Abstract
In forested headwater streams, decomposition of allochthonous organic matter is a fundamental process driven by aquatic microbes and invertebrate shredders. We examined how season and eutrophication affect leaf decomposition and the associated decomposer communities by immersing leaves of a late deciduous species (Quercus robur) in five streams in Portugal along a gradient of eutrophication in autumn and spring. We found hump-shaped relationships between leaf decomposition and total nitrogen and phosphorus in stream water in both seasons. Leaf decomposition and shredder biomass were higher during spring in streams with moderate levels of eutrophication. Fungal sporulation and biomass were stimulated at moderate levels of eutrophication and inhibited at low or high levels of eutrophication. Fungal assemblage composition shifted between seasons and along the gradient of eutrophication. Tricladium chaetocladium increased its contribution to total conidial production in spring, while Dimorphospora foliicola was dominant in the most eutrophic streams where Articulospora tetracladia was almost absent. Invertebrate shredders were the primary decomposers of leaves in streams with moderate levels of eutrophication, particularly in the warmest season. Although the presence of late deciduous plant species, such as oak, in the riparian corridors may help to mitigate food depletion to freshwater decomposers in spring, our results suggest that moderate eutrophication can accelerate decomposition further reducing litter standing stocks in the warmer seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In low-order forested streams, terrestrial derived litter is the main source of nutrients and energy for aquatic biota (Webster and Benfield 1986). In these ecosystems, leaf decomposition is a key ecological process mainly driven by microorganisms and invertebrates (Graça and Canhoto 2006). Among microorganisms, aquatic hyphomycetes play a pivotal role in leaf decomposition by producing a variety of extracellular enzymes that degrade the structural polysaccharides of plant cell walls, and improve leaf palatability for invertebrate shredders (Suberkropp 1998; Gessner et al. 2007).
Anthropogenic activities such as urbanization, industry and intensified agriculture (Malmqvist and Rundle 2002) have contributed significantly to the degradation of water resources (Dudgeon et al. 2006). Eutrophication caused by the increase of nutrients in stream water, mainly phosphorus and nitrogen, can directly affect stream biota and overall ecosystem functioning (Woodward et al. 2012; Rosemond et al. 2015). Previous studies suggested that increased levels of dissolved nutrients in stream water stimulate leaf decomposition and the activity of associated decomposers (Pascoal et al. 2005a; Ferreira et al. 2006c; Woodward et al. 2012; Lima-Fernandes et al. 2015). However, the co-occurrence of increased nutrients and other stressors, such as sedimentation and/or low dissolved oxygen, can inhibit leaf decomposition due to a reduction in fungal and invertebrate activities (Pascoal et al. 2005a; Medeiros et al. 2009). Moreover, inhibition of leaf decomposition may also occur when nutrients, such as ammonia and nitrites, become toxic to decomposers (Lecerf et al. 2006).
Temperature is an important environmental factor determining the activity of organisms and key ecosystem processes in streams (Brown et al. 2004; Friberg et al. 2009). Under controlled conditions, water temperature affects leaf decomposition rates, by enhancing microbial activity on leaves (Ferreira and Chauvet 2011; Fernandes et al. 2014) and stimulates leaf consumption by invertebrate shredders (González and Graça 2003; Friberg et al. 2009). In fact, correlative studies have found a positive relationship between water temperature and decomposition rates of leaves incubated along latitudinal (Boyero et al. 2011) or geothermal gradients (Friberg et al. 2009).
Studies under different seasonal thermal regimes have reported that temperature can have a strong influence on biological processes and freshwater decomposer communities (Swan and Palmer 2004; Nikolcheva and Bärlocher 2005; Friberg et al. 2009), but, how these alterations affect stream-dwelling decomposer communities remains poorly understood in streams impacted by eutrophication. In this study, we examined the effects of season and nutrient concentrations by assessing leaf decomposition of a late-deciduous species (Quercus robur L.) and the associated fungal and invertebrate decomposers in autumn and spring, in streams spanning a gradient of eutrophication. We hypothesized that: (1) augmented temperature in spring would shift the structure of fungal communities towards species more common in warmer seasons and would stimulate the activity of heterotrophic microorganisms resulting in increased leaf consumption by invertebrate shredders, and (2) intermediate levels of eutrophication would shift the structure of fungal communities towards species more tolerant to higher levels of nutrients and would stimulate microbial activity, potentially increasing leaf consumption by invertebrate shredders.
Methods
Sampling sites and experimental design
The study was conducted in five streams of the Ave River basin (northern Portugal): Agra Stream, Oliveira Stream, Andorinhas Stream, Selho River and Couros Stream. Agra is located in Serra da Cabreira in an area with low human influence, while Oliveira and Andorinhas run through areas with some agricultural activities. The other two sites, Selho and Couros are surrounded by agricultural fields downstream of the city of Guimarães. Selho runs near the city through an area with some industrial activities, while Couros crosses the city. Agra is a 3rd order stream, Oliveira, Andorinhas and Couros are 4th order streams, while Selho is a 5th order river (for a more detailed characterization see Duarte et al. 2015; Lima-Fernandes et al. 2015).
The riparian vegetation of the study region was dominated by Alnus glutinosa Gaerth. (alder), Quercus robur (oak) and Castanea sativa Miller (chestnut), which contributed extensively to the natural litter standing stocks of streams of the Ave River basin. Q. robur has been reported as a late-deciduous and sometimes marcescent species, i.e. leaves wither in autumn but remain dry in the trees, shedding throughout winter until spring (Box and Fujiwara 2015). Additionally, alder and chestnut leaves decompose faster than oak (Lima-Fernandes et al. 2015), so the presence of oak leaves in the stream bed during spring is much higher than alder or chestnut (González and Pozo 1996). Thus, we chose oak leaves for this study given their potential year-round prevalence in the streams to assess the effects of season and eutrophication on their decomposition.
All Q. robur leaves used in this study were collected on the same day, from adjacent trees (easily reached at the collecting site), immediately before abscission in autumn 2007. Leaves were mixed (to decrease the variability in litter quality from different trees), air dried and stored until used. Portions of 3 g (±0.01 g) were weighed and placed in plastic coarse-mesh bags (30 × 23 cm; 5-mm mesh size; 4 replicates). The bags were sealed and immersed at each sampling site on 5th November 2012 (autumn) and 29th April 2013 (spring). After 9, 23 and 43 days of stream immersion, four leaf bags were collected from the stream, placed individually in zip-lock plastic bags, and transported in a cool box (4 °C) to the laboratory, where leaves were rinsed with water over an 850-µm mesh sieve to collect invertebrates. The animals were sorted and preserved in 96 % ethanol for further identification and counting. Leaf material was cut into 12-mm leaf disks (from different leaves) and used to estimate fungal biomass, diversity and to induce fungal sporulation.
Stream water parameters
During the study period, water temperature was continuously monitored with a submerged data logger (HOBO Pendant UA-001-08, Onset Computer Corp., Bourne, MA, USA) in all streams. Conductivity, pH and dissolved oxygen were measured in situ with field probes (Multiline F/set 3 no. 400327, WTW, Weilheim, Germany). Stream water samples were collected into plastic bottles, transported in a cool box (4 °C), and used within 24 h for chemical analyses. Concentrations of nitrite (HACH kit, program 371, diazotization method), nitrate (HACH kit, program 351, cadmium reduction method), phosphate (HACH kit, program 490, ascorbic acid method) and ammonia (HACH kit, program 385, salicylate method) were measured using a HACH DR/2000 spectrophotometer (Hach Company, Loveland, CO, USA), according to the manufacturer instructions.
Identification of fungal spores and quantification of fungal biomass
Fungal sporulation was induced by aeration of 10 leaf disks in 75 mL of filtered stream water (0.22-µm pore size, Sarstedt, Nümbrecht, Deutschland) for 48 ± 4 h, at 16 °C. Conidial suspensions were mixed with 0.5 % triton X-100 and appropriate volumes of each replicate were filtered (0.45-µm pore size, Millipore Corp, Billerica, MA, USA), and stained with 0.05 % cotton blue in lactic acid. At least 300 spores per filter were counted and identified under a light microscope to determine the contribution of each aquatic hyphomycete species to the total conidial production in assemblages. Fungal sporulation rates were calculated for each species as the number of spores released per µg of ash free dry mass (AFDM) per day.
Fungal biomass was estimated from ergosterol concentration on leaves according to Gessner (2005). Lipids were extracted from sets of 8 freeze-dried leaf disks by heating (80 °C for 30 min) in 8 g L−1 KOH in methanol. The ergosterol was purified by solid phase extraction, eluted in isopropanol and quantified by high performance liquid chromatography (Dionex UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) using a LiChrospher RP18 column (250 × 4 mm, Merck KGaA, Darmstadt, Germany). The system was run isocratically with HPLC-grade methanol at 1.4 mL min−1 and 33 °C. Ergosterol was detected at 282 nm and quantified based on a standard curve of ergosterol in isopropanol (Sigma-Aldrich, Saint Louis, MO, USA). Ergosterol concentration was converted to fungal biomass assuming 5.5 μg ergosterol mg−1 mycelial dry mass (Gessner and Chauvet 1993).
DNA extraction and amplification
DNA was extracted from 4 freeze-dried leaf disks using a soil DNA extraction kit (PowerSoil® DNA Isolation kit, MoBio Laboratories, Solana Beach, CA, USA) according to the manufacturer instructions, except for the lysis step that was conducted in the FastPrep FP120 instrument (velocity 5.5, duration 30 s, 2 times) (Qbiogene, Heidelberg, Germany). Fungal diversity was assessed with the primer pairs ITS3GC (GC-GCATCGATGAAGAACGCAGC) and ITS4 (TCCTCCGCTTATTGATATGC) (White et al. 1990), which amplify the ITS2 region of fungal rDNA. For PCR reactions, 1× GoTaq Green Master Mix (Promega, Madison, WI, USA), 0.4 µM of each primer, and 1 µL containing 50 ng of DNA were used in a final volume of 25 µL. Fungal PCRs were carried out in a T100™ Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA), using the following program: initial denaturation at 95 °C for 2 min, followed by 36 cycles of denaturation at 95 °C for 30 s, primer annealing at 55 °C for 30 s, and extension at 72 °C for 1 min. The final extension was done at 72 °C for 5 min (Duarte et al. 2008).
Denaturing gradient gel electrophoresis
DGGE analysis was performed using a DCode™ universal mutation detection system (Bio-Rad Laboratories, Hercules, CA, USA). Samples with 15–40 ng of fungal DNA from the amplification products of 380–400 bp (ITS3GC/ITS4) were loaded on 8 % (w/v) polyacrylamide gels in 1× Tris–acetate-EDTA (TAE) with denaturing gradients from 30 to 60 % (100 % denaturant corresponds to 40 % formamide and 7 M urea). The gels were run at 55 V, 56 °C for 16 h and stained with 1x Midori Green (NIPPON Genetics EUROPE GmbH, Düren, Germany) for 10 min. The gel images were captured under UV light in a GenoSmart gel documentation system (VWR International, Radnor, PA, USA). In each DGGE gel, a DNA mixture of the taxa Dimorphospora foliicola Tubaki (UMB-1085.13), Anguillospora filiformis Greath (UMB-1088.13), Tricladium chaetocladium Ingold (UMB-1101.13), Alatospora pulchella Marvanová (UMB-1115.13), Tricladium splendens Ingold (UMB-1117.13), Triscelophorus acuminatus Nawawi (UMB-1118.13) and Articulospora tetracladia Ingold (UMB-1127.13 and UMB-1136.13) was used to calibrate the gels.
Invertebrate identification and biomass
Leaf-associated invertebrates were identified to the family level and categorized as shredders according to Tachet et al. (2010). After counting and identification, invertebrate shredders were oven-dried to constant mass (±80 °C, 48 h) and weighed to the nearest ±0.0001 g.
Leaf dry mass
The remaining leaf material from each bag was oven-dried (80 °C ± 72 h) to constant mass and weighed to the nearest ±0.0001 g. The dried samples were ashed at 550 °C for 6 h at the Centro de Apoio Científico e Tecnolóxico á Investigación (CACTI, University of Vigo, Spain) to determine the organic matter content (AFDM). Four additional 3 g groups of leaves were freeze dried (72 ± 24 h, Christ alpha 2–4; B. Braun, Melsungen, Germany) to constant mass, weighed (±0.0001 g) and ashed (as above) to determine the initial AFDM.
Statistical analysis
Ordination of streams according to the stream water variables was performed using a principal component analysis (PCA), after standardization of data (z-scores; the values of each variable had their mean subtracted and divided by their standard variation; Zar 2010).
In order to standardize the decomposition process for temperature differences among sites, leaf decomposition rates were expressed in degree-days (dd−1) by multiplying the average temperature during the experiment, by the sampling day (Boyero et al. 2011). The AFDM remaining of oak leaves was fit to the exponential model: W t = W 0 × e −kt, where W t is the leaf dry mass remaining at time t, W 0 is the initial leaf dry mass, and k is the leaf decomposition rate. In the model, a fixed intercept was used at W 0 = 100. Leaf breakdown rates were compared by ANCOVA with time (dd) as a continuous variable and season (fixed factor) and stream (random factor) as categorical variables, followed by Tukey´s HSD tests (Zar 2010). Relationships between decomposition rates and total nitrogen and phosphorus in stream water in autumn and spring were assessed by non-linear regressions (Lorentzian model: y = amplitude/(1 + ((x-center)/width) ^ 2); for spring data, a fixed intercept at amplitude = 0.0044 dd−1 was used in the model). Relationships between decomposition rates and shredder biomass on oak leaves in autumn and spring were assessed by linear regressions.
In spring, time was excluded as a factor in ANOVAs because data for fungal and shredder parameters from day 43 were missing in Oliveira and Andorinhas (leaf decomposition was 97 % in Andorinhas and 100 % in Oliveira, and no leaves were remaining to analyze these two variables). A mixed-effect model was used to assess the effects of season (fixed factor) and stream (random factor) on shredder abundance and biomass and fungal sporulation and biomass associated with oak leaves. Two-way ANOVAs were followed by Tukey’s HSD tests (Zar 2010). Data were transformed when necessary to achieve normality.
To assess if season and stream affected the assemblage of aquatic hyphomycetes based on conidial morphology (number of conidia produced by each fungal species per µg of leaf ash free dry mass per day) and DGGE fingerprints (relative intensity of each OTU) two-way PERMANOVAs were used (Anderson 2001). Data were log (x + 1) transformed and converted into a Bray-Curtis similarity matrix, prior to Unweighted Pair-Group Method Average (UPGMA) cluster analysis.
DGGE gels were aligned and normalized using BioNumerics 7.1 (Applied Maths, Sint-Martens-Latem, Belgium). Each DGGE band was considered one operational taxonomic unit (OTU) taking into account that more than one species can co-migrate to the same position in the gel. PCA, PERMANOVA and clusters were done with PRIMER v6 software package (Primer-E Ltd, Ivybridge, UK). Other statistical analyses were done with STATISTICA 8.0 for Windows (StatSoft, Inc., Tulsa, OK, USA).
Results
Physical and chemical characteristics of the stream water
Physical and chemical characteristics of the stream water among the five streams and two seasons are described in Table 1. Stream water temperature ranged from 10.7 to 13.2 °C in autumn, and from 11.3 to 15.7 °C in spring. Agra had the lowest pH (6.1), while Couros had the highest one (7.2). Conductivity was 18 times higher in Couros compared to the lowest conductivity observed in Agra, and dissolved oxygen concentration was 2 times higher in Oliveira than in Couros. Couros had much higher concentrations of N-NO3 − (48 times), N-NO2 − (49 times), N-NH4 + (731 times) and P-PO4 3− (43 times) compared to the lowest nutrient concentrations observed in Agra.
The PCA ordination of the streams according to the stream water variables showed that axes 1 and 2 explained 96.9 % of the total variance (Fig. 1). The first PC axis separated Agra, Oliveira and Andorinhas, from Selho and Couros, mainly due to differences in pH, conductivity, water temperature and inorganic nutrient concentrations (N-NO2 −, N-NO3 −, P-PO4 3− and N-NH4 +) in stream water. Streams were ordinated according to the gradient of eutrophication as follows: Agra < Oliveira < Andorinhas < Selho < Couros.
Shredder abundance and biomass
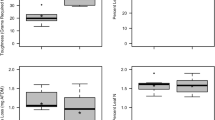
The abundance of shredders associated with oak leaves ranged from 0 to 3.4 individuals leaf bag−1 in autumn, and from 0 to 20.4 individuals leaf bag−1 in spring (Fig. 2a). Stream and season did not affect shredder abundance (Two-Way ANOVA, P > 0.05; Table 2). However, a significant interaction between stream and season was found (Two-Way ANOVA, P < 0.05; Table 2). Shredder abundance was significantly higher in spring, in streams with low (Agra) and moderate (Oliveira) levels of eutrophication (Tukey’s HSD, P < 0.05).
Shredder abundance (a) and biomass (b), and fungal sporulation rates (c) and biomass (d) associated with oak leaves decomposing at the five streams of the Ave River basin, in autumn and spring. Mean ± SEM (n = 6–12). Capital letters correspond to the stream site: AG Agra, OL Oliveira, AN Andorinhas, SE Selho, CO Couros
Shredder biomass ranged from 0 to 13.4 mg DM leaf bag−1 in autumn, and from 0 to 97.1 mg DM leaf bag−1 in spring (Fig. 2b). Stream and season did not affect shredder biomass (Two-Way ANOVA, P > 0.05; Table 2). However, a significant interaction was found between stream and season (Two-Way ANOVA, P < 0.05; Table 2). The highest shredder biomass was observed in spring in streams with moderate (Oliveira and Andorinhas) levels of eutrophication (Tukey’s HSD, P < 0.05).
Fungal sporulation and biomass
In autumn, fungal sporulation rates on decomposing oak leaves varied from 0.1 to 1.2 spores µg−1 AFDM day−1, and from 0.2 to 3.5 spores µg−1 AFDM day−1 in spring (Fig. 2c). Fungal sporulation rates were significantly affected by stream (Two-Way ANOVA, P < 0.05; Table 2), but not by season or the interaction between both factors (Two-Way ANOVA, P > 0.05; Table 2). Fungal sporulation was significantly lower in the most eutrophic stream (Couros) (Tukey’s HSD, P < 0.05), but similar in streams with low (Agra), moderate (Oliveira and Andorinhas) or high (Selho) levels of eutrophication (Tukey’s HSD, P > 0.05).
In autumn, fungal biomass varied from 9.2 to 52.3 mg DM g−1 AFDM, and from 7.9 to 54.3 mg DM g−1 AFDM in spring (Fig. 2d). Fungal biomass was significantly affected by stream (Two-Way ANOVA, P < 0.05; Table 2), but not by season or the interaction between factors (Two-Way ANOVA, P > 0.05; Table 2). Fungal biomass was lowest in the most eutrophic stream and not different among the others.
Fungal assemblages on decomposing leaves
In the oligotrophic stream (Agra), Articulospora tetracladia and Anguillospora filiformis were the species that contributed the most to the total conidial numerical production in autumn (76 %) and spring (86 %; Table 3). Conversely, Dimorphospora foliicola was the dominant species in the most eutrophic stream (Couros), contributing 93 % and 68 % in autumn and spring respectively, to conidial production.
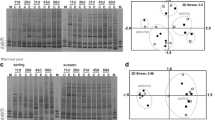
Leaf-associated fungal assemblages (based on conidial morphology or OTUs from DNA fingerprints) were significantly affected by stream, season and the interaction between both factors (PERMANOVA, P = 0.001; Table 4). Based on conidial morphology, autumn and spring assemblages from the oligotrophic stream (Agra) grouped together and separated from the assemblages of other streams (Fig. 3a). Autumn assemblages from streams with moderate (Oliveira and Andorinhas) or high (Selho) levels of eutrophication grouped together and separated from the spring assemblages of Oliveira, Andorinhas, Selho and Couros. On the other hand, based on OTUs from DNA fingerprints, spring assemblages from streams with moderate levels of eutrophication (Oliveira and Andorinhas) grouped together and separated from the assemblages of other streams (Fig. 3b). Autumn assemblages from Oliveira and Andorinhas grouped together, and separated from the autumn and spring assemblages of Agra, Selho and Couros.
Cluster dendograms of fungal communities on decomposing oak leaves, based on fungal conidial morphology (a) and OTUs from DGGE fingerprints (b), in the five streams of the Ave River basin, in autumn and spring. Capital letters correspond to the stream site. Lowercase letters correspond to the season: a autumn, s spring
Leaf decomposition
Decomposition of oak leaves after 43 days of stream immersion varied between 0.00043 (Couros) and 0.00182 dd−1 (Oliveira) in autumn, and 0.00048 (Couros) and 0.00440 dd−1 (Oliveira) in spring (Table 5). Leaf decomposition was significantly affected by time and the interaction between stream and season (ANCOVA, P < 0.05; Table 2), but not by stream or season (ANCOVA, P > 0.05; Table 2). Leaf decomposition was stimulated in streams with moderate levels of eutrophication (Oliveira and Andorinhas) in spring (Tukey’s HSD, P < 0.05), but not in autumn (Tukey’s HSD, P > 0.05; Table 5).
Hump-shaped relationships were found between leaf decomposition rates and the gradient of total nitrogen (Fig. 4a) and phosphorus (Fig. 4b) concentrations in stream water. In both seasons, leaf decomposition increased in streams with moderate levels of eutrophication but was lower at both extremes of the gradient (Lorentzian model). A positive linear relationship between decomposition rates and shredder biomass on leaves was found in spring (linear regression, P < 0.0001), but not in autumn (linear regression, P = 0.1750; Fig. 5).
Relationship between decomposition rates and total nitrogen (a) and phosphorus (b) concentrations in stream water in five streams of the Ave River basin, in autumn (black) and spring (grey). Data were fit to non-linear regressions (Lorentzian model; total nitrogen: autumn, r 2 = 0.85; spring, r 2 = 0.71; phosphorus: autumn, r 2 = 0.72; spring, r 2 = 0.78)
Relationship between decomposition rates and shredder biomass on oak leaves decomposing at the five streams of the Ave River basin, in autumn (black) and spring (grey). Data were fit to linear regressions (autumn, y = 7.6 × 10−5 x + 5 × 10−4, r 2 = 0.51, P = 0.1750; spring, y = 4.2 × 10−5 x + 4 × 10−4, r 2 = 0.99, P < 0.0001)
Discussion
In our study, leaf decomposition and the biomass of shredders were higher in spring in streams with moderate levels of eutrophication (Oliveira and Andorinhas). This agrees with previous studies reporting faster leaf decomposition in warmer seasons, due to a stimulation of microbial and invertebrate activity on leaves by temperature (Swan and Palmer 2004; Friberg et al. 2009; Boyero et al. 2011; Ferreira and Canhoto 2014). However, in this study, the differences observed between seasons cannot be directly connected with differences in temperature, because decomposition rates were normalized by temperature (expressed in dd). Actually, we found a significant linear relationship between decomposition rates and shredder biomass in spring. Thus, the higher decomposition rates observed in the warmer season, particularly in streams with moderate levels of eutrophication (Oliveira and Andorinhas), seemed to be attributed to the higher abundance and biomass of shredders (later instars are more abundant in spring; Basaguren et al. 2002), that may have consumed more leaves (González and Graça 2003), rather than the higher temperatures found in stream water. On the other hand, in these two streams litter standing stocks were reduced in spring, in comparison with autumn stocks (authors pers. obs.). Thus, litter bags immersed in Oliveira and Andorinhas could possibly have acted as food islands in which invertebrates fed at higher rates (Ferreira et al. 2006b).
In this study, streams with moderate levels of eutrophication had higher leaf decomposition rates compared to streams with lower (Agra) or higher levels of eutrophication (Selho and Couros). In fact, hump-shaped relationships between total nitrogen and phosphorus in the stream water and leaf decomposition were found in both seasons, due to nutrient limitation in oligotrophic ecosystems and the excess of nutrients in highly eutrophic streams (this study; Woodward et al. 2012; Lima-Fernandes et al. 2015). Consistent with this prediction, we found that fungal biomass and sporulation, and shredder abundance and biomass on decomposing leaves increased from low to moderate levels of eutrophication and decreased in the most eutrophic stream (Couros). This suggests that increased fungal activity on leaves may have improved litter quality for invertebrate shredders and therefore stimulated leaf decomposition (Gulis and Suberkropp 2003; Pascoal et al. 2005a; Gulis et al. 2006). This is in accordance with previous studies reporting a stimulation of fungal and invertebrate activity on leaves, and consequently leaf decomposition, due to increased nutrient concentrations in stream water (Lecerf et al. 2006; Woodward et al. 2012; Lima-Fernandes et al. 2015). However, the highest levels of ammonium and nitrite, and the lowest dissolved oxygen found in Couros, could have negatively affected fungal and/or invertebrate activity on leaves inhibiting leaf decomposition at this site (Lecerf et al. 2006; Medeiros et al. 2009; Lima-Fernandes et al. 2015). Our data revealed that the effects of eutrophication on leaf decomposition can differ between seasons. Thus, the effects of season on leaf decomposition and associated biota should be taken into account when assessing effects of anthropogenic stress on functional and structural stream condition.
In this study, the structure of aquatic fungal assemblages, based on conidial morphology and OTUs from DGGE fingerprints, clearly differed between seasons. In temperate regions, seasonal alterations in water temperature affected the occurrence and dominance of fungal species (Suberkropp 1984; Nikolcheva and Bärlocher 2005), mostly due to species tolerance to temperature (Duarte et al. 2013). For instance, T. chaetocladium a warm-water species, known to grow and sporulate at higher temperatures (Chauvet and Suberkropp 1998), increased its contribution to the total conidial production during spring. On the other hand, the cold-water species A. tetracladia was dominant on leaves decomposing in Agra, in autumn and spring. In this stream, the community composition of fungi was not affected by season, probably because water temperature did not change much during the study period (10.65–11.25 °C). Although slight differences were found in community structure of fungal species decomposing oak litter between seasons, this was not translated into changes in fungal sporulation and biomass, suggesting redundancy among fungal species that experience seasonal changes in temperature.
The structure of aquatic fungal assemblages on decomposing leaves also shifted along the gradient of eutrophication. Nutrient enrichment has been reported to induce shifts in the dominance of fungal communities on leaves (Pascoal et al. 2005a, b; Duarte et al. 2009). For instance, A. tetracladia was the dominant species on leaves decomposing at streams with low (Agra) or moderate levels of eutrophication (Oliveira and Andorinhas), but was absent from leaves decomposing in the most eutrophic streams (Selho and Couros). Although this species is reported to have a worldwide distribution (Duarte et al. 2016), it appears to be relatively sensitive to eutrophication (Pascoal et al. 2005b; Duarte et al. 2009). On the other hand, in Couros, fungal assemblages were dominated by D. foliicola that is reported to be abundant in nutrient-enriched (Gulis and Suberkropp 2003) and eutrophic streams (Pascoal et al. 2005b; Duarte et al. 2008, 2009). In this eutrophic stream, fungal diversity on leaves appeared to be higher when assessed by DNA-based techniques (data not shown), which is consistent with that found in other streams (Nikolcheva et al. 2005; Fernandes et al. 2015). Thus, DGGE was proven to be a useful technique to assess the diversity of fungi associated with leaves decomposing in hypertrophic streams, where sporulation was strongly inhibited. The shift in fungal species among streams with different trophic status may have also accounted for the differences in fungal biomass and sporulation found among streams. Although aquatic hyphomycete species have been considered functionally redundant (Pascoal et al. 2005a; Ferreira et al. 2006a; Gonçalves et al. 2013), shifts in fungal species composition can affect leaf decomposition directly (Bärlocher and Corkum 2003; Duarte et al. 2006) or indirectly because invertebrate shredders seem to feed preferentially on certain fungal species on decomposing leaves (Suberkropp et al. 1983; Jabiol and Chauvet 2012).
Overall, leaf decomposition rates and shredder biomass were higher in spring in streams with moderate levels of eutrophication, suggesting that shredders were the main decomposers of oak leaves and responsible for the substantial differences found between seasons in these streams. Fungal communities revealed shifts in community composition between seasons and along the gradient of eutrophication. These shifts may affect the ability of fungal communities to decompose leaves directly and also indirectly by changing shredder activity due to differential feeding preferences. Our results suggest that moderately eutrophic streams can face a potential reduction in litter standing stocks in warmer seasons. In temperate deciduous forests, the major input of leaves into streams occurs in autumn (Abelho and Graça 1998), but the presence of late-deciduous species in the riparian corridors, such as oak (Box and Fujiwara 2015), may help to mitigate food depletion that can limit the activity of aquatic decomposers in warmer seasons. Nevertheless, the high activity of invertebrates in moderately eutrophic streams may accelerate leaf decomposition, and, consequently, the disappearance of litter from benthos, with consequences for the stream biota and overall processes in detritus-dependent ecosystems. Thus, seasonal effects should be considered when assessing freshwater ecosystem condition by examining leaf decomposition and aquatic decomposers, particularly in human-impacted eutrophic streams.
References
Abelho M, Graça M (1998) Litter in a first-order stream of a temperate deciduous forest (Margaraça Forest, central Portugal). Hydrobiol 386:147–152. doi:10.1023/A:1003532921432
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi:10.1046/j.1442-9993.2001.01070.x
Bärlocher F, Corkum M (2003) Nutrient enrichment overwhelms diversity effects in leaf decomposition by stream fungi. Oikos 101:247–252. doi:10.1034/j.1600-0706.2003.12372.x
Basaguren A, Riano P, Pozo J (2002) Life history patterns and dietary changes of several caddisfly (Trichoptera) species in a northern Spain stream. Arch für Hydrobiol 155:23–41
Box EO, Fujiwara K (2015) Warm-Temperate Deciduous Forests around the Northern Hemisphere. Spring International Publishing, Switzerland
Boyero L, Pearson RG, Gessner MO et al (2011) A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol Lett 14:289–294. doi:10.1111/j.1461-0248.2010.01578.x
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. doi:10.1890/03-9000
Chauvet E, Suberkropp K (1998) Temperature and sporulation of aquatic hyphomycetes. Appl Environ Microbiol 64:1522–1525
Duarte S, Pascoal C, Cássio F, Bärlocher F (2006) Aquatic hyphomycete diversity and identity affect leaf litter decomposition in microcosms. Oecologia 147:658–666. doi:10.1007/s00442-005-0300-4
Duarte S, Pascoal C, Cássio F (2008) High diversity of fungi may mitigate the impact of pollution on plant litter decomposition in streams. Microb Ecol 56:688–695. doi:10.1007/s00248-008-9388-5
Duarte S, Pascoal C, Garabetian F et al (2009) Microbial decomposer communities are mainly structured by trophic status in circumneutral and alkaline streams. Appl Environ Microbiol 75:6211–6221. doi:10.1128/AEM.00971-09
Duarte S, Fernandes I, Nogueira MJ et al (2013) Temperature alters interspecific relationships among aquatic fungi. Fungal Ecol 6:187–191. doi:10.1016/j.funeco.2013.02.001
Duarte S, Bärlocher F, Trabulo J et al (2015) Stream-dwelling fungal decomposer communities along a gradient of eutrophication unraveled by 454 pyrosequencing. Fungal Divers 70:127–148. doi:10.1007/s13225-014-0300-y
Duarte S, Bärlocher F, Pascoal C, Cássio F (2016) Biogeography of aquatic hyphomycetes: current knowledge and future perspectives. Fungal Ecol 19:169–181. doi:10.1016/j.funeco.2015.06.002
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. doi:10.1017/S1464793105006950
Fernandes I, Seena S, Pascoal C, Cássio F (2014) Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshw Biol 59:2390–2399. doi:10.1111/fwb.12445
Fernandes I, Pereira A, Trabulo J et al (2015) Microscopy- or DNA-based analysis: which methodology gives a truer picture of stream-dwelling decomposer fungal diversity? Fungal Ecol 18:130–134. doi:10.1016/j.funeco.2015.08.005
Ferreira V, Canhoto C (2014) Effect of the experimental and seasonal warming on litter decomposition in a temperate stream. Aquat Sci 76:155–163. doi:10.1007/s00027-013-0322-7
Ferreira V, Chauvet E (2011) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Chang Biol 17:551–564. doi:10.1111/j.1365-2486.2010.02185.x
Ferreira V, Elosegi A, Gulis V et al (2006a) Eucalyptus plantations affect fungal communities associated with leaf-litter decomposition in Iberian streams. Arch für Hydrobiol 166:467–490. doi:10.1127/0003-9136/2006/0166-0467
Ferreira V, Graça MAS, de Lima JLMP, Gomes R (2006b) Role of physical fragmentation and invertebrate activity in the breakdown rate of leaves. Arch für Hydrobiol 165:493–513. doi:10.1127/0003-9136/2006/0165-0493
Ferreira V, Gulis V, Graça MAS (2006c) Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149:718–729. doi:10.1007/s00442-006-0478-0
Friberg N, Dybkjær JB, Olafsson JS et al (2009) Relationships between structure and function in streams contrasting in temperature. Freshw Biol 54:2051–2068. doi:10.1111/j.1365-2427.2009.02234.x
Gessner MO (2005) Ergosterol as measure of fungal biomass. In: MAS Graça, Bärlocher F, Gessner MO (eds) Methods to study litter Decompos. a Pract. Guid. Springer, The Netherlands, pp 189–195
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Gessner MO, Gulis V, Kuehn KA, et al (2007) Fungal decomposers of plant litter in aquatic ecosystems. In: Kubicek CP, Druzhinina IS (eds) Mycota Environ. Microb. relationships, 2nd edn. Springer, Berlin, pp 301–321
Gonçalves AL, Graça MAS, Canhoto C (2013) The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecol 6:546–553. doi:10.1016/j.funeco.2013.07.002
González JM, Graça MAS (2003) Conversion of leaf litter to secondary production by a shredding caddis-fly. Freshw Biol 48:1578–1592. doi:10.1046/j.1365-2427.2003.01110.x
González E, Pozo J (1996) Longitudinal and temporal patterns of benthic coarse particulate organic matter in the Agüera stream (northern Spain). Aquat Sci 58:355–366. doi:10.1007/BF00877475
Graça MAS, Canhoto C (2006) Leaf litter processing in low order streams. Limnetica 25:1–10
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol 48:123–134. doi:10.1046/j.1365-2427.2003.00985.x
Gulis V, Ferreira V, Graça MAS (2006) Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshw Biol 51:1655–1669. doi:10.1111/j.1365-2427.2006.01615.x
Jabiol J, Chauvet E (2012) Fungi are involved in the effects of litter mixtures on consumption by shredders. Freshw Biol 57:1667–1677. doi:10.1111/j.1365-2427.2012.02829.x
Lecerf A, Usseglio-Polatera P, Charcosset J-Y et al (2006) Assessment of functional integrity of eutrophic streams using litter breakdown and benthic macroinvertebrates. Arch für Hydrobiol 165:105–126. doi:10.1127/0003-9136/2006/0165-0105
Lima-Fernandes E, Fernandes I, Pereira A et al (2015) Eutrophication modulates plant-litter diversity effects on litter decomposition in streams. Freshw Sci 34:31–41. doi:10.1086/679223
Malmqvist B, Rundle S (2002) Threats to the running water ecosystems of the world. Environ Conserv 29:134–153. doi:10.1017/S0376892902000097
Medeiros AO, Pascoal C, Graça MAS (2009) Diversity and activity of aquatic fungi under low oxygen conditions. Freshw Biol 54:142–149. doi:10.1111/j.1365-2427.2008.02101.x
Nikolcheva LG, Bärlocher F (2005) Seasonal and substrate preferences of fungi colonizing leaves in streams: traditional versus molecular evidence. Environ Microbiol 7:270–280. doi:10.1111/j.1462-2920.2004.00709.x
Nikolcheva LG, Bourque T, Bärlocher F (2005) Fungal diversity during initial stages of leaf decomposition in a stream. Mycol Res 109:246–253. doi:10.1017/S0953756204001698
Pascoal C, Cássio F, Marcotegui A et al (2005a) Role of fungi, bacteria, and invertebrates in leaf litter breakdown in a polluted river. J North Am Benthol Soc 24:784–797. doi:10.1899/05-010.1
Pascoal C, Cássio F, Marvanová L (2005b) Anthropogenic stress may affect aquatic hyphomycete diversity more than leaf decomposition in a low-order stream. Arch Fur Hydrobiol 162:481–496
Rosemond AD, Benstead JP, Bumpers PM et al (2015) Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science 347:1142–1145. doi:10.1126/science.aaa1958
Suberkropp K (1984) Effect of temperature on seasonal occurrence of aquatic hyphomycetes. Trans Br Mycol Soc 82:53–62. doi:10.1016/S0007-1536(84)80211-9
Suberkropp K (1998) Microorganisms and organic matter decomposition. In: Naiman RJ, Bilby RE (eds) River Ecol. Manag. lessons from Pacific Coast. ecoregion. Springer, New York, pp 120–143
Suberkropp K, Arsuffi TL, Anderson JP (1983) Comparison of degradative ability, enzymatic activity, and palatability of aquatic hyphomycetes grown on leaf litter. Appl Environ Microbiol 46:237–244
Swan CM, Palmer MA (2004) Leaf diversity alters litter breakdown in a Piedmont stream. J North Am Benthol Soc 23:15–28. doi:10.1899/0887-3593(2004)023<0015:LDALBI>2.0.CO;2
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertebrés d’eau douce. Systématique, biologie, écologie. CNRS Editions, Paris, France
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17:567–594
White TJ, Bruns S, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guid. to Methods Appl. Academic Press, pp 315–322
Woodward G, Gessner MO, Giller PS et al (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. doi:10.1126/science.1219534
Zar JH (2010) Biostatistical analysis. Prentice-Hall, Englewood-Cliffs
Acknowledgments
FEDER-POFC-COMPETE (FCOMP-01-0124-FEDER-013954) and the Portuguese Foundation for Science and Technology (FCT) I.P. supported this study (PTDC/AAC-AMB/113746/2009, PTDC/AAC-AMB/117068/2010 and through the strategic funding UID/BIA/04050/2013). Financial support given by FCT to SD (SFRH/BPD/47574/2008, SFRH/BPD/109842/2015) and IF (SFRH/BPD/97656/2013) is also acknowledged. We also want to thank to two anonymous reviewers and to the editorial board for the comments and suggestions made on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, A., Trabulo, J., Fernandes, I. et al. Spring stimulates leaf decomposition in moderately eutrophic streams. Aquat Sci 79, 197–207 (2017). https://doi.org/10.1007/s00027-016-0490-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-016-0490-3