Abstract
Background
As a mediator of ECM homeostasis, connective tissue growth factor (CTGF) appears to be involved in adverse structural remodeling processes in the heart. However, the diagnostic and prognostic value of CTGF levels in acute heart failure (AHF) in addition to natriuretic peptide testing has not yet been evaluated.
Methods and results
A total of 212 patients presenting with acute dyspnea and/or peripheral edema to the Emergency Department were evaluated. CTGF and NT-proBNP plasma levels were measured at the initial presentation. All patients were followed up to 1 and 5 years. The first endpoint tested was the diagnostic non-inferiority of combined CTGF plus NT-proBNP compared to NT-proBNP alone for AHF diagnosis. Afterwards, the additional diagnostic value of CTGF plus NT-proBNP was tested. CTGF levels were higher in NYHA class III/IV and AHA/ACC class C/D patients compared to lower class patients (p = 0.04). Patients with HFREF revealed highest CTGF levels (median 93.3 pg/ml, IQR 18.2–972 pg/ml, n = 48) compared to patients with a normal heart function (i.e., without HFREF and HFPEF) (median 25.9, IQR <1–82.2 pg/ml, n = 37) (p < 0.05), followed by patients with HFPEF (median 82.2 pg/ml, IQR 11.5–447 pg/ml, n = 32) as assessed by echocardiography. Finally, CTGF levels were higher in patients with AHF (median 77.3 pg/ml, IQR 22.5–1012 pg/ml, n = 66) compared to those without (p = 0.002). CTGF plus NT-proBNP was non-inferior to NT-proBNP testing alone for AHF diagnosis (AUC difference 0.01, p > 0.05). CTGF plus NT-proBNP improved the diagnostic capacity for AHF (accuracy 82 %, specificity 83 %, positive predictive value 66 %, net reclassification improvement +0.11) compared to NT-proBNP alone (p = 0.0001). CTGF levels were not able to differentiate prognostic outcomes after 1 and 5 years.
Conclusions
Additional CTGF measurements might lead to a better discrimination of higher functional and structural heart failure stages and might identify patients of an increased risk for an acute cardiac decompensation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
European Acute Heart Failure (AHF) Guidelines recommend the use of natriuretic peptides in patients presenting with an acute onset or worsening of heart failure symptoms, such as acute dyspnea or peripheral edema [1]. Natriuretic peptides should be used to rule out AHF being indicated by a NT-proBNP test result of lower than 300 pg/ml (i.e., class of recommendation IIa) and consequently non-cardiac diseases should be investigated [1]. Natriuretic peptides as a confirmative tool for AHF diagnosis reveal diagnostic grey zones being expressed by a moderate test specificity and positive predictive value (specificity 62 %, PPV 55 %) [2, 3]. Pathologically, several non-heart failure-related diseases impair myocardial function without a clinically overt AHF syndrome [e.g., pulmonary hypertension, pulmonary embolism, acute coronary syndrome, atrial fibrillation (AF), cor pulmonale, anemia] [2, 4–6] and thereby increase the synthesis of natriuretic peptides. Renal failure might prolong natriuretic peptide degradation [2]. In contrast, several pathophysiological conditions, such as a marked amount of myocardial ischemia, electrical disorders or cardiac fibrosis, represent specific causes for the development of AHF rather than the result of a compensatory reaction of AHF being expressed by the extend of natriuretic peptides release [2]. Testing new biomarkers in addition to natriuretic peptides has been discussed to improve the diagnostic decision-making and risk stratification of heart failure patients [7].
Heart failure is caused amongst others by an adverse structural remodeling being characterized by ultra-structural changes of cardiac tissue including enhanced collagen deposition and fibrosis [8–13]. Myocardial fibrosis might lead to an increased mechanical stiffness affecting both diastolic and systolic function of the heart [14, 15]. As a matricellular protein, connective tissue growth factor (CTGF/CCN2) [16, 17] is involved in certain physiological conditions, e.g., endochondral ossification, angiogenesis or cellular differentiation, but also participates in pathological processes such as wound healing or fibrosis [18]. CTGF modulates local factors of extracellular matrix (ECM) homeostasis and has been found in multiple fibrotic disorders of the lung, liver, kidney, skin [18] and specifically within cardiac tissue of mice and humans [19–21]. CTGF mRNA expression is influenced by several stimulating (e.g., mechanical stretch or transforming growth factor beta) or inhibiting factors [e.g., b-type natriuretic peptide (BNP)]. CTGF plasma levels have been shown to be increased in patients suffering from chronic heart failure and AF [15, 22, 23].
However, the diagnostic and prognostic value of CTGF levels in the context of AHF in addition to natriuretic peptide testing has not yet been evaluated. Therefore, the present study investigates the diagnostic and prognostic value of CTGF plasma levels in patients with AHF and in different graduations of heart failure.
Methods
Study patients, design and data collection
The Mannheim NT-proBNP Study (MANPRO, clinicaltrials.gov identifier: NCT00143793) [4] was conducted as a single-centre prospective controlled trial at the University Medical Centre Mannheim, Germany. The study was carried out according to the principles of the Declaration of Helsinki and was approved by the Medical Ethics Commission II of the Faculty of Medicine Mannheim, University of Heidelberg, Germany. Informed consent was obtained from all participating patients or their legal representatives.
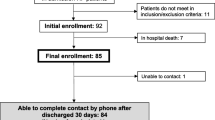
Patients with symptoms of acute dyspnea and/or peripheral edema presenting in the Emergency Department were consecutively included and the underlying diseases were diagnosed. Patients suffering from severe renal disease (defined as serum creatinine level >2.8 mg/dl), anemia (hemoglobin concentration below 8.0 g/dl), obvious traumatic causes of dyspnea, pregnancy, with a status after immediate cardiopulmonary resuscitation, participation in another clinical trial and patients with age under 18 years were excluded. Patient enrolment lasted from August 2005 until March 2006 [4, 24].
Clinical graduations of heart failure
The investigators of the study were neither involved in therapeutic decisions nor in decisions regarding clinical examinations. To determine the main diagnosis of each patient, an independent cardiologist had unrestricted access to the records of the patients, but was blinded to the results of the biomarker measurements. Based on this approach all patients were classified into two categories: (1) symptomatic patients because of AHF, (2) symptomatic patients due to any cause except for AHF. Diagnosis of AHF was based on the European Guidelines for the diagnosis of AHF [1]. Diagnosis of AHF was based on the acute development of typical symptoms (i.e., orthopnea, paroxysmal nocturnal dyspnea, tachypnea, increasing dyspnea or fatigue already starting at lower exercise levels or at rest (i.e., NYHA class III/IV), and a new increase of peripheral edema of the lower extremities. Additionally, specific clinical signs made AHF diagnosis even more likely, such as pulmonary rales, elevated jugular venous pressure, or hepatojugular reflux. At least one of the following technical findings substantiated the diagnosis of AHF, such as radiographic evidence of pulmonary congestion and edema, abnormalities on the ECG [i.e., supraventricular tachycardia (sinus tachycardia, atrial tachycardia, atrial flutter, AF), ventricular arrhythmias, myocardial ischaemia or infarction], or evidence of LV systolic dysfunction (defined as LVEF < 55 %) or diastolic dysfunction as assessed by echocardiography (see below). On the one hand, AHF patients presented with ‘decompensated’ HF, meaning that these patients were previously diagnosed with heart failure, resulting in the acute and rapid progression/development of the described symptoms and signs. On the other hand, AHF patients presented acutely with ‘de novo’ heart failure newly revealing the described symptoms and signs without any prior medical history of heart failure. Patients not fulfilling AHF criteria were allocated to the ‘no AHF’ group. Diagnoses were not based on biomarker levels (such as CTGF or NT-proBNP), because patients and physicians were blinded to biomarker results.
Patients were classified according to the functional NYHA classification and structural ABCD classification of the American College of Cardiology/American Heart Association (ACC/AHA) [1, 25]. Heart failure with a predominant reduced ejection fraction (HFREF) was defined as a left ventricular ejection fraction (LVEF; Simpson’s biplane method) of <55 % as assessed by echocardiography. Heart failure with preserved ejection fraction (HFPEF) accorded to the evidence of diastolic dysfunction and a LVEF > 55 %. Diastolic function was routinely assessed by the E/A ratio (the ratio of the maximum velocities of early (E) to atrial (A) left ventricular filling determined by pulsed-wave Doppler of the mitral valve), deceleration time, isovolumetric relaxation time and ratio of E/E′ (which is used to estimate an increase in left ventricular diastolic pressure, measured at the basal septum). Definitions of diastolic dysfunction corresponded to E/E′ > 15 or stages 1, 2 and 3 [24, 26]. Patients with a normal heart function comprised patients without echocardiographic indices of HFREF or HFPEF.
Measurements of biomarkers
Blood samples for CTGF and NT-proBNP measurements were collected immediately after the initial clinical evaluation in the emergency room. All samples were obtained by venipuncture into ammonium heparin plasma tube for later CTGF/CCN2 and NT-proBNP measurement. Within 30 min all blood samples were centrifuged at 2,000g for 10 min. Plasma was separated, frozen and stored at −80 °C. CTGF measurement was performed with a commercially available immunoassay/ELISA (human connective tissue growth factor, CTGF ELISA kit, Catalog No. E0010h, Uscn Life Science Inc., Wuhan, China). NT-proBNP measurement was performed with a commercially available immunoassay on the Dimension® RxL clinical chemistry system (Flex reagent cartridge PBNP, Dimension System, Dade Behring Ltd., Atterbury Milton Keynes, UK) as previously described [4].
Study endpoints
The first endpoint tested was whether the diagnostic value of combined CTGF plus NT-proBNP was comparable to NT-proBNP alone for diagnosis of AHF.
The second endpoint was to test whether CTGF reveals prognostic information in patients admitted to the Emergency Department with symptoms of acute dyspnea and/or edema (total cohort, n = 212), as well as specifically in those patients of the total cohort suffering from AHF (n = 66). Two different prognostic outcomes were considered: all-cause mortality and AHF-related rehospitalization.
Additionally, revisits at the outpatient clinic and long-term cardiac events occurring in our hospital were documented (e.g., recurrent myocardial infarction, heart rhythms disorders, syncopes, cardiopulmonary resuscitation, stroke, coronary angiography, percutaneous transluminal coronary angioplasty, ICD or pacemaker implantation).
In a first step, our hospital electronic records were screened with regard to in-hospital all-cause mortality, AHF-related rehospitalization, revisits at the outpatient clinic and long-term cardiac events over a follow-up period of at least 5 years. In a second step, family physicians of the patients with incomplete follow-up after step 1 (i.e., either patients without re-admission to our inpatient and outpatient clinics, or patients re-admitted for the last time before expiration of total 5 years follow-up) were contacted to complete survival status. In a third step, survival status was completed by individual telephone visits with the remaining patients, who did not complete total 5 years follow-up after screening by follow-up steps 1 and 2.
Statistical analysis
For normally distributed data, the Student t test was applied. Otherwise, the Mann–Whitney U test was used as nonparametric test. Deviations from a Gaussian distribution were tested by the Kolmogorov–Smirnov test. CTGF and NT-proBNP data were log10 transformed, thereby promoting normality, and the unpaired t test was applied. Spearman’s rank correlation for nonparametric data was used to test the association of CTGF/NT-proBNP blood levels with medical parameters. Qualitative parameters were analyzed by use of a 2 × 2 contingency table and χ 2 test or Fisher’s exact test as appropriate. Quantitative data are presented as mean ± standard error of mean (SEM) or as median and interquartile ranges (25th to 75th percentiles) (=IQR), depending on the distribution of the data. For qualitative parameters absolute and relative frequencies are presented. All analyses were exploratory and utilized a p value of 0.05 (2 tailed) for significance.
Diagnostic value of combined CTGF plus NT-proBNP versus NT-proBNP alone
C-statistics: Receiver-operating characteristic curve (ROC) analyses with areas under the curves (AUC) were calculated for diagnosis of AHF. Two logistic regression models with AHF as dependent variable and the combined biomarkers CTGF plus NT-proBNP as well as NT-proBNP alone (reference biomarker) as independent variables were analyzed. The two areas under the ROC curves were compared using the method by Hanley and McNeil [27]. A margin of AUC difference was set at lower or equal than 0.05 to define diagnostic non-inferiority. The optimal cut-off for the combined biomarkers was chosen where the Youden index (sensitivity + specificity − 1) yielded maximum values. The optimal cut-off for NT-proBNP accorded to the established cut-off value of 300 ng/ml for the diagnosis of AHF [4]. Contingency tables were used to assess the individual diagnostic goodness criteria [i.e., accuracy, specificity, sensitivity, negative/positive predictive values (NPV/PPV)] of the combined biomarkers versus NT-proBNP alone. Accuracy was defined as the sum of true positives plus true negatives divided by the number of measurements (n = 212). Accuracy, specificity, sensitivity were compared by McNemar tests. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated as suggested by Pencina [28, 29]. NRI/IDI close to 0 indicates comparability, a positive NRI/IDI indicates improvement of the combined biomarkers versus NT-proBNP alone. The calculations were performed with InStat and StatMate (GraphPad Software), SPSS software (SPSS Software GmbH), and SAS version, release 9.2 (SAS Institute Inc. Cary, NC, USA).
Results
Patient characteristics
Baseline characteristics of the study patients are summarized in Table 1. Mean age of the patients was 67 years and genders were evenly distributed between AHF and no AHF patients. 31 % (66 out of 212) of the evaluated study patients suffered from AHF. Dyspnea was most commonly observed in 88 % (186 out of 212) of the patients.
CTGF levels are associated with clinical graduations of heart failure
CTGF levels were associated with functional NYHA classification and structural ABCD classification of congestive heart failure: CTGF plasma levels were significantly higher (p = 0.04) in patients being classified to functional NYHA class III/IV (median 74.0 pg/ml, IQR 19.7–1025 pg/ml, n = 66) compared to those of NYHA class I/II (median 34.6 pg/ml, IQR 2.3–441 pg/ml, n = 41) (Fig. 1a). Accordingly, CTGF levels were significantly higher (p = 0.003) in patients being classified to structural ACC/AHA class C/D (median 74.2 pg/ml, IQR 15.4–714 pg/ml, n = 117) compared to patients classified to class A/B (median 24.3 pg/ml, IQR <1–233 pg/ml, n = 77) (Fig. 1b). CTGF levels did not correlate with serum creatinine in all patient groups (p > 0.05).
CTGF levels were significantly increased in patients with functional NYHA class III/IV (n = 66) compared to patients with NYHA class I/II (p = 41) (p = 0.04) (a) and increased in patients with structural AHA/ACC stage C/D (n = 117) compared to patients with stages A/B (n = 77) (p = 0.003) (b). Data are presented as medians with 25th and 75th percentiles (boxes) and 5th and 95th percentiles (whiskers)
Mean left ventricular ejection fraction (LVEF) was 44 % (95 % CI 36–51 %). Patients suffering from HFREF (LVEF < 55 %) revealed highest CTGF levels (median 93.3 pg/ml, IQR 18.2–972 pg/ml, n = 48) compared to patients with a globally normal left ventricular function (median 25.9, IQR <1–82.2 pg/ml, n = 37, p < 0.05), followed by patients with HFPEF (LVEF ≥ 55 %) (median 82.2 pg/ml, IQR 11.5–447 pg/ml, n = 32) (ANOVA p = 0.03) (Fig. 2).
Finally, CTGF levels were able to differentiate patients with a history of AHF (median 77.3 pg/ml, IQR 22.5–1012 pg/ml, n = 66) from those without (median 36.1 pg/ml, IQR <1–318 pg/ml, n = 146) (p = 0.002) (Fig. 3). Even after excluding patients with AF (n = 28 in the no AHF group, n = 32 in the AHF group), CTGF levels were still significantly higher in AHF patients (median 81.4, IQR 51.7–651.1, n = 34) compared to patients without AHF (median 36.1, IQR 36.1–492.1, n = 118) (p = 0.02). When tested in all patients as well as in AHF patients, CTGF levels did not differ between patients with AF and those without AF (p > 0.05). CTGF did not correlate with left atrial size being assessed by echocardiography (r = −0.11, p = 0.3). Also CTGF did not correlate with creatinine levels (r = 0.09, p = 0.2).
Diagnostic value of CTGF in combination with NT-proBNP for AHF
The AUC for the combined biomarkers of CTGF plus NT-proBNP was non-inferior to the AUC of NT-proBNP alone to diagnose AHF (AUC difference 0.01, p > 0.05) (CTGF plus NT-proBNP, AUC 0.88, 95 % CI 0.83–0.93, NT-proBNP, AUC 0.89, 95 % CI 0.83–0.93) (Fig. 4).
The combination of CTGF plus NT-proBNP revealed a significantly (p = 0.0001) greater accuracy, specificity and PPV compared to NT-proBNP alone (accuracy: combined 82 %, 95 % CI 77–87 % versus NT-proBNP 66 %, 95 % CI 59–72 %; specificity: combined 83 %, 95 % CI 77–89 % versus NT-proBNP 53 %, 95 % CI 45–61 %; PPV: combined 66 %, 95 % CI 55–77 % versus NT-proBNP 46 %, 95 % CI 37–55 %). Sensitivity (combined: 78 %, 95 % CI 67–89 %; NT-proBNP: 97 %, 95 % CI 92–99 %), NPV (combined: 90 %, 95 % CI 81–92 %; NT-proBNP: 97 %, 95 % CI 94–99 %) (Table 2). Accordingly, NRI to correctly diagnose AHF by combined CTGF plus NT-proBNP versus NT-proBNP alone was +0.11, corresponding to an IDI of −0.02. CTGF did not correlate with NT-proBNP in univariate analyses (r = 0.10, p = 0.2).
Prognostic value of CTGF and NT-proBNP
Median follow-up period was 5.0 years (IQR 2.1–5.0 years) regarding survival status. Follow-up with regard AHF-related rehospitalization was completed in all patients after 5 years. CTGF levels were not able to differentiate prognostic outcomes (i.e., AHF-related rehospitalization and all-cause mortality) within 1 and 5 years neither in all patients (n = 212) nor in patients suffering from AHF (n = 66) (p > 0.05) (Tables 3, 4). Accordingly, Kaplan–Meier curves did not show significant differentiation with regard to each outcome (data not shown). CTGF levels did not differ significantly with regard to revisits at the outpatient clinic and with regard to long-term cardiac events occurring in our hospital (e.g., recurrent myocardial infarction, heart rhythms disorders, syncopes, cardiopulmonary resuscitation, stroke, coronary angiography, percutaneous transluminal coronary angioplasty, ICD or pacemaker implantation) after 1 and 5 years (data not shown). In contrast, NT-proBNP levels were significantly higher in patients who died after 1 and 5 years, as well as in patients being rehospitalized because of AHF after 1 and 5 years (p = 0.0001).
Discussion
The present study evaluated the diagnostic and long-term prognostic value of CTGF levels in symptomatic patients with acute dyspnea and peripheral edema being suspected of AHF. CTGF levels were associated with more severe stages of functional NYHA and structural AHA/ACC classification of heart failure. Higher CTGF levels discriminated patients with a predominant HFREF from those with a normal left ventricular function as assessed by echocardiography. Finally, CTGF levels were able to differentiate AHF patients from patients with other causes of dyspnea and peripheral edema. Combining CTGF with NT-proBNP measurements revealed a better overall accuracy and specificity to diagnose AHF compared to NT-proBNP testing alone. However, in this cohort CTGF levels could not differentiate prognostic outcomes (e.g., all-cause mortality, AHF-related and all-cause rehospitalization, long-term cardiac events) after 1 and 5 years [30, 31].
To the best of our knowledge, the present trial represents the first to investigate the diagnostic and prognostic value of CTGF plasma levels in a realistic cohort of patients as they presented to the Emergency Department with acute dyspnea and/or peripheral edema and being specifically suspected of AHF.
Clinical evidence of CTGF as a potential biomarker in heart failure is limited to two clinical studies recruiting 52 Japanese patients suffering from chronic HF [22] and 46 Japanese patients with normal or slightly reduced left ventricular heart function [21]. These studies demonstrated increased levels of CTGF both in chronic systolic and diastolic heart failure [21, 22, 32, 33].
CTGF is supposed to be one of the earliest molecular mediators of cardiac fibrosis and remodeling [34–36]. Rapid up-regulation of CTGF mRNA transcription is caused by several stimulating factors including mechanical stretch, G-protein coupled receptors, growth factors, energy substrates like free fatty acids [37, 38] and several neurohormones like endothelin-1, angiotensin II and phenylephrine [18, 36]. CTGF expression was shown to be up-regulated in experimental models both in cardiomyocytes and fibroblasts after acute myocardial infarction and in failing rat hearts [39, 40]. It was demonstrated that specifically neurohormones—e.g., phenylephrine, endothelin-1 and angiotensin II—rapidly induce both CTGF mRNA expression already within 1 h and CTGF protein synthesis within 2–24 h in cardiac myocytes [41, 42]. These experimental observations suggest CTGF to be of potential value in the setting of symptomatic patients suffering from AHF [33].
Additionally, the amount of reparative and reactive fibrosis in patients with chronic HF as substrates of an adverse structural remodeling [8] is supposed to be higher in more severe stages of HF (i.e., NYHA stages III/IV based on symptoms and specifically AHA/ACC stages C/D being additionally based on cardiovascular risk factors and cardiac pathologies) [34, 35], thereby increasing the risk of the deterioration of cardiac function leading to AHF [1, 43].
The described underlying pathophysiological processes might be reflected in the additional diagnostic value of CTGF in combination with NT-proBNP being evaluated within the present study. Identifying heart failure patients of increased risk with regard to a clinical deterioration is recommended [1, 43]. As shown in this trial, the practical clinical use of CTGF measurements to diagnose AHF might be justified in the finding that a combined biomarker strategy of CTGF plus NT-proBNP improves the diagnostic capacity of NT-proBNP alone (i.e., improved test accuracy, specificity and PPV), specifically when applied in those patients with NT-proBNP levels above the established cut-off of 300 pg/ml for AHF diagnosis, where NT-proBNP alone reveals an even weaker accuracy, specificity and PPV [1].
The diagnostic performance of NT-proBNP in the MANPRO study was previously discussed as being slightly lower compared to other studies. Inclusion criteria of both symptoms of acute dyspnea and peripheral edema might have caused a lower prevalence of AHF in the MANPRO study population, whilst representing a realistic subset of Emergency Department patients [4, 44].
Taken together, additional CTGF measurements might lead to a better discrimination of higher functional and structural heart failure stages and might identify patients with an increased risk for an acute cardiac decompensation.
Study limitations
The present study was conducted as a single-center study with a relatively small number of patients. Despite the diagnostic value of CTGF in AHF, CTGF represents a central regulator of fibrotic diseases of various etiologies [45–51]. Inclusion criteria within this study are in accordance with selection criteria of previous clinical trials evaluating CTGF in heart failure [21, 22]. Confirming AHF diagnosis by more than one independent cardiologist might have further improved diagnostic accuracy. HFPEF was not additionally tested by invasive diagnostics (e.g., increased LV end-diastolic pressure or mean pulmonary capillary wedge pressure). They were only assessed by echocardiographic examinations during routine clinical care. Although CTGF has been shown to be related to kidney function in a small selected cohort [52], the present trial did not find any significant correlation between creatinine and CTGF levels. However, our results need to be confirmed in larger prospective clinical trials specifically investigating the prognostic impact of CTGF in heart failure patients [46–51].
Conclusions
This study demonstrated that CTGF levels were associated with higher stages of functional NYHA and structural AHA/ACC classification as well as with left ventricular dysfunction. Plasma levels of CTGF were able to differentiate AHF patients from patients with other causes of dyspnea and peripheral edema. CTGF levels added significantly to the diagnostic performance of NT-proBNP for AHF. CTGF levels did not reveal prognostic information with regard to prognostic outcomes (i.e., all-cause mortality, AHF-related and all-cause rehospitalization, long-term cardiac events).
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K et al (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847
Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG et al (2008) State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 10:824–839
Metra M, Bettari L, Pagani F, Lazzarini V, Lombardi C, Carubelli V et al (2012) Troponin T levels in patients with acute heart failure: clinical and prognostic significance of their detection and release during hospitalisation. Clin Res Cardiol 101:663–672
Behnes M, Brueckmann M, Ahmad-Nejad P, Lang S, Wolpert C, Elmas E et al (2009) Diagnostic performance and cost effectiveness of measurements of plasma N-terminal pro brain natriuretic peptide in patients presenting with acute dyspnea or peripheral edema. Int J Cardiol 135:165–174
Lok DJ, Lok SI, Bruggink-Andre de la Porte PW, Badings E, Lipsic E, Van Wijngaarden J et al (2013) Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol 102:103–110
Neuberger HR, Cacciatore A, Reil JC, Graber S, Schafers HJ, Ukena C et al (2012) Procollagen propeptides: serum markers for atrial fibrosis? Clin Res Cardiol 101:655–661
Braunwald E (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159
Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA (2006) Controversies in ventricular remodelling. Lancet 367:356–367
Towbin JA (2007) Scarring in the heart: a reversible phenomenon? N Engl J Med 357:1767–1768
Sun Y, Weber KT (1998) Cardiac remodelling by fibrous tissue: role of local factors and circulating hormones. Ann Med 30(Suppl 1):3–8
Weber KT (1997) Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation 96:4065–4082
Behnes M, Brueckmann M, Lang S, Espeter F, Weiss C, Neumaier M, et al. Diagnostic and prognostic value of osteopontin in patients with acute congestive heart failure. Eur J Heart Fail 2013. [Epub ahead of print]
Behnes M, Hoffmann U, Lang S, Weiss C, Ahmad-Nejad P, Neumaier M et al (2011) Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol 100:335–342
Brown RD, Ambler SK, Mitchell MD, Long CS (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45:657–687
Weber KT (1997) Monitoring tissue repair and fibrosis from a distance. Circulation 96:2488–2492
Brigstock DR (1999) The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 20:189–206
Brigstock DR (2010) Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 4:1–4
Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA (2009) Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 195:321–338
de Sousa Chuva, Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S et al (2004) Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn 231:542–550
Friedrichsen S, Heuer H, Christ S, Cuthill D, Bauer K, Raivich G (2005) Gene expression of connective tissue growth factor in adult mouse. Growth Factors 23:43–53
Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y et al (2007) Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 49:1120–1127
Koitabashi N, Arai M, Niwano K, Watanabe A, Endoh M, Suguta M et al (2008) Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail 10:373–379
Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S et al (2010) Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol 55:469–480
Behnes M, Lang S, Breithardt OA, Kaden JJ, Haghi D, Ahmad-Nejad P et al. (2008) Association of NT-proBNP with severity of heart valve disease in a medical patient population presenting with acute dyspnea or peripheral edema. J Heart Valve Dis 17:557–565
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013. [Epub ahead of print]
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172 (discussion 207–12)
Cook NR, Ridker PM (2009) Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med 150:795–802
Zobel C, Dorpinghaus M, Reuter H, Erdmann E (2012) Mortality in a cardiac intensive care unit. Clin Res Cardiol 101:521–524
Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD et al (2011) Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol 100:755–764
Reed AL, Tanaka A, Sorescu D, Liu H, Jeong EM, Sturdy M et al (2011) Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol 301:H824–H831
Lampe B, Hammerstingl C, Schwab JO, Mellert F, Stoffel-Wagner B, Grigull A et al (2012) Adverse effects of permanent atrial fibrillation on heart failure in patients with preserved left ventricular function and chronic right apical pacing for complete heart block. Clin Res Cardiol 101:829–836
Ahmed MS, Gravning J, Martinov VN, von Lueder TG, Edvardsen T, Czibik G et al (2011) Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 300:H1291–H1302
Dendooven A, Gerritsen KG, Nguyen TQ, Kok RJ, Goldschmeding R (2011) Connective tissue growth factor (CTGF/CCN2) ELISA: a novel tool for monitoring fibrosis. Biomarkers 16:289–301
Matsui Y, Sadoshima J (2004) Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol 37:477–481
Leask A (2010) Getting to the heart of the matter: CCN2 plays a role in cardiomyocyte hypertrophy. J Cell Commun Signal 4:73–74
Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM (2009) Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol 297:C1490–C1500
Ahmed MS, von Lueder TG, Oie E, Kjekshus H, Attramadal H (2005) Induction of myocardial connective tissue growth factor in pacing-induced heart failure in pigs. Acta Physiol Scand 184:27–36
Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M et al (1998) Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol 30:2411–2422
Kemp TJ, Aggeli IK, Sugden PH, Clerk A (2004) Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol 37:603–606
Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M (2003) Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 108:1499–1505
Bohm M, Voors AA, Ketelslegers JM, Schirmer SH, Turgonyi E, Bramlage P et al (2011) Biomarkers: optimizing treatment guidance in heart failure. Clin Res Cardiol 100:973–981
Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG et al (2005) The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95:948–954
Rachfal AW, Brigstock DR (2005) Structural and functional properties of CCN proteins. Vitam Hormone 70:69–103
Arnott JA, Lambi AG, Mundy C, Hendesi H, Pixley RA, Owen TA et al (2011) The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr 21:43–69
Boor P, Floege J (2011) Chronic kidney disease growth factors in renal fibrosis. Clin Exp Pharmacol Physiol 38:441–450
Dhar A, Ray A (2010) The CCN family proteins in carcinogenesis. Exp Oncol 32:2–9
Jungel A, Distler JH, Gay S, Distler O (2011) Epigenetic modifications: novel therapeutic strategies for systemic sclerosis? Expert Rev Clin Immunol 7:475–480
Li GM, Fan JG (2011) The role of CTGF in mediating hepatocytes epithelial-to-mesenchymal transition and hepatic fibrogenesis. Zhonghua Gan Zang Bing Za Zhi 19:795–797
Phanish MK, Winn SK, Dockrell ME (2010) Connective tissue growth factor-(CTGF, CCN2)—a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol 114:e83–e92
Gerritsen KG, Abrahams AC, Peters HP, Nguyen TQ, Koeners MP, den Hoedt CH et al (2012) Effect of GFR on plasma N-terminal connective tissue growth factor (CTGF) concentrations. Am J Kidney Dis 59:619–627
Acknowledgments
The study was supported by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung—German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
Conflict of interest
There is no conflict of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behnes, M., Brueckmann, M., Lang, S. et al. Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol 103, 107–116 (2014). https://doi.org/10.1007/s00392-013-0626-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-013-0626-6