Abstract
We aimed to investigate plasma connective tissue growth factor (CTGF) levels in pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD) (PAH–CHD) in children and the relationships of CTGF with hemodynamic parameters. Plasma CTGF levels were calculated in 30 children with CHD, 30 children with PAH–CHD and 25 health volunteers, using the subtraction method. Cardiac catheterization was performed to measure clinical hemodynamic parameters. Plasma CTGF levels were significantly higher in PAH–CHD than in those with CHD and health volunteers (p < 0.01). In cyanotic PAH–CHD, plasma CTGF levels were significantly elevated compared with acyanotic PAH–CHD in the same group (p < 0.05). Plasma CTGF levels showed positive correlation with B-type natriuretic peptide (BNP) in PAH–CHD (r = 0.475, p < 0.01), while oxygen saturation was inversely related to plasma CTGF levels (r = −0.436, p < 0.05). There was no correlation between CTGF and hemodynamic parameters. Even though the addition of CTGF to BNP did not significantly increase area under curve for diagnosis of PAH–CHD compared with BNP alone (p > 0.05), it revealed a moderately better specificity, positive predictive value and positive likelihood ratio than BNP alone. Plasma CTGF levels could be a promising diagnostic biomarker for PAH–CHD in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a severe progressive disease with high mortality and morbidity, ultimately leading to elevated pulmonary vascular resistance, right heart failure and death [34]. The increased pulmonary blood flow in congenital heart disease (CHD) with left-to-right shunts induces endothelial cell injury and apoptosis and increases pulmonary arterial pressure, eventually triggering the neointimal development and pulmonary vascular remodeling. Therefore, PAH increasingly arises in pediatric patients with CHD [11, 35]. Though specific PAH-target drugs have improved the quality of life and survival by preventing pulmonary vascular remodeling [28, 31], PAH carried a poor prognosis in children with CHD [30, 32]. Over the past years, some blood biomarkers in PAH such as B-type natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), endothelin-1, growth differentiation factor-15 and miRNAs have been used to access diagnosis and prognosis [4, 12], but none of them was validated to be a ideal biomarker.
Connective tissue growth factor (CTGF), also known as CCN2, is a 38-kDa, cysteine-rich secreted peptide and belongs to the member of the CCN (acronym of Cyr61/CEF-10, CTGF/Fisp-12 and Nov) family of growth factors that was originally isolated from umbilical vein endothelial cells [6]. CTGF is involved in normal physical condition, but also participates in pathological processes such as angiogenesis and wound healing, as well as extracellular matrix production, adhesion, proliferation and apoptosis [14]. CTGF functions as a downstream mediator of transforming growth factor-β (TGF-β) signaling which is implicated in the pro-fibrotic actions of TGF-β [8]. More importantly, there are several observations, demonstrating that CTGF is intimately correlated with pulmonary vascular remodeling. Firstly, Lee et al. [24] and Zhu et al. [38] found that abundant CTGF mRNA was expressed in monocrotaline-induced rats with pulmonary hypertension and rats with increased pulmonary blood flow-induced PAH. Secondly, Liu et al. [26] also found that simvastatin could prevent pulmonary vascular remodeling via down-regulation of CTGF mRNA expression in monocrotaline + pneumonectomy-induced rat pulmonary hypertension model. Finally, both Wang et al. and Li et al. found CTGF contributed to the proliferation of pulmonary artery smooth muscle cells [25, 37]; on the contrary, knockdown of CTGF by shRNA led to substantial attenuation of pulmonary vascular remodeling in cigarette smoke-exposed rats [36]. Collectively, these results suggest that CTGF should play an important role in the development of pulmonary vascular remodeling.
Interestingly, its overexpression was mainly associated with diverse fibrosis diseases such as liver fibrosis [33], myocardial fibrosis [21] and diabetic retinopathy [17], and indeed, plasma CTGF levels are shown to be useful in diagnosing or predicting heart failure [3], idiopathic pulmonary fibrosis [20] and airway obstruction in adult patients with asthma [16] and also correlate with the severity of fibrotic diseases [9]. We therefore hypothesize that secreted CTGF levels could be a clinical biomarker in children with PAH associated with CHD (PAH–CHD). However, no reports are available on the associations between plasma CTGF levels and hemodynamic parameters in children with PAH–CHD. Based on these understandings, the aim of the present study was to investigate plasma CTGF levels in children with PAH–CHD and their relationships with hemodynamic parameters.
Materials and Methods
Study Population
Sixty children evaluated at Affiliated Hospital of Sichuan medical university, with left-to-right shunt CHD, were included in the study from September 2012 to December 2014. All the cardiac diagnosis based on clinical and laboratory examinations. According to the diagnostic criteria of PAH [2], there were 30 children with PAH–CHD among them. Healthy control group (HCG) consisted of 25 healthy children. Detailed history and physical examination including age, gender, weight, height, body surface area (BSA), body mass index (BMI), electrocardiography and heart catheterization findings were recorded. The individuals included in the study were screened for disorders such as skin, pancreas, liver or kidney diseases, systemic hypertension. The local ethics committee approved the study protocol. Informed consents were obtained from the parents of the subjects.
Cardiac Catheterization
Routine cardiac catheterization and hemodynamic studies were performed with general anesthesia and systemic heparinization. Details of the procedure were described previously [10]. Pressure measurements were recorded using fluid-filled catheters connected to pressure transducers, including pulmonary artery systolic pressure (PASP), pulmonary artery diastolic pressure (PADP), mean pulmonary artery pressure (mPAP), right ventricular systolic pressure (RVSP) and right ventricular diastolic pressure (RVDP). Oxygen consumption was estimated based on age, sex and heart rate, according to the method of LaFarge and Miettinen [22]. Pulmonary (Qp) blood flow and systemic (Qs) blood flow were calculated using the Fick equation and indexed for body surface area. The ratio of Qp/Qs blood flow, the pulmonary (Rp) and systemic (Rs) vascular resistance and the Rp/Rs ratio were calculated according to the standard formulas [1].
Assay for Plasma CTGF and BNP Level
After overnight fasting, blood samples for CTGF and BNP measurements were collected at 08–09 am, immediately transferred to chilled polypropylene tubes containing EDTA-2Na and centrifuged at 4 °C and 2080 g for 15 min. The plasma samples were stored at −80 °C and then thawed by incubating at 25 °C for 5 min in a water preceding the CTGF assay. BNP was measured using the chemiluminescent microparticle immunoassay on the ARCHITECT 2000i System (Abbott; Abbott Park, IL, USA).
Anti-CTGF antibodies were prepared, and each sandwich enzyme-linked immunosorbent assay (ELISA) was executed as described previously [27]. The full-length CTGF levels were detected by a sandwich ELISA using two monoclonal antibodies against modules 1 and 4. Total CTGF levels were determined by a sandwich ELISA using two monoclonal antibodies against modules 1 and 2. N-terminal CTGF levels were calculated using a subtraction method, as follows: (N-terminal CTGF Level) = (Total CTGF Level) − (Full-Length CTGF Level). All analyses were performed in duplicate, and the mean value is reported for each.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) Program version 13.0 was used in the analysis of data. The values were used to describe mean ± standard deviation. Comparison between groups was made using the Mann–Whitney U test, Chi-square or rank sum test, as appropriate. Correlations between variables were explored with Spearman’s correlation coefficient. Differences in CTGF levels in New York Heart Association (NYHA) classes were analyzed by nonparametric Jonckheere–Terpstra. Receiver operating curve (ROC) analyses were assessed for diagnosis of PAH–CHD with MedCalc statistical software version 11.5.0. The differences between area under curve (AUC) were evaluated by Hanley and McNeil methods [13]. p < 0.05 was considered statistically significant.
Results
The study enrolled 60 individual, including 30 children with CHD (all with left-to-right shunting but no PAH), 30 children with PAH–CHD (13 children with ventricular septal defects, three children with patent ductus arteriosus, one child with atrial septal defect, five children with both ventricular septal defects and patent ductus arteriosus, two children with double-outlet right ventricle, three children atrioventricular septal defect, two children with endocardial cushion defects, one child with total anomalous pulmonary venous return) and 25 health control subjects. Of the patients with PAH, 17 were acyanotic and 13 were cyanotic. The health control group and patient groups did not differ significantly in terms of age, gender and BSA (p > 0.05). Height, weight and BMI were significantly lower in the both CHD group and PAH–CHD group compared with the HCG (p < 0.05), while no difference was found between CHD group and PAH–CHD group (p > 0.05) (Table 1). In addition, BNP levels were higher in PAH–CHD group than not only health control group but also CHD group (Table 1). Table 2 depicts that compared with CHD group, PAH–CHD group had higher PASP, PADP, RVSP, mPAP, Qp, Rp and Rp/Rs (p < 0.01).
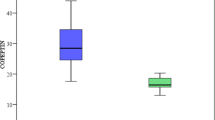
As shown in Fig. 1, plasma CTGF levels were significantly higher in PAH–CHD group (1685.8 ± 460.7 pmol/L) than in HCG (1089.1 ± 261.9 pmol/L) and CHD group (1123.7 ± 344.9 pmol/L) (all p < 0.01), while there was no significant difference between HCG and CHD group (p > 0.05). Figure 2 shows that cyanotic PAH–CHD (1956.5 ± 506.5 pmol/L) had significantly higher levels of plasma CTGF than not only acyanotic PAH–CHD (1528.7 ± 409.5 pmol/L) (p < 0.05) but also CHD (p < 0.01) and that plasma CTGF levels were also higher in acyanotic PAH–CHD than in CHD (p < 0.01).
The results of Spearman’s correlation coefficient of the variables in the PAH–CHD patients are given in Table 3. Plasma CTGF levels were not correlated with PASP, PADP, RVSP, RVDP, mPAP, Qp, Qs, Qp/Qs, Rp, Rs or Rp/Rs, respectively. A positive correlation was found between plasma CTGF levels and BNP (r = 0.475, p < 0.01), while plasma CTGF levels were negatively related to oxygen saturation (r = −0.436, p < 0.05). In addition, plasma CTGF levels were significantly correlated with NYHA classes (p < 0.01).
Through ROC analysis, even though the combination of CTGF with BNP did not significantly increase AUC for diagnosis of PAH–CHD compared with BNP alone (AUC difference 0.061, p = 0.19) (combination, AUC 0.841, BNP, AUC 0.78), it revealed a moderately better specificity, positive predictive value (PPV) and positive likelihood ratio than BNP alone (specificity: combined 93 % vs. BNP 76 %; PPV: combined 91 % vs. BNP 71 %; positive likelihood ratio: combined 10.0 vs. BNP 2.4) (Fig. 3 and Table 4).
Discussion
The available information confirms that a large amount of full-length CTGF which were known to be present in platelets were shown to be released into plasma by platelet activation during or after blood collection and may interfere with determination of plasma CTGF levels existing as N-terminal CTGF fragments [7], which is considered to reflect fibrosis in a variety of organs [9]. We therefore focused our investigation on a novel subtraction method for accurately determining plasma N-terminal CTGF levels [27].
To our knowledge, we demonstrated for the first time that plasma CTGF levels were elevated in children with PAH–CHD. Cyanotic PAH–CHD had significantly higher levels of plasma CTGF than acyanotic PAH–CHD, but plasma CTGF levels also were higher in acyanotic PAH–CHD compared with CHD. Plasma CTGF levels were not correlated with PASP, PADP, RVSP, RVDP, mPAP, Qp, Qs, Qp/Qs, Rp, Rs or Rp/Rs, respectively. BNP levels were positively correlated with plasma CTGF levels, while there was a negative relation between plasma CTGF levels and oxygen saturation. Plasma CTGF levels were associated with NYHA classes in PAH–CHD. Furthermore, even though the addition of CTGF to BNP did not significantly increase AUC for diagnosis of PAH–CHD compared with BNP alone, it revealed a moderately better specificity, PPV and positive likelihood ratio than BNP alone.
Accumulating data demonstrate that CTGF expression was regulated by several stimulating factors, for example mechanical stretch, pressure overload, TGF-β, oxidative and angiotensin II [5]. Thus, it is reasonable that plasma CTGF levels were markedly elevated due to abnormal hemodynamics in children with PAH–CHD. On the other hand, as an important induced factor for PAH, hypoxia increased secretion of CTGF [15]. Our results indicate that the mean mixed venous oxygen saturation of the children with PAH–CHD was approximately 88.9 %, suggesting that tissue hypoxia was slightly present; cyanotic PAH–CHD had significantly higher levels of plasma CTGF than acyanotic PAH–CHD, but also oxygen saturation was negatively correlated with plasma CTGF levels. We therefore speculate that higher plasma CTGF levels in cyanotic PAH–CHD children than in other group that we studied may be explained, at least in part by this mechanism.
Several studies have found that CTGF participated in the development of pulmonary vascular remodeling [24–26, 37, 38]. Conversely, plasmid-based shRNA against CTGF prevented pulmonary vascular remodeling in cigarette smoke-exposed rats [36]. These experimental observations suggest that CTGF expression promotes accumulation of extracellular matrix and proliferation of pulmonary arterial smooth muscle cell and eventually leads to vascular remodeling. In previous studies, CTGF has been involved in many disease states characterized by fibrosis and also has been proposed as a biomarker of fibroproliferative disease [3, 19, 23]. Currently, hemodynamic parameters are key indicators for assessing progression and diagnosis of PAH, but alternative parameters to monitor disease severity and diagnosis which are noninvasive, reliable and relatively quick are urgently needed in children with PAH–CHD. It has been widely accepted that plasma BNP levels as a biomarker for PAH correlated with hemodynamic parameters in PAH [12], while in the present study, the significant relations of elevated plasma CTGF levels with hemodynamic parameters (i.e., PASP, PADP, RVSP, RVDP, mPAP, Qp, Qs, Qp/Qs, Rp, Rs or Rp/Rs) have not been observed, indicating that BNP and CTGF may capture different aspects of PAH–CHD pathophysiology. Immunohistochemical stains of lungs from rat PAH model showed that CTGF was markedly expressed in pulmonary arterial smooth muscle cells [26, 38]. Moreover, vascular remodeling closely associates with pulmonary arterial smooth muscle cells proliferation in PAH. Increased plasma levels of CTGF seem to mirror remodeling processes. Taken together, CTGF is unable to directly relate to and cannot be considered a indicator for the severity of PAH–CHD; thus, CTGF has no prognostic potential. Additionally, Kono et al. [20] demonstrated that plasma CTGF levels were higher in idiopathic pulmonary fibrosis patients and negatively correlated with 6-month change in force vital capacity. Elevated plasma CTGF levels in adult patients with asthma which negatively correlated with force vital capacity, forced expiratory volume in 1 s, were considered to be a clinical biomarker of asthma [16].
BNP is secreted mainly from ventricle in response to volume load and pressure load. Increased plasma BNP levels were correlated with NYHA classes in PAH [12]. We also confirmed an association of plasma CTGF levels with NYHA classes in our study population. Furthermore, although exogenous BNP inhibited the expression of CTGF [18], increased plasma CTGF levels correlated with BNP in our trial. These findings indicate that plasma CTGF levels may reflect, to some extent, cardiac pathologies, especially right heart dysfunction in PAH–CHD. Supporting this hypothesis, similar relations between plasma levels of CTGF and BNP have been observed in adult patients with heart failure [18, 19]. Owing to pathobiology of PAH induced by multifactor, a single biomarker will not be precise for the diagnosis, prognosis and aspects of the underlying disease process for PAH. Thus, a combination of several biomarkers could be the optimal proposal to improve the diagnosis and prognosis. Recently, Nickel et al. [29] demonstrated that the addition of growth differentiation factor-15 to NT-proBNP could improve prognostic value in idiopathic PAH. Notably, as shown in this trial, the combination of CTGF with BNP did not significantly increase AUC for diagnosing PAH–CHD compared with BNP alone in this study, whereas its specificity, PPV and positive likelihood ratio reached 93, 91 and 10.0 %, respectively. These findings clearly indicate that CTGF could be a promising diagnostic biomarker for PAH–CHD in children.
There were several limitations in our study. Firstly, this is a single-center study and sample size needs to be enlarged. Secondly, a study with a longer follow-up period should be executed to evaluate the relation between plasma CTGF levels and clinical hemodynamic parameters. Thirdly, there will be a topic of future studies to investigate the plasma CTGF levels in various conditions of PAH. Fourthly, more work is required to validate the supposition that CTGF is a novel diagnostic biomarker.
Conclusions
To summarize, plasma CTGF levels were significantly elevated in children with PAH–CHD and were positively correlated with BNP and inversely with oxygen saturation. There was no correlation between CTGF and hemodynamic parameters. Plasma CTGF levels could be a promising diagnostic biomarker for PAH–CHD in children.
References
Abdulla R (1998) The science and practice of pediatric cardiology. Pediatr Cardiol 19:211
Barst RJ, McGoon M, Torbicki A, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S (2004) Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 43:40S–47S. doi:10.1016/j.jacc.2004.02.032
Behnes M, Brueckmann M, Lang S, Weiss C, Ahmad-Nejad P, Neumaier M, Borggrefe M, Hoffmann U (2014) Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol 103:107–116. doi:10.1007/s00392-013-0626-6
Bienertova-Vasku J, Novak J, Vasku A (2015) MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens 9:221–234. doi:10.1016/j.jash.2014.12.011
Blom IE, Goldschmeding R, Leask A (2002) Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy. Matrix Biol 21:473–482
Bradham DM, Igarashi A, Potter RL, Grotendorst GR (1991) Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 114:1285–1294
Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M (2004) Activated human platelets release connective tissue growth factor. Thromb Haemost 91:755–760
Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR (1999) Connective tissue growth factor mediates transforming growth factor β-induced collagen synthesis: down-regulation by cAMP. FASEB J 13:1774–1786
Dziadzio M, Usinger W, Leask A, Abraham D, Black CM, Denton C, Stratton R (2005) N-terminal connective tissue growth factor is a marker of the fibrotic phenotype in scleroderma. QJM 98:485–492
Feltes TF, Bacha E, Beekman RH et al (2011) Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123:2607–2652. doi:10.1161/CIR.0b013e31821b1f10
Gatzoulis MA, Alonso-Gonzalez R, Beghetti M (2009) Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev 18:154–161. doi:10.1183/09059180.00003309
Giannakoulas G, Mouratoglou SA, Gatzoulis MA, Karvounis H (2014) Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease. A systematic review. Int J Cardiol 174:618–623. doi:10.1016/j.ijcard.2014.04.156
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T (1999) Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem 274:37461–37466
Huang X, Zou L, Yu X, Chen M, Guo R, Cai H, Yao D, Xu X, Chen Y, Ding C, Cai X, Wang L (2015) Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J Mol Cell Cardiol 82:153–166. doi:10.1016/j.yjmcc.2015.03.005
Kato M, Fujisawa T, Hashimoto D, Kono M, Enomoto N, Nakamura Y, Inui N, Hamada E, Miyazaki O, Kurashita S, Maekawa M, Suda T (2014) Plasma connective tissue growth factor levels as potential biomarkers of airway obstruction in patients with asthma. Ann Allergy Asthma Immunol 113:295–300. doi:10.1016/j.anai.2014.05.026
Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO (2015) The role of CTGF in diabetic retinopathy. Exp Eye Res 133:37–48. doi:10.1016/j.exer.2014.10.016
Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y, Maeno T, Nishida T, Kubota S, Takigawa M, Kurabayashi M (2007) Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 49:1120–1127
Koitabashi N, Arai M, Niwano K, Watanabe A, Endoh M, Suguta M, Yokoyama T, Tada H, Toyama T, Adachi H, Naito S, Oshima S, Nishida T, Kubota S, Takigawa M, Kurabayashi M (2008) Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail 10:373–379. doi:10.1016/j.ejheart.2008.02.011
Kono M, Nakamura Y, Suda T, Kato M, Kaida Y, Hashimoto D, Inui N, Hamada E, Miyazaki O, Kurashita S, Fukamachi I, Endo K, Ng PS, Takehara K, Nakamura H, Maekawa M, Chida K (2011) Plasma CCN2 (connective tissue growth factor; CTGF) is a potential biomarker in idiopathic pulmonary fibrosis (IPF). Clin Chim Acta 412:2211–2215. doi:10.1016/j.cca.2011.08.008
Koshman YE, Patel N, Chu M, Iyengar R, Kim T, Ersahin C, Lewis W, Heroux A, Samarel AM (2013) Regulation of connective tissue growth factor gene expression and fibrosis in human heart failure. J Card Fail 19:283–294. doi:10.1016/j.cardfail.2013.01.013
LaFarge CG, Miettinen OS (1970) The estimation of oxygen consumption. Cardiovasc Res 4:23–30
Leask A, Parapuram SK, Shi-Wen X, Abraham DJ (2009) Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease. J Cell Commun Signal 3:89–94. doi:10.1007/s12079-009-0037-7
Lee YS, Byun J, Kim JA, Lee JS, Kim KL, Suh YL, Kim JM, Jang HS, Lee JY, Shin IS, Suh W, Jeon ES, Kim DK (2005) Monocrotaline-induced pulmonary hypertension correlates with upregulation of connective tissue growth factor expression in the lung. Exp Mol Med 37:27–35
Li G, Hu Y, Jia P, Fu J, Lu CX, Sun YQ, Liu B (2011) Integrin β3 pathway mediated connective tissue growth factor-induced proliferation, migration and extracellular matrix deposition of pulmonary arterial smooth muscle cells. Zhonghua Er Ke Za Zhi 49:895–900
Liu B, Wang XM, Zhou TF, Hua YM, Liu HM, Wei L, Qiao LN, Wang XQ, Zhao SS, Shi K (2008) Expression of connective tissue growth factor and its down-regulation by simvastatin administration in pulmonary hypertensive rats. Zhonghua Er Ke Za Zhi 46:359–365
Miyazaki O, Kurashita S, Fukamachi I, Endo K, Ng PS, Takehara K (2010) Subtraction method for determination of N-terminal connective tissue growth factor. Ann Clin Biochem 47:205–211. doi:10.1258/acb.2010.009182
Montani D, Chaumais MC, Guignabert C, Gunther S, Girerd B, Jais X, Algalarrondo V, Price LC, Savale L, Sitbon O, Simonneau G, Humbert M (2014) Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther 141:172–191. doi:10.1016/j.pharmthera.2013.10.002
Nickel N, Kempf T, Tapken H, Tongers J, Laenger F, Lehmann U, Golpon H, Olsson K, Wilkins MR, Gibbs JS, Hoeper MM, Wollert KC (2008) Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178:534–541. doi:10.1164/rccm.200802-235OC
Ploegstra MJ, Zijlstra WM, Douwes JM, Hillege HL, Berger RM (2015) Prognostic factors in pediatric pulmonary arterial hypertension: a systematic review and meta-analysis. Int J Cardiol 184:198–207. doi:10.1016/j.ijcard.2015.01.038
Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR (2009) Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther 121:69–88. doi:10.1016/j.pharmthera.2008.10.002
Rosenzweig EB, Feinstein JA, Humpl T, Ivy DD (2009) Pulmonary arterial hypertension in children: diagnostic work-up and challenges. Prog Pediatr Cardiol 27:4–11
Sakai K, Jawaid S, Sasaki T, Bou-Gharios G, Sakai T (2014) Transforming growth factor-β-independent role of connective tissue growth factor in the development of liver fibrosis. Am J Pathol 184:2611–2617. doi:10.1016/j.ajpath.2014.06.009
Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A (2004) Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43:5S–12S
Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR (2012) Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 40:1555–1565. doi:10.1183/09031936.00046612
Wang R, Xu YJ, Liu XS, Zeng DX, Xiang M (2011) Knockdown of connective tissue growth factor by plasmid-based short hairpin RNA prevented pulmonary vascular remodeling in cigarette smoke-exposed rats. Arch Biochem Biophys 508:93–100. doi:10.1016/j.abb.2011.01.019
Wang R, Xu YJ, Liu XS, Zeng DX, Xiang M (2012) CCN2 promotes cigarette smoke-induced proliferation of rat pulmonary artery smooth muscle cells through upregulating cyclin D1 expression. J Cell Biochem 113:349–359. doi:10.1002/jcb.23361
Zhu R, He L, Xu J, Zhang Y, Hu Y (2012) Changes of TGF-β1 and CTGF in rats with increased blood flow-induced pulmonary artery hypertension. Zhong Nan Da Xue Xue Bao Yi Xue Ban 37:1013–1020. doi:10.3969/j.issn.1672-7347.2012.10.008
Acknowledgments
We thank Fan Yang and Changxue Wu (Department of Cardiothoracic Surgery, Affiliated Hospital of Sichuan Medical University, 646000, Sichuan, China). This study was supported by the Talent Foundation Sichuan Medical University (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All of the authors declare that they have no conflicts of interest regarding this paper.
Rights and permissions
About this article
Cite this article
Li, G., Tang, L., Jia, P. et al. Elevated Plasma Connective Tissue Growth Factor Levels in Children with Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. Pediatr Cardiol 37, 714–721 (2016). https://doi.org/10.1007/s00246-015-1335-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1335-x