departamento de Biologia, Universidade dos Açores, Ponta Delgada: 14.
Graça, M. A. S., 2001. The role of invertebrates on leaf litter decomposition in streams—a review. International Review of Hydrobiology 86: 383–393.
Graça, M. A. S. & C. Canhoto, 2006. Leaf litter processing in low order streams. Limnetica 25: 001–010.
Graça, M. A. S. & C. Cressa, 2010. Leaf quality of some tropical and temperate tree species as food resource for stream shredders. International Review of Hydrobiology 95: 27–41.
Graça, M. A. S., F. Bärlocher & M. O. Gessner, 2005. Methods to study litter decomposition: a practical guide. Springer, New York.
Gulis, V. & K. Suberkropp, 2003. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biology 48: 123–134.
Gulis, V., L. Marvanová & E. Descals, 2005. An illustrated key to the common temperate species of aquatic hyphomycetes. Methods to Study Litter Decomposition. Springer, Dordrecht: 153–167.
Gulis, V., V. Ferreira & M. A. S. Graça, 2006. Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshwater Biology 51: 1655–1669.
Hieber, M. & M. O. Gessner, 2002. Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83: 1026–1038.
Hughes, S. J., 2003. A study of the freshwater macroinvertebrate fauna of Madeira and its application in a regional ecological assessment system (Doctoral dissertation). King’s College, University of London, London.
Hughes, S. J., 2005. Application of the water framework directive to Macaronesian freshwater systems. In: Biology and Environment: Proceedings of the Royal Irish Academy: 185–193.
Hughes, S. J., 2006. Temporal and spatial distribution patterns of larval trichoptera in Madeiran streams. Hydrobiologia 553: 27–41.
Kennedy, K. T. & R. W. El-Sabaawi, 2017. A global meta-analysis of exotic versus native leaf decay in stream ecosystems. Freshwater Biology 62: 977–989.
Kriska, G., 2013. Freshwater Invertebrates in Central Europe: A Field Guide. Springer, Heidelberg, Berlin, New York.
Larned, S. T., 2000. Dynamics of coarse riparian detritus in a Hawaiian stream ecosystem: a comparison of drought and post-drought conditions. Journal of the North American Benthological Society 19: 215–234.
Larned, S. T., R. A. Kinzie, A. P. Covich & C. T. Chong, 2003. Detritus processing by endemic and non-native Hawaiian stream invertebrates: a microcosm study of species-specific effects. Archiv für Hydrobiologie 156: 241–254.
Li, A. O. Y. & D. Dudgeon, 2009. Shredders: species richness, abundance, and role in litter breakdown in tropical Hong Kong streams. Journal of the North American Benthological Society 28: 167–180.
Longo, M. & J. F. Blanco, 2014. Shredders are abundant and species-rich in tropical continental-island low-order streams: Gorgona Island, Tropical Eastern Pacific, Colombia. Revista de Biología Tropical 62: 85–105.
Machado, A. L. F. B. & D. Gonçalves, 2004. Influência do habitat na distribuição da Galinhola (Scolopax rusticolà) na ilha de S. Miguel (Açores) durante a época de reprodução. Universidade do Porto, Porto.
MacKenzie, R. A., T. N. Wiegner, F. Kinslow, N. Cormier & A. M. Strauch, 2013. Leaf-litter inputs from an invasive nitrogen-fixing tree influence organic-matter dynamics and nitrogen inputs in a Hawaiian river. Freshwater Science 32: 1036–1052.
Malmqvist, B., 2002. Aquatic invertebrates in riverine landscapes. Freshwater Biology 47: 679–694.
Martínez, A., A. Larrañaga, J. Pérez, E. Descals, A. Basaguren & J. Pozo, 2013. Effects of pine plantations on structural and functional attributes of forested streams. Forest Ecology and Management 310: 147–155.
McArdle, B. H. & M. J. Anderson, 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82: 290–297.
Pascoal, C., L. Marvanová & F. Cássio, 2005. Aquatic hyphomycete diversity in streams of Northwest Portugal. Fungal Diversity 19: 109–128.
Pereira, A. P., M. A. S. Graca & M. Molles, 1998. Leaf litter decomposition in relation to litter physico-chemical properties, fungal biomass, arthropod colonization, and geographical origin of plant species. Pedobiologia 42: 316–327.
Pozo, J., J. Casas, M. Menéndez, S. Mollá, I. Arostegui, A. Basaguren, C. Casado, E. Descals, J. García-Avilés, J. M. González & A. Larrañaga, 2011. Leaf-litter decomposition in headwater streams: a comparison of the process among four climatic regions. Journal of the North American Benthological Society 30: 935–950.
Pringle, C. M., G. A. Blake, A. P. Covich, K. M. Buzby & A. Finley, 1993. Effects of omnivorous shrimp in a montane tropical stream: sediment removal, disturbance of sessile invertebrates and enhancement of understory algal biomass. Oecologia 93: 1–11.
Raposeiro, P. M., A. C. Costa & S. J. Hughes, 2011. Environmental factors–spatial and temporal variation of chironomid communities in oceanic island streams (Azores archipelago). EDP Sciences. Annales de Limnologie-International Journal of Limnology 47: 325–338.
Raposeiro, P. M., M. A. Cruz, S. J. Hughes & A. C. Costa, 2012. Azorean freshwater invertebrates: status, threats and biogeographic notes. Limnetica 31(1): 0013–0022.
Raposeiro, P. M., S. J. Hughes & A. C. Costa, 2013. Environmental drivers – spatial and temporal variation of macroinvertebrate communities in island streams: the case of the Azores Archipelago. Fundamental and Applied Limnology/Archiv für Hydrobiologie 182: 337–350.
Raposeiro, P. M., G. M. Martins, I. Moniz, A. Cunha, A. C. Costa & V. Gonçalves, 2014. Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates vs. microbial decomposition of native vs. exotic plant species. Limnologica 45: 80–87.
Raposeiro, P. M., V. Ferreira, G. Gea & V. Gonçalves, 2018. Contribution of aquatic shredders to leaf litter decomposition in Atlantic island streams depends on shredder density and litter quality. Marine and Freshwater Research 69: 1432–1439.
Rincón, J. & A. P. Covich, 2014. Effects of insect and decapod exclusion and leaf litter species identity on breakdown rates in a tropical headwater stream. Revista de Biología Tropical 62: 143–154.
Rosa, J. S., C. Mascarenhas, L. Oliveira, T. Teixeira, M. C. Barreto & J. Medeiros, 2010. Biological activity of essential oils from seven Azorean plants against Pseudaletia unipuncta (Lepidoptera: Noctuidae). Journal of Applied Entomology 134: 346–354.
Santos, F. D., M. A. Valente, P. M. A. Miranda, A. Aguiar, E. B. Azevedo, A. R. Tomé & F. Coelho, 2004. Climate change scenarios in the Azores and Madeira islands. World Resource Review 16: 473–491.
Schmidt-Kloiber, A. & D. Hering, 2012. The taxa and autecology database for freshwater organisms, version 5.0. https://www.freshwaterecology.info. Accessed October 2017.
Seena, A., F. Bärlocher, O. Sobral, M. O. Gessner, D. Dudgeon, B. G. Mckie, E. Chauvet, L. Boyero, V. Ferreira, A. Frainer, A. Bruder, C. D. Matthaei, S. Fenoglio, K. R. Sridhar, R. J. Albariño, M. Douglas, A. C. Encalada, E. Garcia, S. D. Ghate, D. P. Giling, V. Gonçalves, T. Iwata, A. Landeira-Dabarca, D. McMaster, A. O. Medeiros, J. Naggea, J. Pozo, P. M. Raposeiro, C. M. Swan, N. S. D. Tenkiano, C. M. Yule & M. A. S. Graça, 2019. Biodiversity of leaf litter fungi in streams along a latitudinal gradient. Science of the Total Environment 661: 306–315.
Silva, L. & C. W. Smith, 2004. A characterization of the non-indigenous flora of the Azores Archipelago. Biological Invasions 6: 193–204.
Skalar, 2004. The San Plus Continuous Flow Analyzer E User Manual: Section 8 e Operating the SFA Analyzer. Skalar Analytical BV, Breda.
Smith, G. C., A. P. Covich & A. M. Brasher, 2003. An ecological perspective on the biodiversity of tropical island streams. AIBS Bulletin 53: 1048–1051.
Strahler, A. N., 1957. Quantitative analysis of watershed geomorphology. Eos, Transactions American Geophysical Union 38: 913–920.
Tachet, H., P. Richoux, M. Bournaud & P. Usseglio-Polatera, 2000. Invertébrés d’eau douce: Systématique, Biologie, Écologie. CNRS éditions, Paris.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 1997. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277: 102–104.
Whittaker, R. J. & J. M. Fernández-Palacios, 2007. Island Biogeography: Ecology, Evolution, and Conservation. Oxford University Press, Oxford.
Wright, M. S. & A. P. Covich, 2005. The effect of macroinvertebrate exclusion on leaf breakdown rates in a tropical headwater stream 1. Biotropica 37: 403–408.
Yeung, A. C., D. P. Kreutzweiser & J. S. Richardson, 2019. Stronger effects of litter origin on the processing of conifer than broadleaf leaves: a test of home-field advantage of stream litter breakdown. Freshwater Biology 64: 1755–1768.
Acknowledgements
This work was funded by FEDER—European Fund for Regional Development through the COMPETE—Operational Programme for Competitiveness Factors and by national funds through FCT—Foundation for Science and Technology under the UID/BIA/50027/2013 and POCI-01-0145-FEDER-006821. VF and PMR acknowledge the financial support from the FCT (IF/00129/2014 and SFRH/BPD/99461/2014, respectively). We thank the Freshwater Ecology Group from the University of the Azores for all the help in the field and the anonymous reviewers for their comments on an earlier version of the manuscript. The field surveys comply with the current laws of Portugal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Checo Colón-Gaud.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balibrea, A., Ferreira, V., Balibrea, C. et al. Contribution of macroinvertebrate shredders and aquatic hyphomycetes to litter decomposition in remote insular streams. Hydrobiologia 847, 2337–2355 (2020). https://doi.org/10.1007/s10750-020-04259-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04259-1