Abstract
Fish living in the João Dias creek (southern Brazil) have to deal with trace-metal contamination in the long-term basis, as this aquatic environment has been historically impacted by copper mining activities. In order to survive in this harsh environment, the local biota had to develop adaptations related to pollution tolerance. The aim of this study was to test if biochemical mechanisms related to osmoregulation were among these adaptations, using translocation experiments. Water ionic and trace-metal compositions were measured in a nonmetal impacted site (NMIS) and in a metal impacted site (MIS) of this creek. Also, whole-body metal accumulation, ion concentration and branchial enzyme activity (Na,K-ATPase and carbonic anhydrase) were evaluated in Hyphessobrycon luetkenii. In both NMIS and MIS, fish were collected and immediately stored, kept caged or translocated from sites. The result shows that waterborne Cu was 3.4-fold higher at the MIS. Accordingly, animals that had contact with this site showed elevated whole-body Cu levels. Moreover, both translocated groups showed elevated Na,K-ATPase activity. Additionally, fish translocated from the NMIS to the MIS showed lower carbonic anhydrase activity. These findings indicate that H. luetkenii chronically or acutely exposed to naturally elevated waterborne Cu showed a rapid Cu bioaccumulation but was unable to readily excrete it. Moreover, classic Cu osmoregulatory toxicity related to Na,K-ATPase inhibition was not observed. Conversely, impacts in ammonia excretion related to carbonic anhydrase inhibition may have occurred.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Trace-metals are one of the most frequent pollutants released into natural areas (IPCC 2014; Zebral et al. 2019a). Some of these elements are essential components for life due to their redox ability, such as cooper ions (Cu+2). Indeed, this metal is used to form cuproenzymes, molecules involved in several biological functions acting as enzymes, transporters and signaling transducers (Wood et al. 2011; Zhao et al. 2014). Cu is an essential element for life but can be toxic at elevated concentrations (Zebral et al. 2020). In several freshwater animals, including fish, this metal has been reported to inhibit key enzymes such as Na/K-ATPase (Laurén and McDonald 1987) and carbonic anhydrase (Zimmer et al. 2012) leading to disfunction in ionic regulation (Grosell et al. 2002; Grosell 2011). Indeed, pollution is now considered as one of the greatest challenges that humans and other animals have to face (IPCC 2014; Zebral et al. 2019a).
The Na/K-ATPase is abundant in epithelial tissues, where this enzyme is used for the maintenance of intracellular ionic gradients used for absorption or excretion of compounds and for cellular homeostasis maintenance (McCormick et al. 2009). At the gills of freshwater fish, this enzyme can be majorly found in specialized cells responsible for ion uptake, the chloride cells (McCormick et al. 2009). Within this organ, Na/K-ATPase is responsible for the reduction of the intracellular concentration of Na+, producing the gradient used for the entry of this ion through sodium channels (McCormick et al. 2009). On the other hand, the carbonic anhydrase is a ubiquitous enzyme responsible for catalyzing the reversible hydration of CO2 to form H+ and HCO3− (Zebral et al. 2019a). This reaction is important to many biochemical processes, such as acid–base regulation, ammonia excretion and ionic and osmotic regulation (Zebral et al. 2019a).
In order to adapt and survive in metal-contaminated environments, aquatic fauna has to develop physiological mechanisms associated with adjustments in metal uptake, storage and excretion, leading to reduced sensitivity to these elements (Depledge and Rainbow 1990; Whitehead et al. 2011; Romero et al. 2012; Uren Webster et al. 2013; Hamilton et al. 2016). Within this context, field experiments of reciprocal translocation are elegant strategies to elucidate such physiological mechanisms (Larsen et al. 2011). For example, the fish Fundulus heteroclitus locally adapted to different levels of chemical contamination showed divergent physiological patterns among populations. These patterns were related with the negative impacts caused by long-term exposure to contaminants such as polychlorinated biphenyls, dioxins, polycyclic aromatic hydrocarbons, trace-metals and pesticides (Whitehead et al. 2011).
The sub-basin of João Dias creek, a tributary of the Camaquã River located at Camaquã municipality (Caçapava do Sul city, southern Brazil), was directly impacted by Cu mining activity for more than a century (1870–1996) (Laybauer 1998) and even though the extraction has been discontinued for almost two decades, elevated levels of Cu can still be found in water and sediments from this area (Laybauer 1998; Ronchi and Lobato 2000; Bidone et al. 2001; Abril et al. 2018). To give an example, a recent study reported Cu waterborne levels corresponding to 8.5 µg/L in the region. Interestingly, the same study also showed that a non-impacted portion of this creek, located upstream the mining area, had Cu concentrations corresponding to 4.3 µg/L (Abril et al. 2018). In face of the presented facts, it is clear that the João Dias creek is an interesting environment to perform studies related to chronic exposure of aquatic organisms to naturally elevated levels of Cu. This kind of study is majorly needed, taking into consideration that most of the studies on the toxic effects of this metal were conducted under laboratory conditions and throughout acute exposure periods (Zebral et al. 2018). As a result of that, our knowledge related to Cu chronic effects on biochemical and physiological parameters is still limited, especially in wild fish populations (Whitehead et al. 2011; Uren Webster et al. 2013; Hamilton et al. 2016; Abril et al. 2018; Zebral et al. 2018; Anni et al. 2019a; Anni et al. 2019b; Zebral et al. 2019b; Zebral et al. 2021).
Despite the chronic contamination of João Dias creek, 36 fish species can still be found at the region, representing around 50% of all fish species reported for Camaquã river basin (Konrad and Paloski 2000). Among them, the lambari Hyphessobrycon luetkenii is a small fish that can be found at Rio Grande do Sul water basins (southern Brazil), at coastal rivers of Rio de Janeiro (southeastern Brazil), and at Paraguay and Uruguay rivers. At Rio Grande do Sul, this species can be found at the Patos Lagoon system and at the drainage basin of the following rivers: Camaquã, Uruguay, Negro, Paraguay, Tramandaí, Mampituba (Weiss 2013) and Guaíba (Konrad and Paloski 2000). H. luetkenii is known to form localized populations that usually swims in small groups of two to five individuals (Lima et al. 2008). These small (7 cm) animals are omnivorous freshwater fish, with a diet composed of small invertebrates, detritus and algae (Graciolli et al. 2003). Unfortunately, studies describing H. luetkenii life history are still scarce, but it is known that this fish species are fast growing animals, with parceled spawning of adhesive eggs. It is also interesting to comment that H. luetkenii is not migratory and completes its full reproductive cycle in localized environments (Giora et al. 2000; Lima et al. 2008).
The objective of this work was to search for physiological adaptations involved in osmoregulation in wild populations of the fish H. luetkenii living in the pollution portion of João Dias creek. Additionally, acute physiological adjustments were also evaluated, using translocation experiments. Physiological parameters evaluated were whole-body metal (Cd, Cu, Fe, Mn, Pb and Zn) and major ions (Mg, Na, K and Ca) concentrations and the activity of branchial enzymes involved in ionic regulation (Na/K-ATPase and carbonic anhydrase). To achieve our goal, fish living in a nonmetal impacted site (NMIS) and in a metal impacted site (MIS) of João Dias creek were collected for analysis. Also, fish collected at the NMIS were kept caged at the collection site or translocated and kept caged at the MIS and vice versa. Following 96 h of experiment, fish were collected for analysis. Our hypothesis was that animals chronically exposed to Cu contamination would show elevated Cu bioaccumulation and unimpacted levels of branchial enzymes and major ions, suggesting the presence of physiological adjustments at the populational level. On the other hand, it was hypothesized that animals translocated from the NMIS to the MIS would show clear signs of physiological impacts, such as inhibition in Na/K-ATPase and carbonic anhydrase and reduced levels of major ions.
Materials and Methods
Fish Translocation Experiment
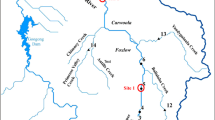
The present work was performed in two areas of the João Dias creek located at Minas do Camaquã, southern Brazil. These areas were composed by a NMIS located at 7 km upstream the place where the mining activity occurred (30°53′47''S–53°25′28''W) and a historically MIS located within the mining area (30°52′55''S–53°27′11''W). An aerial photograph showing the collection points can be found in Fig. 1, and photos of each site can be found in the supplementary material (Fig. 1a). At each site, 30 individuals divided among male and female fish (mean total body length = 4.32 cm) were caught using a fish trap and were divided into six groups. The first two groups were composed by animals collected at each site and immediately anesthetized with benzocaine, quickly rinsed in MilliQ water, euthanized by spine cord sectioning and stored in liquid nitrogen for the analysis of whole-body metal concentrations. Additionally, another group of animals were collected and euthanized as stated above but had their gills dissected and stored in liquid nitrogen for enzyme activity determination (Na/K-ATPase and carbonic anhydrase). The animals immediately collected at the NMIS and at the MIS were designated as control (C; n = 5 for Cu body burden and enzyme analysis) and polluted (P; n = 5 for Cu body burden and enzyme analysis), respectively.
Aerial photograph showing the sites where the freshwater fish Hyphessobrycon luetkenii was collected. The blue circle is indicating the precise location of the unimpacted site (NMIS) and the red circle is indicating the precise location of the metal impacted site (MIS). This aerial photograph was obtained using the software Google Earth Pro 7.3
The other experimental groups were composed by animals collected and kept caged at the NMIS (CCC; n = 5 for Cu body burden and enzyme analysis), collected and kept caged at the MIS (CCP; n = 5 for Cu body burden and enzyme analysis), collected at the NMIS and translocated to MIS (CP; n = 5 for Cu body burden and enzyme analysis) and animals collected at the MIS and translocated to the NMIS (PC; n = 5 for Cu body burden and enzyme analysis). Fish translocation was performed immediately following collection and animals were transported in buckets filled with local water continuously aerated with mobile air pumps. At the final destination, animals were acclimated to the new conditions by the addition of small volumes of water from the translocation experiment to the bucket. Following an hour, animals were transferred to the containment cages. Fish from CCC, CP, CCP and PC groups were kept caged for 96 h. This is the most traditional experimental period used in acute ecotoxicological experiments, therefore, using it allows direct comparisons with previous and future studies. Additionally, longer experimental periods could implicate in the intensification of possible cofounding factors related with caging the animals, such as different patterns of food availability and less water renew inside some of the cages resulting from mesh clogging with algae and detritus.
Following this period, animals were anesthetized with benzocaine, quickly rinsed in MilliQ water, humanely euthanized by spine cord sectioning and stored in liquid N2 for determination of whole-body metal concentrations and enzyme analysis. Cages had a total volume of 5L and were surrounded by holes blocked with a fine mesh, in order to allow water flow. Cages were anchored with local stones using ropes. Considering the total volume of the cages and fish mean weight, it is possible to state that animals were stocked at < 0.1 g fish/L. It is important to state that no fish mortality was observed, in any of the experimental groups.
Environmental Parameters
Water quality parameters, including dissolved O2, pH and temperature, were daily monitored during fish collection and during translocation experiments. Also, water samples were collected, acidified (final concentration: 1%) with 65% HNO3 (Suprapur) and kept at 4 °C until analysis. In these water samples, we determined the levels of total carbon concentrations (Total Organic Carbon analyzer; 5050A, Shimadzu, Japan), Mg, Na, K, Ca (flame photometer, model B262, Micronal, São Paulo, Brazil), Fe, Cd, Cu, Pb, Mn and Zn (HR-CS GF AAS; model Control-A 700; Analytik Jena, Germany). Standard curves were made by serially dilutions of 1000 mg/L certified stock solutions (Multi-Element Standards Certipur®, Merck, Darmstadt, Germany). Methodological procedures were performed accordingly to Marques et al. (2019), additionally, LOD and LOQ are shown in supplementary material (Table A1). Metal recovery ranged from 92.6 to 102.8%. All reagents were of high-purity grade. Water used for preparing reagents and reference solutions was deionized and further purified using a Milli-Q system (Millipore Corp., Bedford, USA).
Whole-Body Metal Concentration
Whole-body metal determination was performed according to Marques et al. (2019) with minor modifications. Fish were weighed before and after drying in an oven at 60 °C and were completely digested with HNO3 (65%; Suprapur, Merck, Darmstadt, Germany). Mg, Na, K and Ca concentrations were measured using a flame photometer (São Paulo, Brazil, Micronal, model B262). Metal (Fe, Cu, Cd, Zn, Pb and Mn) concentrations were determined using a high-resolution atomic absorption spectrometer coupled with a graphite furnace (HR-CS GF AAS) (Germany, Analytik Jena, model Control-A 700). Spiked matrices and regular blank analysis were used as quality control and quality assurance for metal quantifications. Standard curves were made using standard solutions (Multi-Element Standards Certipur®, Merck, Darmstadt, Germany). Additionally, certified reference material (Fish protein DORM-3, National Research Council Canada, Ottawa, Canada) was analyzed to confirm extraction efficiency. Standard reference material was prepared as described for tissue samples and were used for the calculation of metal recovery. This evaluation was performed similarly to Sahuquillo et al. (1999) and Qu et al. (2014a, 2014b). Metal recovery ranged from 91.1 to 106.4%. All procedures were performed in triplicate.
Enzyme Activities
Na/K-ATPase activity was measured in gill homogenates following procedures described by Bianchini and Wood (2003), with modifications. Tissue samples were homogenized in 0.5 mL of ice-cold buffer solution containing 150 mM sucrose, 50 mM imidazole, 10 mM ethylenediaminetetraacetic acid (EDTA) and 11.5 mM sodium deoxycholate. Homogenates were centrifuged at 4 °C, for 30 s, at 5,000 g and the supernatant was used as enzyme source. Two reaction mixtures were assayed: salt solution A (10.5 mM MgCl2, 100 mM NaCl, 30 mM KCl and 50 mM imidazole, pH 7.5) and salt solution B (50 mM imidazole, 10.5 mM MgCl2, 130 mM NaCl and 1 mM ouabain; pH 7.5). Reaction mixture A was made with 20 µL of sample homogenates and 200 µL of salt solution A. Reaction mixture B was made with 20 µL of sample homogenates and 200 µL of salt solution B. Enzyme assays were run in duplicate at 20 °C (room temperature) during 10 min. For reaction cessation, 0.2 mL of trichloroacetic acid (50%) was added to reaction medium. Inorganic phosphate (Pi) concentrations in the reaction solution were assessed by commercial reagent kit (Fosfato, Doles, Goiânia, Brazil), based on the colorimetric method described by Fiske and Subbarow (1925).
The enzyme activity corresponded to the difference in Pi concentration produced in the two reaction mixtures (A and B). The idea behind this method is that the activity of Na/K-ATPase is directly related with elevated levels of Pi only on reaction mixture A, because the ouabain present in reaction mixture B is a strong inhibitor of this enzyme. Therefore, reaction mixture A yields Pi background levels present in homogenates together with the Pi produced by Na/K-ATPase activity during the assay. On the other hand, reaction mixture B only yields Pi background levels already present in the homogenates. Analyses were performed together with blank samples. Enzyme activity was normalized considering the protein content in sample homogenates, measured with Bradford reagent (Bio-Rad, Richmond, CA, USA). Total protein content was estimated using standard curves and blank analyses. Finally, enzyme activity was expressed as µmoles ADP/mg protein/h. Absorbance for Na/K-ATPase and Bradford analysis was made using a microplate reader (ELx-800, Biotek, Winooski, VT, USA).
Carbonic anhydrase activity was determined in gill homogenates using the delta pH method (Henry 1991) with modifications. This method is based in pH decrease following H+ release upon the catalytic hydration of CO2. Reaction mixtures contained 15 mM sucrose, 225 mM mannitol, 10 mM phosphate and 10 mM Tris-Base (pH 8.5). Sample homogenates (10 µl) were added to 2 ml of reaction solution. The enzyme substrate (260 µl of MiliQ-water saturated with CO2) was then added to the mixture to start the reaction. Reaction mixture pH was measured every 5 s for up to 30 s. Blank reactions were prepared by replacing the sample homogenate with the buffer solution (10 µl) used for sample homogenization. Sample and blank measurements were taken simultaneously. The slope of pH values with time was estimated by a linear regression model. The catalyzed reaction ratio was considered as being the regression slope obtained for each individual sample homogenate. In turn, the non-catalyzed reaction ratio was considered as being the regression slope obtained in the blank measurement. Data were normalized based on the total protein content in sample homogenates, measured with Bradford reagent (Bio-Rad, Richmond, CA, USA). Enzyme activity was expressed as enzyme units/mg protein. Carbonic anhydrase analysis was made using a microplate reader (ELx-800, Biotek, Winooski, VT, USA).
Data Presentation and Statistical Analysis
Data are shown as mean ± standard error. Endpoints were analyzed using Analysis of Variance (One-way ANOVA) followed by the Tukey post hoc test. Parametric assumptions were graphically verified by residuals analysis (data normality) and the by the Cochran C test (homogeneity of variances). The significance level adopted was 95% (α = 0.05). Analyses were performed using the software Statistica 12.0.
Ethics and Legal Statements
All experimental and laboratorial procedures performed in the present work were previously approved by the university ethics committee (CEUA; protocol # 23,116.001365/2015-44) and by the Brazilian Ministry of the Environment (MMA; research license # 44,769-1).
Results
Environmental Parameters
Water pH (MIS: 5.02 ± 0.02; NMIS: 5.08 ± 0.06) and dissolved oxygen (NMIS: 7.07 ± 0.10 mg O2/L; MIS: 6.95 ± 0.11 mg O2/L) showed no significant differences when the two sites were compared. Additionally, no significant differences were observed when major cations concentrations (Ca, K, Na and Mg) were compared (Table 1). However, a significant difference between sites in water temperature (MIS: 26.8 ± 0.37 °C; NMIS: 24.6 ± 0.39 °C) was observed, as well as in the Cu concentration. As expected, the MIS showed higher concentrations (~ twofold) in comparison to the NMIS (Table 1). Additionally, no other significant differences among trace-metals (Cd, Fe, Mn, Pb and Zn) were found when the MIS and the NMIS were compared (Table 1).
Whole-Body Metal and Major Cation Content
As expected, whole-body Cu content in P, CCP, CP and PC fish was elevated in comparison to C and CCC fish (Fig. 2). Conversely, no differences were observed for H. luetkenii Cd, Pb (Fig. 2), Fe, Mn and Zn (Fig. 3) whole-body concentrations among all experimental groups. In accordance, no differences were observed for whole-body content of Na, K, Ca and Mg among the experimental groups evaluated (Fig. 4).
Whole-body Cd, Pb and Cu concentrations in the freshwater fish H. luetkenii collected at a nonmetal impacted site (C), kept caged at this site (CCC) or translocated to the metal impacted site (CP) for 96 h. Also, fish were collected at the metal impacted site (P), kept caged in this site (CCP) or translocated to the nonmetal impacted site (PC fish) for 96 h. Data are expressed as mean ± standard error (n = 5). Different letters indicate significant differences among fish groups for the same parameter analyzed (ANOVA followed by Tukey test; P < 0.05)
Whole-body Fe, Mn and Zn concentrations in the freshwater fish H. luetkenii collected at a nonmetal impacted site (C), kept caged at this site (CCC) or translocated to the metal impacted site (CP) for 96 h. Also, fish were collected at the metal impacted site (P), kept caged in this site (CCP) or translocated to the nonmetal impacted site (PC fish) for 96 h. Data are expressed as mean ± standard error (n = 5). Different letters indicate significant differences among fish groups for the same parameter analyzed (ANOVA followed by Tukey test; P < 0.05)
Whole-body major cations (Na, K, Ca and Mg) content in the freshwater fish H. luetkenii collected at the nonmetal impacted site (C), kept caged at this site (CCC) or translocated to the metal impacted site (CP) for 96 h. Also, fish were collected at the metal impacted site (P), kept caged in this site (CCP) or translocated to the nonmetal impacted site (PC fish) for 96 h. Data are expressed as mean ± standard error (n = 5). Different letters indicate significant differences among fish groups for the same parameter analyzed
Na/K-ATPase and Carbonic Anhydrase Activities
No significant differences were observed in Na, K- ATPase branchial activity among non-translocated fish (C, CCC, P and CCP). Conversely, this enzyme activity was significantly elevated in translocated animals (CP and PC) in comparison to non-translocated fish (Fig. 5). Despite that, no differences were observed between CP and PC fish (Fig. 5). In the case of carbonic anhydrase activity, the only group that showed significant differences was the CP fish, as an enzymatic inhibition was observed in these animals in comparison to all other groups (Fig. 6).
Gill Na,K-ATPase activity in the freshwater fish H. luetkenii collected at the nonmetal impacted site (C), kept caged at this site (CCC) or translocated to the metal impacted site (CP) for 96 h. Also, fish were collected at the metal impacted site (P), kept caged in this site (CCP) or translocated to the nonmetal impacted site (PC fish) for 96 h. Data are expressed as mean ± standard error (n = 5). Different letters indicate significant differences among fish groups for the same parameter analyzed
Gill carbonic anhydrase activity in the freshwater fish H. luetkenii collected at the nonmetal impacted site (C), kept caged at this site (CCC) or translocated to the metal impacted site (CP) for 96 h. Also, fish were collected at the metal impacted site (P), kept caged in this site (CCP) or translocated to the nonmetal impacted site (PC fish) for 96 h. Data are expressed as mean ± standard error (n = 5). Different letters indicate significant differences among fish groups for the same parameter analyzed
Discussion
In agreement with the elevated levels of Cu found in the water samples, the whole-body content of this metal was significantly higher in P fish in comparison to C animals, indicating that chronic exposure to Cu under natural conditions resulted in the bioaccumulation of this metal. Interestingly, the same results have been reported by similar studies (Pyle et al. 2005; Couture and Pyle 2008; Uren Webster et al. 2013). Additionally, a significant increase in whole-body Cu burden was also observed in CP fish, indicating that H. luetkenii, like other freshwater fishes (Grosell and Wood 2002; Grosell 2011; Eyckmans et al. 2012, Zebral et al. 2019c), can readily accumulate Cu following an acute exposure (96 h) to increased levels of this metal. Indeed, Cu tissue uptake and accumulation by fish is known to be a fast (Grosell and Wood 2002; Grosell 2011; Eyckmans et al. 2012). Despite the aforementioned results, animals translocated from the MIS to the NMIS did not have any reduction in whole-body Cu concentration. This was an unexpected result, considering that one could have hypothesized that translocation from a contaminated environment to a non-contaminated area would result in a clearance process. One possible explanation to this apparent contradiction is that 96 h was not enough time for an efficient metal depuration. Further supporting this idea, Subathra and Karuppasamy (2008) showed that the fish Mystus vittatus took 21 and 39 days to depurate the Cu bioaccumulated in gills and kidney, respectively, following exposure to 5.98 mg/L for 28 days. It was also possible to observe that none of the other trace-metals assessed in the water or in H. luetkenii whole-body were elevated in the MIS in comparison to the NMIS. This is a strong evidence showing that the water bodies impacted by the mining activity at Minas do Camaquã District are contaminated with Cu specifically, and not with trace-metals in general.
Moving forward, it is well known that the main mechanism involved in Cu toxicity in freshwater fish is associated with inhibition of brachial Na/K-ATPase activity, resulting in ionic and osmoregulatory disturbances (Grosell and Wood 2002; Grosell 2011). With that in mind, we expected that the elevated levels of whole-body Cu observed in P, CP, PC and CCP would be accompanied by reductions in the tissue content of major cations (Na, K and Ca) due to Na/K-ATPase disruption. However, no significant differences were observed. For the case of P fish, this lack of disturbances was also paralleled by unaltered branchial Na/K-ATPase and carbonic anhydrase activities. Altogether, these findings indicate that H. luetkenii wild individuals chronically exposed to Cu were able to deal with the excess of this metal in its tissues, showing no disturbances in ionic content. This result may indicate that the H. luetkenii population living in the MIS has developed local adaptations to deal with Cu contamination. These adaptations could be related with the production of Na/K-ATPase and carbonic anhydrase isoforms that are less susceptible to Cu. Interestingly, as it can be observed in Fig. 1 (supplementary material), the populations of H. luetkenii living at the MIS and at the NMIS are separated by a dam catchment. This may result in reproductive isolation among them. Additionally, H. luetkenii are fast growing animals (Lima et al. 2008). Together, these two facts support the idea that animals living at the MIS have developed local adaptations at the populational level. Although extremely interesting, this point is still very hypothetical and further studies comparing genetic variations among populations living at the NMIS and in the MIS are still needed. There is no doubt that this is a prolific line of work and our future studies will be further assessing this matter.
For the case of Na/K-ATPase in translocated animals, an unexpected result was observed as both CP and PC fish had augmented levels of this enzyme in the gills. Despite that, one can hypothesize that the physiological basis for each of these outcomes are likely to be different. For example, Abril et al. (2018) showed in a companion paper that PC fish had a reduction in brachial Cu content, explaining the elevation in branchial Na/K-ATPase activity seen in the present study, as a reduction in the inhibition effect induced by this metal would also be expected. On the other hand, the induction of this enzyme in the gills of CP fish may indicate the activation of compensatory mechanisms to avoid osmoregulatory disturbances associated with higher Cu accumulation (Grosell 2011), such as the diffusive loss of Na due to oxidative damage to the gills (Craig et al. 2007; Wang et al. 2015; Ransberry et al. 2016). In this case, the suggested compensatory mechanisms could be related with the intensification of the ionic gradient produced by Na/K-ATPase in the gills, the force that drives Na absorption in this tissue (McCormick et al. 2009), counteracting any osmotic disturbances induced by Cu toxicity. It is interesting to comment that this was an unexpected result, considering that in most studies this enzyme was unaffected (Zimmer et al. 2012; Moyson et al. 2016; Canli et al. 2016) or was inhibited (Grosell and Wood 2002; Grosell 2011; Chowdhury et al. 2016) by Cu. It is important to remember that experimental animals were exposed to naturally elevated Cu levels together with a complex mixture of other environmental cues. In this context, the slightly elevated water temperature observed at the MIS could also help to understand this unexpected elevation in Na/K-ATPase of CP fish, as this parameter is known to impact this enzyme activity (Schwarzbaum et al. 1992; Yang et al. 2018; Monroe et al. 2019; Vargas-Chacoff et al. 2020). Therefore, the results of the present study may indicate that under a real-case scenario, the physiological mechanisms related to Cu-induced osmoregulatory toxicity may actually be more complex than previously expected. Certainly, this is an exciting field to be further assessed in future studies.
For the case of carbonic anhydrase, a significant reduction in this enzyme activity was observed in the gills of CP fish. In fact, Cu is known to be potent inhibitor of this enzyme (Grosell 2011; Zebral et al. 2019a). The cytosolic form of carbonic anhydrase is responsible for the intracellular supply of H+ to H-ATPase and Na/H-exchanger through hydration of intracellular CO2, facilitating Na uptake (Weihrauch et al. 2009; Wright and Wood 2009). Therefore, the inhibition of branchial carbonic anhydrase activity observed in CP fish could have led to disruption in Na+ balance, but the whole-body content of this cation was unaltered in these animals. This apparent contradiction can be explained by the fact that, as discussed above, CP animals showed an elevation in Na/K-ATPase branchial activity, counteracting any possible ionoregulatory disturbances. On the other hand, the apical membrane-bound form of carbonic anhydrase is responsible, together with H-ATPase and Na/H-exchanger, to induce the acidification of the apical gill boundary layer, allowing the unprotonated form of ammonia to be excreted by facilitated diffusion (Weihrauch et al. 2009; Wright and Wood 2009), therefore, it is unwise to neglect a hypothetical impact in the excretion of nitrogenous compounds in CP animals. As a matter of fact, many studies have already demonstrated that Cu-induced toxicity and mortality in fish may be attributed to an inhibition in ammonia excretion thought the gills (Grosell et al. 2003, Grosell et al., 2004a; Blanchard and Grosell 2006; Zimmer et al. 2012; Lim et al. 2015; Sinha et al. 2016), although the mechanism related to this toxic effect is not yet clear (Zimmer et al. 2012). In this regard, there is a strong hypothesis attributing this Cu-dependent disruption in ammonia clearance to an inhibition in the activity of carbonic anhydrase in fish gills (Grosell, 2011). The results obtained in the present study strengthens this hypothesis. Actually, as far as we know, this is the first evidence of a Cu-dependent inhibition in fish carbonic anhydrase activity in a field study. It is worth noting that any of the biological parameters assessed in the present study were different among C and CCC fish. Also, no significant differences were observed between P and CCP fish. These findings indicate that the significant effects observed in CP and PC fish can be attributed to animals’ translocation and not to the fact that fish were kept caged during the experimental period (96 h).
Conclusions
In conclusion, H. luetkenii chronically or acutely exposed to naturally elevated levels of Cu rapidly accumulated this metal, but was unable to readily excrete it when transferred to an uncontaminated environment. Moreover, classical Cu toxic effects related to ionic and osmotic disturbances, such as inhibition in Na/K-ATPase activity and reduced levels of major ions, were not observed. Despite that, a reduction in carbonic anhydrase activity was seen, indicating that the excretion of nitrogenous compounds may have been compromised. Finally, it is concluded that populations of H. luetkenii living in an environment chronically contaminated by Cu-developed biochemical mechanisms to sustain osmoregulation even in the face of elevated accumulation of this metal.
References
Abril SIM, Costa PG, Bianchini A (2018) Metal accumulation and expression of genes encoding for metallothionein and copper transporters in a chronically exposed wild population of the fish Hyphessobrycon luetkenii. Comp Biochem Physiol C 211:25–31
Albrecht M (2012) Influência da mata ciliar em parâmetros da ictiocenose e em parâmetros populacionais de quatro espécies de peixes em riachos no sul do Brasil. MSc. Thesis, Universidade do Rio dos Sinos (unisinos), São Leopoldo, RS, Brazil
Aldrovandi P, Pestana MHD (2012) Avaliação da contaminação por metais na área das Minas do Camaquã. http://www.lume.ufrgs.br/handle/10183/64071.
Amiard-Triquet C, Amiard JC, Mouneyrac C (2015) Predictive ecotoxicology and environmental assessment. Aquatic ecotoxicology. Academic Press, Cambridge, pp 463–496
Andrian IF, Peretti D, Lambrecht D (2006) Recursos alimentares explorados por Astyanax (Characiformes, Characidae) em diferentes bacias hidrográficas. Arq Mudi 10:21–27
Anni ISA, Zebral YD, Afonso SB, Jorge MB, Abril SIM, Bianchini A (2019a) Life-time exposure to waterborne copper II: patterns of tissue accumulation and gene expression of the metal-transport proteins ctr1 and atp7b in the killifish Poecilia vivipara. Chemosphere 223:257–262
Anni ISA, Zebral YD, Afonso SB, Abril SIM, Lauer MM, Bianchini A (2019b) Life-time exposure to waterborne copper III: effects on the energy metabolism of the killifish Poecilia vivipara. Chemosphere 227:580–588
Bianchini A, Wood CM (2003) Mechanism of acute silver toxicity in Daphnia magna. Environ Toxicol Chem 22:1361–1367
Bidone ED, Laybauer L, Castilhos ZC, Maddock JL (2001) Environmental risk increase due to heavy metal contamination caused by a copper mining activity in Southern Brazil. An Acad Bras Ciênc 73:277–286
Blanchard J, Grosell M (2006) Copper toxicity across salinities from freshwater to seawater in the euryhaline fish Fundulus heteroclitus: is copper an ionoregulatory toxicant in high salinities? Aquat Toxicol 80:131–139
Canli EG, Atli G, Canli M (2016) Responses of the antioxidant and osmoregulation systems of fish erythrocyte following copper exposures in differing calcium levels. Bull Environ Contam Toxicol 97:601–608
Chowdhury MJ, Girgis M, Wood CM (2016) Revisiting the mechanisms of copper toxicity to rainbow trout: Time course, influence of calcium, unidirectional Na+ fluxes, and branchial Na+, K+ ATPase and V-type H+ ATPase activities. Aquat Toxicol 177:51–62
Couture P, Pyle G (2008) Live fast and die young: metal effects on condition and physiology of wild yellow perch from along two metal contamination gradients. Hum Ecol Risk Assess 14:73–96
Craig PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 293:R1882–R1892
Depledge MH, Rainbow PS (1990) Models of regulation and accumulation of trace metals in marine invertebrates. Comp Biochem Physiol C 97:1–7
Dias TS, Giora J, Gelain D, Malabarba LR, Fialho CB (2000) Fecundidade de Hyphessobrycon luetkenii (Boulenger 1887) na lagoa Fortaleza, município de Cidreira, RS, Brasil. Salão de Iniciação Científica livro de resumos. Porto Alegre: UFRGS, 2000
Durrant CJ, Stevens JR, Hogstrand C, Bury NR (2011) The effect of metal pollution on the population genetic structure of brown trout (Salmo trutta L.) residing in the River Hayle, Cornwall, UK. Environ Pollut 159:3595–3603
Eyckmans M, Benoot D, Van Raemdonck GAA, Zegels G, Van Ostade XWM, Witters E, Blust R, De Boeck G (2012) Comparative proteomics of copper exposure and toxicity in rainbow trout, common carp and gibel carp. Comp Biochem Physiol Part D Genom Proteom 7:220–232
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Giora J, Dias TS, Gelain D, Fialho CB (2000) Tipo de desova de Hyphessobrycon luetkenii (Boulenger 1887) na lagoa Fortaleza, município de Cidreira, RS, Brasil (Characiformes; Characidae). Salão de Iniciação Científica livro de resumos. Porto Alegre: UFRGS, 2000
Graciolli G, Azevedo MA, Melo FAG (2003) Comparative study of the diet of landulocaudinae and Tetragonopterinae (Ostariophysi: Characidae) in a small stream in Southern Brazil. Stud Neotropical Fauna Environ 38:95–103
Grosell M (2011) Copper. In: Wood CM, Farrell T, Brauner CJ (eds) Homeostasis and toxicology of essential metals. Fish Physiology. Academic Press, San Diego, pp 53–133
Grosell M, Wood CM (2002) Copper uptake across rainbow trout gills: mechanisms of apical entry. J Exp Biol 205:1179–1188
Grosell M, Wood CM, Walsh PJ (2003) Copper homeostasis and toxicity in the elasmobranch Raja erinacea and the teleost Myoxecephalus octodecemspinosus during exposure to elevated waterborne copper. Comp Biochem Physiol Part C 135:179–190
Grosell M, McDonald MD, Wood CM, Walsh PJ (2004) Effects of prolonged copper exposure in the marine gulf toadfish (Ospanus beta) I. Hydromineral balance and plasma nitrogenous waste products. Aquat Toxicol 68:249–262
Hahn ME (1998) Mechanism of innate and acquired resistance to dioxin-like compounds. Rev Toxicol 2:395–443
Hamilton PB, Cowx IG, Oleksiak MF, Griffiths AM, Grahn M, Stevens JR, Carvalho GR, Nicol E, Tyler CR (2016) Population-level consequences for wild fish exposed to sublethal concentrations of chemicals – a critical review. Fish Biol 17:545–566
Henry RP (1991) Techniques for measuring carbonic anhydrase activity in vitro: the electrometric delta pH and pH stat assay. In: Dodgson SJ, Tashian RE, Gros G, Carter ND (eds) The carbonic anhydrases: cellular physiology and molecular genetics. Plenum, New York, pp 119–126
IPCC (2014) The fifth assessment report of the intergovernmental panel on climate. Available online: https://www.ipcc.ch/assessment-report/ar5/ Accessed from 20 Feb 2019
Konrad HG, Paloski NI (2000) Fauna da região das minas do camaquã, sub-bacia do arroio joão dias. in: minas do camaquã, um estudo multidisciplinar. São Leopoldo unisinos 85–108
Larsen PF, Schulte PM, Nielsen EE (2011) Gene expression analysis for the identification of selection and local adaptation in fishes. J Fish Biol 78:1–22
Laurén DJ, McDonald DG (1987) Acclimation to copper by rainbow trout, Salmo gairdneri: physiology. Can J Fish Aquat Sci 44:99–104
Laybauer L (1998) Influência da mineração no incremento e na disponibilidade geoquímica de cobre em sedimentos fluviais – o caso das minas do Camaquã, RS, Brasil. Pesquisas 23:51–61
Leung KP, Chen D, Chan KM (2014) Understanding copper sensitivity in zebrafish (Danio rerio) through the intracellular localization of copper transporters in a hepatocyte cell-line ZFL and the tissue expression profiles of copper transporters. Metallomics 6:1057–1067
Lim MYT, Zimmer AM, Wood CM (2015) Acute exposure to waterborne copper inhibits both the excretion and uptake of ammonia in freshwater rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 168:48–54
Lima SM, Cunha AA, Sánchez-Botero JI, Caramaschi ÉP (2008) Vertical segregation of two species of Hyphessobrycon (Characiformes: Characidae) in the Cabiúnas coastal lagoon, southeastern Brazil. Neotropical Ichthyol 6:683–688
Lionetto MG, Caricato R, Giordano ME, Schettino T (2016) The complex relationship between metals and carbonic anhydrase: new insights and perspectives. Int J Mol Sci 17:127
Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B, Bugeon J (2016) How muscle structure and composition influence meat and flesh quality. Sci World J 2016:3182746
Marques D, Costa PG, Souza GM, Cardozo JG, Barcarolli IF, Bianchini A (2019) Selection of biochemical and physiological parameters in the croaker Micropogonias furnieri as biomarkers of chemical contamination in estuaries using a generalized additive model (GAM). Sci Total Environ 647:1456–1467
McCormick SD, Regish AM, Christensen AK (2009) Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J Exp Biol 212:3994–4001
Millward RN, Grant A (2000) Pollution-induced tolerance to copper of nematode communities in the severely contaminated Restronguet Creek and adjacent estuaries, Cornwall, United Kingdom. Environ Toxicol Chem 19:454–461
Monroe I, Wentworth S, Thede K, Aravindabose V, Garvin J, Packer RK (2019) Activity changes in gill ion transporter enzymes in response to salinity and temperature in fathead minnows (Pimephales promelas). Comp Biochem Physiol A Mol Integr Physiol 228:29–34
Moyson S, Liew HJ, Fazio A, Van Dooren N, Delcroix A, Faggio C, De Boeck G (2016) Kidney activity increases in copper exposed goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol 190:32–37
Pyle GG, Rajotte JW, Couture P (2005) Effects of industrial metals on wild fish populations along a metal contamination gradient. Ecotoxicol Environ Saf 61:287–312
Qu R, Feng M, Wang X, Qin L, Wang C, Wang Z, Wang L (2014a) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16
Qu R, Wang X, Wang Z, Wei Z, Wang L (2014b) Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J Hazard Mater 275:89–98
Ransberry VE, Blewett TA, McClelland GB (2016) The oxidative stress response in freshwater-acclimated killifish (Fundulus heteroclitus) to acute copper and hypoxia exposure. Comp Biochem Physiol C Toxicol Pharmacol 179:11–18
Ronchi LH, Lobato AOC (2000) Avaliação do estado da qualidade das águas e da contaminação dos sedimentos recentes do arroio João Dias após encerramento das atividades das minas do Camaquã, RS. In: Ronchi, L.H., Lobato, A.O.C (Eds.), Minas do Camaquã, um Estudo Multidisciplinar. unisinos Canoas RS Brazil
Sahuquillo A, Lopez-Sanchez JF, Rubio R, Rauret G, Thomas RP, Davidson CM, Ure AM (1999) Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal Chim Acta 382:317–327
Santos EM, Ball JS, Williams TD, Wu H, Ortega F, van Aerle R, Katsiadaki I, Falciani F, Viant MR, Chipman JK, Tyler CR (2010) Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a fish model. Environ Sci Technol 44:820–826
Schwarzbaum PJ, Niederstätter H, Wieser W (1992) Effects of temperature on the (Na++ K+)-ATPase and oxygen consumption in hepatocytes of two species of freshwater fish, roach (Rutilus rutilus) and brook trout (Salvelinus fontinalis). Physiol Zool 65:699–711
Sinha AK, Kapotwe M, Dabi SB, da Silva MC, Shrivastava J, Blust R, De Boeck G (2016) Differential modulation of ammonia excretion, rhesus glycoproteins and ion regulation in common carp (Cyprinus carpio) following individual and combined exposure to waterborne copper and ammonia. Aquat Toxicol 170:129–141
Subathra S, Karuppasamy R (2008) Bioaccumulation and depuration pattern of copper in different tissues of Mystus vittatus, related to various size groups. Arch Environ Contam Toxicol 54:236–244
Uren Webster TM, Bury N, van Aerle R, Santos EM (2013) Global transcriptome profiling reveals molecular mechanisms of metal tolerance in a chronically exposed wild population of brown trout. Environ Sci Technol 47:8869–8877
Vargas-Chacoff L, Arjona FJ, Ruiz-Jarabo I, García-Lopez A, Flik G, Mancera JM (2020) Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J Therm Biol 88:102526
Wang B, Feng L, Jiang WD, Wu P, Kuang SY, Jiang J, Zhou XQ (2015) Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: preventive role of arginine. Aquat Toxicol 158:125–137
Weihrauch D, Wilkie MP, Walsh PJ (2009) Ammonia and urea transporters in gills of fish and aquatic crustaceans. J Exp Biol 212:1716–1730
Weiss FE (2013) Sistemática e taxonomia de Hyphessobrycon luetkenii (Boulenger, 1887) (Characiformes: Characidae). PhD. Thesis Universidade Federal do Rio Grande do Sul (ufrgs) Porto Alegre RS Brazil.
Whitehead A, Galvez F, Zhang S, Williams LM, Oleksiak MF (2011) Functional genomics of physiological plasticity and local adaptation in killifish. J Hered 102:499–511
Wood CM, Farrell AP, Brauner CJ (2011) Homeostasis and toxicology of essential metals. Fish Physiol 31:520
Wright PA, Wood CM (2009) A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol 212:2303–2312
Yang S, Yan T, Zhao L, Wu H, Du Z, Yan T, Xiao Q (2018) Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J Therm Biol 72:155–160
Zebral YD, Anni ISA, Afonso SB, Abril SIM, KleinBianchini RDA (2018) Effects of life-time exposure to waterborne copper on the somatotropic axis of the viviparous fish Poecilia vivipara. Chemosphere 203:410–417
Zebral YD, Anni ISA, Junior ASV, Corcini CD, da Silva JC, Caldas JS, Bianchini A (2019b) Life-time exposure to waterborne copper IV: sperm quality parameters are negatively affected in the killifish Poecilia vivipara. Chemosphere 236:124332
Zebral YD, da SilvaFonseca J, Marques JA, Bianchini A (2019a) Carbonic anhydrase as a biomarker of global and local impacts: insights from calcifying animals. Int J Mol Sci 20:3092
Zebral YD, Roza M, da Silva Fonseca J, Costa PG, de Oliveira CS, Zocke TG, Pizzol JLD, Robaldo RB, Bianchini A (2019c) Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linkingoxidative stress in the liver with reduced organismal thermal performance. Aquat Toxicol 209:142–149
Zebral YD, Silva Fonseca J, Roza M, Costa PG, Robaldo RB, Bianchini A (2020) Combining elevated temperature with waterborne copper: Impacts on the energy metabolism of the killifish Poecilia vivipara. Chemosphere 253:126631
Zebral YD, Righi BDP, Abou Anni IS, Escarrone ALV, Roza M, Vieira CED, Bianchini A (2021) Pollution levels and biomarker responses in zooplankton from three hydrographic regions of southern Brazil: an integrated approach for water quality monitoring. J Environ Chem Eng 9(5):106180
Zhao L, Xia Z, Wang F (2014) Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism. Front Pharmacol 5:33
Zimmer A, Barcarolli IF, Wood CM, Bianchini A (2012) Waterborne copper exposure inhibits ammonia excretion and branchial carbonic anhydrase activity in euryhaline guppies acclimated to both fresh water and sea water. Aquat Toxicol 122–123:172–180
Acknowledgements
Thanks to Dr. Sandra Isabel Moreno Abril for her help in the field experiment. The present study was financially supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT—Toxicologia Aquática # 573949/2008-5), CAPES (# 230/2010), and IDRC (Ottawa, ON, Canada, # 104519-027). A.B (Proc. # 307647/2016-1) is a CNPq fellow was supported by IDRC.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT—Toxicologia Aquática # 573949/2008–5), CAPES (# 230/2010), and IDRC (Ottawa, ON, Canada, # 104519–027). A.B (Proc. # 307647/2016–1).
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Conflicts of interest
There were no conflict of interests.
Ethics Approval
All experimental and laboratorial procedures performed in the present work were previously approved by the university ethics committee (CEUA; protocol # 23116.001365/2015–44) and by the Brazilian Ministry of the Environment (MMA; research license # 44769–1).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borges, V.D., Zebral, Y.D., Costa, P.G. et al. Metal Accumulation and Ion Regulation in the Fish Hyphessobrycon luetkenii Living in a Site Chronically Contaminated by Copper: Insights from Translocation Experiments. Arch Environ Contam Toxicol 82, 62–71 (2022). https://doi.org/10.1007/s00244-021-00895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00895-3