Abstract
Objective
The goal of this study was to determine plasma levels of hepatocyte growth factor (HGF) in patients with pulmonary artery hypertension (PAH), and to explore the diagnostic value of plasma HGF for PAH.
Methods
Sixty subjects were divided into a control group of healthy individuals (N = 15) and a PAH group (N = 45). The PAH group was divided into three groups (N = 15 each) according to disease severity: mild PAH (group L), moderate PAH (group M), and severe PAH (group H). Plasma HGF levels in PAH patients were collected on the morning after admission to the hospital. Mean pulmonary arterial pressure was measured by right heart catheterization.
Results
Plasma HGF levels were significantly higher in the PAH group than in the control group (P < 0.001), and significantly higher in group H than in group M (P < 0.001) and group L (P < 0.001). There was no statistically significant difference in plasma HGF levels between patients with PAH of idiopathic etiology and those with PAH of secondary etiology (P = 0.595). The HGF level was positively correlated with mean pulmonary arterial pressure (Pearson correlation coefficient 0.967, P < 0.001).
Conclusion
Plasma levels of HGF in PAH patients with mild disease were significantly higher than those in healthy controls, suggesting that plasma HGF has potential utility as a diagnostic biomarker for early PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There have been many basic studies of hepatocyte growth factor (HGF) and pulmonary artery hypertension (PAH), but fewer clinical studies. |
The present study showed that serum HGF levels were elevated in patients with PAH and were positively correlated with the mean pulmonary arterial pressure. Although many clinical conditions may contribute to HGF levels, these findings are still exciting. |
HGF may be a biomarker for early diagnosis of PAH, rather than a specific predictor. |
1 Introduction
Pulmonary artery (PA) hypertension (PAH), a clinically progressive syndrome characterized by restriction of pulmonary vascular circulation [1], is the first category of disease in the World Health Organization (WHO) classification of pulmonary hypertension (PH) [2]. The mechanism of PAH pathology involves proliferation and apoptosis of endothelial cells (ECs) and smooth muscle cells (SMCs) in the PA, resulting in remodeling, damage, and reduced circulatory effectiveness of the pulmonary vasculature [3]. Pathological features of PAH include PA vasospasm, hyperplasia and remodeling of the intimal-media layer, in situ thrombosis, and vascular plexus lesions, as well as elevations of PA pressure and pulmonary vascular resistance (PVR), eventually leading to right heart failure and death. PAH has a poor prognosis, with the available treatments being unable to completely reverse the pathological remodeling of the PA. Early diagnosis and intervention can slow the pathological progression and improve prognosis [4].
Unfortunately, early diagnosis of PH is difficult. Common PAH symptoms of shortness of breath, dizziness, fainting, and chest pain are prevalent in other cardiovascular diseases. Furthermore, as the incidence of PAH is not high, some physicians may be unfamiliar with the disease. This lack of exposure, combined with nonspecific symptoms, can make early diagnosis of PAH difficult. However, the most important reason for the difficulty of early diagnosis is the lack of efficient and effective diagnostic biomarkers for early screening of PAH.
The current gold standard for PAH diagnosis is a mean PA pressure (mPAP) of 25 mmHg or greater on right heart catheterization (RHC) [5]. However, RHC is an invasive technique and thus is not suitable for routine screening. A noninvasive method of PAH diagnosis is use of echocardiography to calculate PA systolic pressure (PASP) through tricuspid regurgitation. However, there is limited agreement between PASP values estimated by echocardiography and those estimated by RHC, with echocardiography having only about 50 % accuracy in estimating PASP [6, 7].
Measuring circulating biomarkers for PAH would be an ideal diagnostic approach for the disease, as blood tests for biomarkers are simple and easy to perform. Levels of B-type natriuretic peptide (BNP) and its precursor, the N-terminal fragment of BNP (NT-proBNP), have been suggested as valuable biomarkers for determining risk stratification and prognosis in PAH [8]. Increased levels of BNP and NT-proBNP in patients with PAH are associated with increased mortality, whereas lower BNP levels have been associated with reduced mortality and good treatment outcomes. In comparison with BNP, NT-proBNP is less influenced by diverse patient-related factors [9]. Nevertheless, neither biomarker is suitable for early diagnosis of PAH.
Hepatocyte growth factor (HGF) is a major multifunctional cytokine derived from liver mesenchymal cells. HGF is an EC-specific growth factor [10], which promotes vascular EC proliferation; demonstrates anti-apoptotic, anti-inflammatory, and endothelial nitric oxide synthase–activating vasodilatory effects; and inhibits interstitial lung remodeling [11]. Some researchers have found elevated serum levels of HGF in patients with acute lung injury [12]. Traniyama et al. [13] studied rats with PAH that were transfected with the gene encoding HGF. They showed that HGF could reduce the pressure and endothelial injury in the PA, and this suggested the existence of an association between HGF and PH. However, serum levels of HGF in patients with PAH have not been reported. Therefore, the purposes of our study were to detect plasma HGF levels in patients with PAH and to explore the value of plasma PAH as a biomarker for early diagnosis of PAH.
2 Materials and Methods
2.1 Study Population and Grouping
Forty-five patients with PAH were admitted to our hospital between January 2014 and July 2015, including 20 patients with idiopathic PAH and 25 patients with secondary PH caused by congenital heart disease (10 patients), left heart disease (5 patients), chronic pulmonary embolism (5 patients), or connective tissue disease (5 cases). PAH was diagnosed on the basis of the Diagnosis of Pulmonary Artery Hypertension Treatment Guidelines drafted by the European Society of Cardiology in 2009. All patients provided informed consent for inclusion in this study. The study was approved by the local institutional review board (approval no. LC20140105).
PAH patients were divided into three groups on the basis of disease severity. Patients with mPAP of 25–30 mmHg were diagnosed with mild PAH (group L). Patients with mPAP >30 mmHg but <50 mmHg were diagnosed with moderate PAH (group M). Patients with mPAP ≥50 mmHg were diagnosed with severe PAH (group H). The control group included 15 healthy people who, on the basis of their physical examination, were free of hypertension, diabetes, kidney disease, obesity, and other organic diseases.
2.2 Outcome Measures
Peripheral blood samples were obtained from all participants. Serum was isolated from the blood and stored at −80 °C until analysis. Serum analyte levels were measured by sandwich enzyme-linked immunosorbent assays (ELISAs), according to the manufacturers’ protocols. The optical density values of the samples were measured by use of a human HGF ELISA kit (Boster Biological Engineering Co. Ltd., Wuhan, China). The HGF concentration values were determined from the curve of absorbance versus concentration. Levels of all analytes were measured at least in duplicate and were quantified in comparison with dilutions of recombinant protein standards and standardized serum samples. In addition, NT-proBNP, C-reactive protein (CRP), and alanine aminotransferase (ALT) were detected by an DXC800 automatic biochemical analyzer (Beckman Coulter, Inc., USA). Demographic information—including age, sex, and comorbidities (e.g., systemic hypertension, diabetes, smoking)—was collected from all participants. The drug prescribed for the treatment of each PAH patient was checked before admission.
2.3 Right Heart Catheterization
With the patient supine, routine disinfection was performed, followed by local anesthesia with 2 % lidocaine. Blood vessel access was achieved by the Seldinger method. The right femoral vein was punctured, and an 8F arterial sheath was placed and flushed with heparin–saline solution. Next, the catheter was moved into the main PA through the 6F sheath. A multichannel polygraph was placed in a side hole at the end of the catheter, allowing mPAP and other indicator data (PVR, cardiac index [CI]) to be obtained by tracing of the pressure curve. Finally, the catheter and arterial sheath were removed, the puncture was bandaged, and the surgery was completed.
2.4 Statistical Analysis
Statistical analysis was performed by use of the SPSS 12.0 statistical software package (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA). Measured data were assessed for normality by use of the Kolmogorov–Smirnov test. Normally distributed data were represented as means ± standard deviations. Data were compared between groups by use of a t test for normally distributed data or the Mann–Whitney test otherwise. Linear correlation analysis was used to determine the relationship between mPAP and HGF levels. For count data, a chi-squared test was used. Differences with P values of <0.05 were considered statistically significant, and those with P values of <0.01 were considered highly significant.
3 Results
3.1 Study Population and HGF Levels
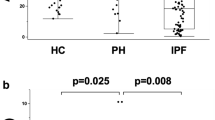
The characteristics of patients in the three PAH groups (L, M, and H) are summarized in Table 1. The three groups showed no differences in age, sex, obesity, or comorbidities. Significantly higher levels of plasma HGF were observed in the PAH group than in the healthy controls (2.220 ± 1.670 versus 0.152 ± 0.038 ng/mL, P < 0.001). HGF levels increased with increasing severity of disease (Fig. 1), with values of 0.685 ± 0.348 ng/mL in group L, 1.808 ± 0.446 ng/mL in group M, and 4.168 ± 1.292 ng/mL in group H (P < 0.001 for groups M or L versus group H; other comparisons were not statistically significant). Additionally, HGF levels in the group with mild PAH were significantly higher than those in the healthy control group (P < 0.001). No statistically significant difference in HGF levels was observed between patients with idiopathic PAH (2.100 ± 1.554 ng/mL) and those with secondary PH (2.370 ± 1.833 ng/mL) (P = 0.595); there was also no statistically significant difference between the four secondary PH groups (P > 0.05; Table 1).
3.2 Clinical Variables in Each Group
Table 2 shows that the differences in levels of PVR and mPAP between the three PAH groups were statistically significant (P < 0.001 for groups M or L versus group H and for groups M versus L); CI values were decreased with elevating mPAP and were lower in group H [2.17 ± 0.06 L/(min·m2)] and group M [2.26 ± 0.10 L/(min·m2)] than in group L [2.48 ± 0.15 L/(min·m2)] (P < 0.001), while those in group H were also lower than those in group M (P = 0.006).
Serum levels of NT-proBNP (median 2500 pg/mL [interquartile range (IQR) 1000–6237]) and CRP (median 16.9 mg/L [IQR 3.45–88.25]) were higher in patients with PAH than in the control group (P < 0.001). NT-proBNP levels increased with higher mPAP or PVR, and there was a statistically significant difference between the three PAH groups (P = 0.006 for groups M versus L, P = 0.002 for groups H versus M, and P < 0.001 for groups H versus L). CRP levels were significantly higher in group H than in groups M and L (P < 0.001); however, no statistically significant difference was observed between groups M and L (P = 0.148). There was no statistically significant difference between the control group (21 U/L [IQR 19–30]) and the PAH group (26 U/L [IQR 19–42], P = 0.104) for ALT levels, nor was there any significant difference between the three PAH groups (P > 0.05). None of the PAH therapies was associated with biomarker levels (Table 2).
3.3 Associations Between mPAP and HGF and Other Clinical Readouts
Linear correlation analysis confirmed the positive correlation between HGF levels and mPAP (Pearson correlation coefficient 0.967, P < 0.001; Fig. 2). Table 2 shows the correlations between HGF and PVR and between HGF and CI; HGF levels also had a high correlation with PVR (r = 0.922, P < 0.001) and a negative correlation with CI (r = −0.839, P < 0.001); NT-proBNP and CRP levels had positive correlations with mPAP (r = 0.755, P < 0.001; r = 0.82, P < 0.001); however, there was no correlation between ALT and mPAP (r = 0.243, P = 0.108).
4 Discussion
In this study, we compared plasma levels of HGF between healthy controls and patients with PAH of varying levels of severity. Plasma HGF levels were significantly higher in PAH patients and in patients with mild PAH than in healthy controls. Furthermore, HGF levels increased as mPAP or PVR increased and CI decreased, revealing a relationship between plasma HGF levels and the extent of PAH. HGF is a pleiotropic factor produced by mesenchymal cells, and it acts on a wide variety of factors (including mitogen and motogen) and influences morphogenesis. Active HGF is a heterodimer composed of an α chain (molecular weight [MW] 69 kD) and a β chain (MW 34 kD) [14]. HGF is widely distributed in the body, being found in the small intestine, brain, thymus, bronchial and alveolar epithelial cells, syncytiotrophoblast cells, and vascular SMCs [15]. Circulating HGF can be detected in blood from healthy humans.
Many recent studies have identified HGF as a crucial proangiogenic factor, which plays important roles in peripheral vascular disease, ischemic heart disease, and ischemic lung disease [16]. Kimihiko et al. [12] measured plasma levels of HGF in patients with acute lung injury and severe lung disease, and found that these patients had elevated levels of HGF protein in serum and HGF mRNA in lung tissue, in comparison with normal values. They concluded from these findings that HGF may promote lung tissue repair and regeneration after acute lung injury. Similarly, we detected significantly higher plasma levels of HGF in PAH patients than in the normal population. Plasma HGF levels were also significantly higher in patients with mild PAH, indicating that HGF concentrations begin to increase in the early period of PAH. A significant relationship was observed between plasma HGF levels and the extent of PAH, as indicated by mPAP.
The mechanism underlying the observed elevated plasma HGF levels in PAH patients is not entirely clear. Various pro-inflammatory factors, including interleukins (ILs) 21α and 21β, can lead to the increased gene and protein expressions seen in HGF [17]. The human HGF gene exists in an IL-6 response element, and IL-6 nuclear factor binding occurs in the transcription initiation region. These observations suggest that IL-6 can induce transcription of HGF. As plasma IL-6 levels are elevated in patients with acute lung injury [18], increased release of inflammatory cytokines may be one important factor that contributes to elevations in plasma HGF levels. Serum CRP levels were elevated with higher mPAP values and showed a positive correlation, which indicated that high levels of CRP relate to both PAH progression and elevated HGF levels. Furthermore, plasma levels of tumor necrosis factor (TNF)-α and nuclear factor (NF)-κB were increased in patients with PAH [19], and TNF-α has been shown to induce expression of HGF via NF-κB [20].
NT-proBNP levels were increased and correlated with mPAP. The association between BNP and HGF is unknown, but heart failure may be indicated directly, which may contribute to HGF elevation [21]. Abnormal liver function may be another factor with an influence on HGF. The ALT levels we measured did not differ significantly between the four groups; however, patients with a higher mPAP were more likely to have complications with abnormal liver function and increased levels of ALT, which may upregulate serum HGF levels [22].
Several limitations of this study should be mentioned. First, many factors may contribute to levels of HGF, such as the drugs used to treat PAH patients and primary diseases (left heart disease, chronic pulmonary embolism or connective tissue disease) leading to PAH. Given the small sample size in this study, this result might have been different if the patient cohort had been larger. Second, HGF levels are likely to be increased in a variety of diseases or conditions such as PA embolism, heart failure, inflammation of the lung or liver, and abnormal liver function. In the above cases, HGF as a biomarker for diagnosis of PAH might have high sensitivity but low specificity. Third, we did not observe a relationship between the duration of PH and HGF, and the long-term prognosis of PAH, which would also be very important to assess in future clinical studies.
The long-term prognosis of patients with PAH is poor but can be significantly improved by early diagnosis and treatment. The results of the present study suggest that HGF has potential as a biomarker for early diagnosis of secondary PH or for screening. However, as the present study enrolled only a small number of cases, the early diagnostic and prognostic value of HGF in PAH will require large-scale experimental verification.
References
Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109(2):159–65.
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94.
Bazan IS, Fares WH. Pulmonary hypertension: diagnostic and therapeutic challenges. Ther Clin Risk Manag. 2015;11:1221–33.
Cordier JF, Chabot F, Dromer C, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30.
Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:34–41.
Kiatchoosakun S, Wongvipaporn C, Nanagara R, Hoit BD. Right ventricular systolic pressure assessed by echocardiography: a predictive factor of mortality in patients with scleroderma. Clin Cardiol. 2011;34:488–93.
Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21.
Giannakoulas G, Dimopoulos K, Bolger AP, et al. Usefulness of natriuretic peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol. 2010;105:869–73.
Takatsuki S, Wagner BD, Ivy DD. B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide in pediatric patients with pulmonary arterial hypertension. Congenit Heart Dis. 2012;7:259–67.
Dimitri Y, Chirgadze JH, Byrd Andrew, et al. Insights into the structur of hepatocyte growth factor/scatter factor (HGF/SF) and implications for receptor activation. FEBS Lett. 1998;430:126–9.
Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):924–40.
Yanagita Kimihiko, Matsumoto Kunio, Sekiguchin Kiyoshi, et al. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:212–7.
Traniyama Y, Morishita R, Aoki M, et al. Therepeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindlimb ischemia models preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8:181–9.
Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology. 1992;15(1):149–55.
Comoglio PM. Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells. EXS. 1993;65:131–65.
Ono K, Matsumori A, Shioi T, et al. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997;95:2552–8.
Ramadori G, Neubauer K, Odenthal M, et al. The gene of hepatocyte growth factor is expressed in fat-storing cells of rat liver and is downregulated during cell growth and by transforming growth factor-beta. Biochem Biophys Res Commun. 1992;183:739–42.
Miyazawa K, Kitamura A, Kitamura N. Structural organization and the transcription ination site of the human hepatocyte growth factor gene. Biochemistry. 1991;30:9170–6.
Price LC, Caramori G, Perros F, et al. Nuclear factor κ-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PloS One. 2013;8:e75415.
Bigatto V, De Bacco F, Casanova E, et al. TNF-α promotes invasive growth through the MET signaling pathway. Mol Oncol. 2015;9:377–88.
Rychli K, Richter B, Hohensinner PJ, et al. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart. 2011;97:1158–63.
Karabulut S, Tas F, Akyüz F, et al. Clinical significance of serum hepatocyte growth factor (HGF) levels in hepatocellular carcinoma. Tumour Biol. 2014;35:2327–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mingting Liang, Ying Pang, Shuguang Zhang, and Mei Zhang declare that they have no conflicts of interest.
Funding
The authors have no funding to declare.
Ethical approval and informed consent
All patients provided informed consent, and the studies were approved by the local ethics committee at Liaocheng City People’s Hospital.
Additional information
M. Liang and Y. Pang contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Liang, M., Pang, Y., Zhang, S. et al. Utility of Hepatocyte Growth Factor as a Biomarker for Early Diagnosis of Pulmonary Artery Hypertension. Mol Diagn Ther 20, 463–468 (2016). https://doi.org/10.1007/s40291-016-0214-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-016-0214-3