Abstract
Freshwater fish Oreochromis niloticus were exposed to Cu in differing Ca2+ levels (15, 30 and 90 mg/L), using acute (0.3 µM, 3 d) and chronic (0.03 µM, 30 d) exposure protocols and enzyme activities related to the antioxidant (catalase, CAT, EC 1.11.1.6; superoxide dismutase, SOD, EC 1.15.1.1; glutathione peroxidase, GPx, EC 1.11.1.9) and osmoregulation (Total, Na+/K+-ATPase, EC 3.6.3.9, Mg2+-ATPase, EC 3.6.3.2) systems in the erythrocytes were measured. Activities of antioxidant enzymes generally decreased significantly following either Ca2+ alone or Ca2++Cu combinations in both acute and chronic exposures. Na+/K+-ATPase activity significantly decreased in chronic exposures, though there was no clear trend in acute exposures. Mg2+-ATPase activity increased significantly in acute exposures, but not in chronic ones. There were more significant alterations in acute exposure compared to chronic ones. There was no clear trend regarding Cu toxicity and its relationship with Ca2+, which may possibly be prompted by the compensatory mechanisms of the enzymes. It may be concluded that freshwater fish erythrocytes may face different degrees of more physiological stress from different waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fish encounter metals in their environments originated from both natural and man-made sources. As fish take up metals from the water and food, metal levels in their tissues can increase depending on physicochemical properties of waters such as salinity, hardness and pH. After certain thresholds, whether essential or not, all metals can be toxic, causing dysfunction or alteration in some vital systems (Heath 1995; Jorgensen 2010; Wood et al. 2012a, b). Although copper is an essential metal (e.g., cytochrome c oxidase) for respiration in the eukaryote cells, it can be highly toxic above tolerable threshold levels. Copper is an essential metal as a co-factor for several respiratory enzymes such cytochrome c oxidase in the eukaryote cells. However, Cu is also known such as a redox active metal, catalyzing Fenton reaction that promotes cellular reactive oxygen species production. Copper concentrations in unpolluted fresh waters can be between 0.2 and 30 µg/L, though this level could be as high as 50–560 µg/L in polluted waters (USEPA 2007).
Water chemistry such as salinity, hardness, pH, etc., affects metal bioavailability, which alters the uptake of metals by aquatic organisms, including fish (Jorgensen 2010; Wood et al. 2012a, b). The hardness of water is the result of the sum of the divalent ion concentrations (e.g., Ca2+ and Mg2+). It is also one of the most important abiotic factors affecting metal uptake by fish. As Ca2+ in fresh waters affects metal bioavailability, it also affects metal toxicity in fish, metals being more toxic in soft water compared to hard water (Grosell et al. 2002; Morgan et al. 2005; Monserrat et al. 2007; Saglam et al. 2013).
Erythrocytes deliver oxygen to body cells and is the most common type of blood cell. Erythrocytes may face a great amount of xenobiotic flowing in the blood that makes them one of the most vulnerable cells, as xenobiotics are mostly transferred through the blood. It has been shown that environmental stressors, such as metals are able to alter the levels of blood parameters. Therefore, researchers suggested that different parameters of the blood may be good biomarkers of fish health to identify their metabolic status and target organs affected by metals (Jorgensen 2010; Wood et al. 2012a, b; Canli and Canli 2015).
Osmoregulation system maintains the concentration of electrolytes and the fluid balance in proper levels with a process called the homeostasis, meaning it regulates the osmotic pressure of organism’s body fluids. In other words, osmoregulation is a process to maintain osmotic concentrations in extracellular fluids, in spite of the osmolarity of the surrounding environment. Membrane-bound enzymes such as Na+/K+-ATPase, regulate the cellular volume, osmotic pressure, and membrane permeability due to the transport of ions through biological membranes. Na+/K+-ATPase transports Na+ and K+ through cell membranes and regulates the osmotic pressure, cellular volume and permeability of membranes (Sancho et al. 2003; Atli et al. 2015). Mg2+-ATPase is responsible for trans-epithelial regulation of Mg2+ and plays an important role in oxidative phosphorylation (Parvez et al. 2006). Therefore, determination of ATPase responses in fish to metal exposures may be used as an “early warning signal” of metal-induced damage to the osmoregulation system (Grosell et al. 2002; Monteiro et al. 2005; Atli and Canli 2007, 2011).
Oxidative stress, potentially, is experienced by all aerobic life when antioxidant defenses are overcome by prooxidant forces, and is the basis of many physiological aberrations (Winston 1991). Reactive oxygen species (ROS) such as hydrogen peroxide, superoxide radical, hydroxyl radical are continuously being released through biochemical processes in eukaryotic organisms. There is a balance between ROS and antioxidant system which is essential for the survival of organisms. However, xenobiotics such as metal ions may contribute additional ROS production, which alters the balance between ROS and antioxidants (Atli et al. 2006; Kanak et al. 2014; Eroglu et al. 2015). Antioxidant enzymes contribute to the maintenance of a relatively low level of the reactive hydroxyl radical (Hidalgo et al. 2002; Sanchez et al. 2005). Therefore, antioxidant enzymes are important in coping with oxidative stress caused by metabolisms itself and various environmental factors such as metals. The most important three antioxidant enzymes are CAT, SOD and GPx (Winston 1991). CAT reduces hydrogen peroxide to water, SOD converts superoxide anion radical to hydrogen peroxide and GPx detoxifies hydrogen peroxide.
Natural waters have different Ca2+ levels depending on their geographic location which affect the metal toxicity for fish in these waters. Therefore, it is important to examine the toxic effects of metals in differing Ca2+ levels to estimate better the consequences of metal contamination on fish physiology. The aim of this study is to determine the influence of Ca2+ on Cu toxicity, based on the responses of osmoregulation and antioxidant systems in the erythrocytes of freshwater fish Oreochromis niloticus. Copper toxicity was studied in two different exposure protocol, namely acute (0.3 µM Cu, 3 d) and chronic (0.03 µM Cu, 30 d), because fish may encounter both low and high copper loads in different aquatic systems.
Materials and Methods
Freshwater fish (O. niloticus) have been being cultured for more than 25 years in the culture pools in Çukurova University (Adana, Turkey). Therefore, fish with the same genetic background were obtained from this centre and brought to the laboratory where they were acclimatized to the new temperature conditions (21 ± 1°C) and light regime (12 h light: 12 h dark) for 1 month. The experiments were conducted in glass aquariums sized 40 × 40 × 100 cm containing 120 L of Nestle water (Nestle Pure Life, Turkey). The aquaria were aerated with air stones attached to an air compressor to saturate with oxygen (7.03 ± 0.81 mg O2/L). The other parameters of the Nestle water were as follows; pH 6.83 ± 0.01, conductivity (77 µS/cm), total hardness 105.4 ± 20.8 mg CaCO3/L, alkalinity 76.2 ± 13.0 mg CaCO3/L. There was no contaminant in this water, according to the Nestle quality control document (Nestle, Turkey). Fish (1 year old) were randomly allocated to the aquaria and fish size (15.4 ± 1.33 cm) and weight (57.5 ± 16.4 g) did not differ significantly (p > 0.05) among different aquaria. All chemicals used were the products of Merck or Sigma, unless otherwise stated.
All experiments were conducted by the Nestle Water as it contained relatively low Ca2+ levels (15 mg Ca2+/L). Calcium levels of this water were increased by adding CaCl2. So, levels of Ca2+ were increased to 30 and 90 mg/L to test the effects of Ca2+. Calcium levels in the exposure media were measured using the Calgon Titration Method and levels were measured to within 5 % of the nominal target Ca2+ levels. Ca2+ control groups were named as follows; lowest Ca (L-Ca), medium Ca (M-Ca) and highest Ca (H-Ca) groups. Total hardness levels were measured as 105.4 ± 20.8, 194.0 ± 12.3 and 318.0 ± 13.0 mg CaCO3/L, respectively. The pH values were in the range of 6.83–6.73 ± 0.01 among the groups. Fish were exposed to copper using acute (0.3 µM, 3 d) and chronic (0.03 µM, 30 d) exposure protocols at three Ca2+ levels. These were named as: lowest Ca + Cu (L-Ca + Cu), medium Ca + Cu (M-Ca + Cu) and highest Ca + Cu (H-Ca + Cu), respectively. Copper concentrations in the exposure media ranged within 5 % of the desired concentrations measured as 0.290 ± 0.01 µM for acute and 0.032 ± 0.003 µM for chronic exposures. Copper level in the Nestle water was below the detection limit (0.002 µg/mL) of flame AAS (Perkin Elmer 3100). Accuracy and measurement validity of AAS were controlled with a reference material (TORT 1 lobster hepatopancreas, National Research Council, Canada). Mean values and standard deviations of the reference material were 5 % of the range.
A total of six fish (as 3 g/L) was used for each treatment. Exposure media were renewed every 3 d just after feeding (2 % of their body weight) to prevent the contamination of the environment with food remains. All experiments were ended in the same day for better comparison (acute experiments began 27th day of chronic experiments). After exposures, fish were killed by transaction of the spinal cord, according to the decision of an Ethic Committee of Çukurova University and immediately blood samples were taken from each fish by puncture of the caudal vessel. Blood samples were centrifuged at 3000×g (Hettich Universal 30 RF, Germany) for 5 min (4°C) to obtain the cells. Cells were washed three times with 0.09 % NaCl and frozen (Revco Ultima II, Newsbreak, UK) at −80°C until the analysis.
ATPase activities in the erythrocytes were measured using the method of Atkinson et al. (1973). Specific Na+/K+-ATPase (EC 3.6.3.9) activity was calculated from the inorganic phosphate liberated from ATP using the differences between the presence (Mg2+-ATPase EC 3.6.3.2) and absence (total-ATPase) of ouabain. ATPase activities were calculated as µmol Pi/mg prot./h. Details were given in our previous papers (Atli and Canli 2011). CAT (EC 1.11.1.6) activity was measured using the method of Lartillot et al. (1988). It was calculated as µmol H2O2/mg prot./min. The GPx (EC 1.11.1.9) activity was measured using the method of Livingstone et al. (1992) and calculated as µmol/mg prot./min. SOD (EC 1.15.1.1) activity was measured by the indirect method involving the inhibition of cytochrome c reduction at 550 nm for 1 min (McCord and Fridovich 1969). The SOD activity was calculated as Unit/mg prot. Details were given in our previous papers (Atli et al. 2006; Eroglu et al. 2015). Total protein levels were determined according to Lowry et al. (1951) and bovine serum albumin was used as a standard.
An SPSS statistical package program (SPSS 13, Chicago, IL, USA) was used for the analysis of data and expressed as mean and standard error (N = 6). Before the statistical analysis, homogeneity of variance was checked among different exposure periods to evaluate the distribution of data by using Kolmogorov–Smirnov normality test. All data from and acute and chronic exposures were compared individually with One-way ANOVA followed by Duncans’ test (p < 0.05). First, three Ca2+ media (15, 30 and 90 mg Ca2+/L) were statistically compared to determine the effects of Ca2+ ions alone on the studied parameters. Then, data from copper exposed fish at three Ca2+ levels were individually compared to estimate the effects of Ca ions on copper toxicity. For example, data from fish exposed to copper in 90 mg Ca2+/L were compared with its own control (90 mg Ca2+/L). T-test was applied for the two group comparisons. The other copper exposures received the same statistical treatment. Acute and chronic exposures were handled separately.
Results and Discussion
Acute and chronic copper exposure of O. niloticus caused some significant alterations in the activities of the antioxidant and osmoregulation system enzymes, which were summarized in Table 1. No fish mortality occurred within 30 days in the experimental conditions and fish seemed healthy.
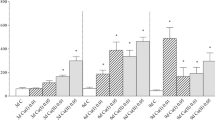
In acute exposure groups, CAT activity did not change in any of Ca2+ alone media (p > 0.05), though activities of SOD and GPx decreased in M-Ca and H-Ca groups when compared to L-Ca group. CAT and GPx activities did not change significantly after Cu exposures in differing Ca2+ levels, except a decrease in GPx activity in L-Ca + Cu. SOD activity decreased in L-Ca + Cu group, but increased in M-Ca + Cu and H-Ca + Cu groups. In chronic exposure groups, the trends of the enzymes were very clear and all antioxidant enzyme activities decreased in both Ca2+ alone and Ca + Cu exposed groups, except M-Ca + Cu group (Fig. 1).
The activities of CAT (a), SOD (b) and GPx (c) in the erythrocytes of O. niloticus exposed to acute (0.3 µM Cu, 3 d) and chronic (0.03 µM Cu, 30 d) copper in differing Ca2+ levels. Base Ca2+ level of experimental water was 15 mg Ca2+/L (L-Ca group). Data are expressed as mean (N = 6) ± standard error.*indicates significant (p < 0.05) changes between L-Ca group and the calcium added M-Ca (60 mg Ca2+/L) and H-Ca (90 mg Ca2+/L) groups, while # indicates significant (p < 0.05) changes between Ca + Cu exposed groups (L-Ca + Cu, M-Ca + Cu and H-Ca + Cu) and their Ca control groups (L-Ca, M-Ca and H-Ca)
In acute exposure, Ca2+ alone exposures decreased the activities of total-ATPase, Na+/K+-ATPase and Mg2+-ATPase, but ATPase activity increased in H-Ca groups (except Mg2+-ATPase). After Cu exposure, total-ATPase and Mg2+-ATPase activities significantly increased in all Cu2+ exposed groups when compared to their own controls. Nevertheless, Na+/K+-ATPase activity showed variations (an increase in M-Ca + Cu and a decrease in H-Ca + Cu) compared to its own controls. In chronic exposure, Na+/K+-ATPase in all Ca2+ alone groups and total-ATPase in H-Ca group decreased significantly (p > 0.05), while Mg2+-ATPase activity did not change in any Ca2+ alone groups (p > 0.05). Copper exposures did not change significantly Mg2+-ATPase activity and total-ATPase at the lower Ca2+ containing media, but increased their activity at the highest Ca2+ containing media. Na+/K+-ATPase showed the opposite trend as its activity did not change at the highest Ca2+, but varied at the lower Ca2+ containing media (Fig. 2).
The activities of total ATPase (a), Mg2+-ATPase (b) and Na+/K+-ATPase (c) in the erythrocytes of O. niloticus exposed to acute (0.3 µM Cu, 3 d) and chronic (0.03 µM Cu, 30 d) copper in differing Ca2+ levels. See Fig. 1 for details
Our previous studies and preliminary experiments showed that Cu was extremely lethal for O. niloticus when given in soft water and large variations in Cu accumulation in fish tissues were measured depending on water Ca2+ levels (Saglam et al. 2013), possibly due to increase in bioavailability and toxicity of metals in soft waters. Therefore, it can be said that the toxicity of metals largely depends on the accumulation of metals which are metabolically available. For instance, competitive interaction between metals such as Cd, Pb and Ca2+ could also explain the protective effects of water hardness as demonstrated in fish exposed to these metals (Rogers et al. 2003; Jorgensen 2010).
As it is well known, metal toxicity in fish mostly depends on free ion levels of waters and also exposure duration and concentration. Calcium is one of the predominant factors in fresh water that decreases Cu bioavailability due to the increased competition between Ca2+ and Cu ions for uptake in organisms and consequent toxicity. Data showed that there is a negative relationship between metal toxicity and water hardness (Ca2+ levels) in the fresh waters (Saglam et al. 2013; Canli and Canli 2015). Thus, protective effects of Ca2+ on metal toxicity have been shown for different freshwater fish species (Richard and Playle 1999; Hollis et al. 2000; Franklin et al. 2005; Abdel-Tawwab et al. 2007; Canli and Canli 2015). However, the present study showed that Cu was able to alter enzyme activities in certain levels in all Ca2+ concentrations, suggesting the compensatory mechanisms of the enzymes.
In the present study, data showed that the activities of all antioxidant enzymes in the erythrocytes generally decreased significantly following Ca2+ alone and Ca + Cu combination exposures. It is interesting to note that the Ca2+ addition to water altered antioxidant enzyme activities, indicating increased Ca2+ in the blood caused a stress for the erythrocytes. Data also emphasized that copper exposure of fish in differing Ca2+ levels decreased antioxidant enzyme activities in the red blood cells, regardless of Ca2+ presence, except SOD activity following acute M-Ca + Cu and H-Ca-Cu exposures. CAT and GPx are two enzymes that compete for hydrogen peroxide removal (Debnath and Mandal 2000). Increased SOD activity indicates the increased hydrogen peroxide formation. GPx activity may be reduced without an elevation in CAT activity (Duzguner and Erdogan 2010). As is known, the erythrocyte is a single cell with large surface area that makes them vulnerable target cells for contaminants flowing in the blood. Possible damage or adhesion of contaminants on the membrane surface of the erythrocytes may change the transport of essential elements across the membrane and thus may cause alteration in enzyme activities. Zikic et al. (1996) studied the effects of Cd on the activities of SOD and CAT in the erythrocyte of carp Cyprinus carpio in an acute exposure protocol. It was shown that the activity of SOD in the erythrocytes decreased after 12, 18 and 24 h of Cd exposure, while CAT activity increased, indicating the oxidative stress caused by acute Cd exposure. Akahori et al. (1999) studied the effects of Zn on the erythrocyte of C. carpio after exposing the fish to various Zn (0.01-1 mM) concentrations. Results showed that there were a marked decrease in CAT and GPx activities, while there were no significant changes in SOD activity. The authors suggested that Zn could affect transport systems across the erythrocyte and therefore, increased the permeability of the membranes to small molecules and led to haemolysis. Zinc ions could act as a potential cell toxicant, leading to disturbances in the functions of the antioxidant defense system and to alterations in the erythrocyte membrane properties. Firat and Kargin (2010) studied the effects of Zn and Cd individually and in combination to erythrocyte antioxidant systems of O. niloticus. They found that GSH level and CAT activity in the erythrocyte increased in response to single and combined exposure to Zn and Cd, predominant effect being caused by Cd alone exposure. It can be evaluated that a decrease in CAT activity may be related to direct binding of metal ions to -SH groups in the enzyme molecule, while an increase may be due to increased oxidative stress caused by metals for compensation of antioxidant defense system maintenance. There was evidence in the literature for increased CAT activity after Cu and Cd exposures in different fish species (Basha and Rani 2003; Sanchez et al. 2005; Atli et al. 2006). Sensitivity of SOD and CAT activities to metal exposures was also supported with our previous results (Atli and Canli 2010). They concluded that toxicants may induce different antioxidant/prooxidant responses, depending on their ability to produce ROS. GPx activity can also be considered complementary to CAT activity that was also supported with the present data. Activity of GPx in the liver of O. niloticus decreased after exposure to metals and it was the most responsive GSH dependent antioxidant enzyme, though it was less affected than CAT (Saglam et al. 2014). The authors indicated that the decreasing trend in GPx activity could be attributed to the direct effects of metal ions in the active site of enzyme molecules. Orun et al. (2008) also indicated the significant alterations in GPx activity together with SOD and CAT activities in the tissues of rainbow trout Onchorhynchus mykiss after Cd and Cr exposures. It can be evaluated that a decrease in GPx activity could be related to the toxic effects of metal ions on the active site of the enzyme molecule, while an increase in GPx activity could be attributed to a stress that fish face. Considering above statements, one may say that the erythrocyte of fish generally did not suffer from oxidative stress, but lost antioxidant enzyme activities in the present study. Interestingly, all enzyme activities, especially in chronic exposures decreased following copper exposure in differing Ca2+ levels, meaning direct Cu toxicity on enzyme molecule occurred and Ca2+ could not prevent this.
The response of the antioxidant system could differ when organisms are exposed to metals and some other factors. For instance, Garcia Sampaio et al. (2008) showed that single-factor Cu exposure was found to be insufficient to decrease the SOD activity in fish (Piaractus mesopotamicus) whereas under hypoxia and combined-factors of hypoxia Cu led a significant decrease in its activity. Similarly, some other studies showed that the activities of the antioxidant enzymes differed after metal exposures between soft and hard waters (Saglam et al. 2014). Physiological activity of fish may also play a significant role in metal stress in fish. For example, Kanak et al. (2014) showed that small O. niloticus exposed to Cu and Cr in acute studies compared to the larger O. niloticus, had significant increases in SOD and CAT activity, suggesting the influence of metabolic activities of different sizes.
ATPases such as Na+/K+-ATPase and Mg2+-ATPase are very important enzymes for the osmoregulation system of fish, as they are responsible in the balance of ions such as Na+, K+ and Mg2+. In the present study, it was evident that most significant alterations occurred in Na+/K+-ATPase activity. Nevertheless, there were more significant alterations in acute exposure compared to chronic ones, indicating an activity change could be compensated in chronic exposures. Data emphasized that copper exposure of fish in differing Ca2+ levels altered Na+/K+-ATPase activity in the red blood cells regardless of Ca2+ presence. Although it seems that in vitro exposures of metals inhibit Na+/K+-ATPase, this inhibition can be compensated by homeostatic regulation in vivo conditions (Atli and Canli 2011). It is likely that the recovery of lost enzymes may occur by increasing the turnover rates of the present enzymes and/or increasing the number of enzyme molecules. An alteration in ATPase activity may also become due to membrane integrity which is affected by ions, as they have major roles in the stability of membrane permeability (Jorgensen 2010; Wood et al. 2012a, b). Thus, decreased Na+/K+-ATPase activity could also be occurred due to the changes in ion levels and the adverse effects of metal exposures as results of metal binding on enzyme molecules (Canli and Stagg 1996). Garcia-Santos et al. (2006) demonstrated that plasma Na+ levels were unaffected and Ca2+ levels decreased despite unaltered Na+/K+-ATPase activity in the gill of O. niloticus following acute Cd exposure indicating the involvement of the compensation mechanism. Similarly, Morgan et al. (2005) showed that increased water hardness reduced the effect of silver on ion levels and gill Na+/K+-ATPase activity, suggesting the nature of the protective effect of hardness on the ionoregulatory disturbance associated with Ag exposure in rainbow trout. Bury et al. (1999) reported similar conclusion, while showing the inhibition of gill Na+/K+-ATPase activity after Ag exposure of rainbow trout acclimated to soft water. In our earlier study, we showed that Na+/K+-ATPase activity in the gill and kidney of O. niloticus was increased by Cu and Cd in hard water, but decreased in soft water (Saglam et al. 2013). The activities of total ATPases and Mg2+-ATPase also increased following copper exposures in all Ca2+ levels. This increase might be due to disturbed magnesium metabolisms and also increased energy demand following the exposures of fish. Mg2+-ATPase is mainly found in the endoplasmic reticulum and mitochondria, which is involved in the respiratory pathway (Canli and Stagg 1996). It is also evident that enzyme recovery may occur by increasing the number of enzyme molecules and/or increasing the turnover rates of the enzyme present in order to compensate for the activity of lost enzymes. As we discuss over enzyme activities, it is not possible to know what happens to individual enzyme molecule after Ca2+ and/or Cu exposures. It seems that in overall, Na,K-ATPase is more altered compared to Mg2+-ATPase, possibly due to their locality differences.
The inhibition of Na+/K+-ATPase probably disturbs Na+ and K+ pump in cells, resulting in an uncontrollable entry of Na+ into the cell along the concentration gradient, followed by water molecule along the osmotic gradient (Thaker et al. 1996). Studies have shown that Cu can enter the cells through Na-sensitive pathways and can cause toxicity by inhibition of Na+/K+-ATPase in cells with consequential whole-body ionoregulatory disturbance (Morgan et al. 1997; Grosell et al. 2004). Osmoregulatory disturbances induced by metals were associated with an increased epithelial permeability and inhibition of active ion uptake, subsequently to reduction of Na+/K+-ATPase activity and a decrease in the number of active chloride cells (Monserrat et al. 2007; Eyckmans et al. 2011). Mg2+-ATPase responds somewhat different than Na+/K+-ATPase to metal exposures. It is perhaps due to lower sensitivity of Mg2+-ATPase to metals or their locality differences between two enzyme groups within the cell.
The erythrocyte has vital roles in fish metabolism and faces xenobiotics flowing in the blood with it on all surface areas. The effects of metals on the antioxidant and osmoregulation systems were generally shown in the liver and gill of fishes, respectively. So, there are few studies to our knowledge that explored to the toxic effects of copper as occurred in the present protocol, which was not entirely comparable to studies in the literature. It could be said that both system enzymes in the erythrocyte are sensitive to copper and/or Ca2+ exposures. Ca2+ ions in the exposure media could not always protect fish from copper toxicity. It is important to note that the toxicity experiments carried out in different laboratories should be as similar as possible concerning the hardness of experimental waters to obtain comparable data. This is also an important issue in the field studies, as the hardness of natural waters vary greatly.
References
Abdel-Tawwab M, Mousa MAA, Ahmad MH, Sakr SFM (2007) The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.) Aquaculture 264:236–246
Akahori A, Gabryelak T, Józwiak Z, Gondko R (1999) Zinc - induced damage to carp (Cyprinus carpioL.) erythrocytesin vitro. IUBMB Life 47(1):89–98
Atkinson A, Gatemby AO, Lowe AG (1973) The determination of inorganic ortophosphate in biological systems. Biochim Biophys Acta 320:195–204
Atli G, Canli M (2007) Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp Biochem Physiol 145 C:282–287
Atli G, Canli M (2010) Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol Environ Saf 73:1884–1889
Atli G, Canli M (2011) Essential metal (Cu, Zn) exposures alter the activity of ATPases in gill, kidney and muscle of tilapia Oreochromis niloticus. Ecotoxicology 20:1861–1869.
Atli G, Alptekin Ö, Tükel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol 143C:218–224
Atli G, Ariyurek SY, Kanak EG, Canli M (2015) Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environ Toxicol Pharm 40 (2):508–515
Basha PS, Rani AU (2003) Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicol Environ Saf 56:218–221
Bury NR, McGeer JC, Wood CM (1999) Effects of altering freshwater chemistry on physiological responses of rainbow trout to silver exposure. Environ Toxicol Chem 18:49–55
Canli EG, Canli M (2015) Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environl Toxicol Pharm 39 (2):606–613
Canli M, Stagg RM (1996) The effects of in vivo exposure to cadmium, copper, and zinc on the activities of gill ATPases in the Norway lobster Nephrops norvegicus. Arch Environ Contam Toxicol 31:491–501
Debnath D, Mandal TK (2000) Study of quinalphos (an environmental oestrogenic insecticide) formulation (Ekalux 25 EC) - induced damage of the testicular tissues and antioxidant defence systems in Sprague - Dawley albino rats. J App Toxicol 20(3):197–204
Duzguner V, Erdogan S (2010) Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pest Biochem Physiol 97:13–18
Eroglu A, Dogan Z, Kanak EG, Atli G, Canli M (2015) Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ Sci Pollut Res 22(5):3229–3237
Eyckmans M, Celis N, Horemans N, Blust R, De Boeck G (2011) Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol 103:112–120
Firat Ö, Kargın F (2010) Effects of zinc and cadmium on erythrocyte antioxidant systems of a freshwater fishOreochromis niloticus. J Biochem Mol Toxicol 24(4):223–229
Franklin NM, Glover CN, Nicol JA, Wood CM (2005) Calcium/Cadmium interactions at uptake surfaces in rainbow trout: waterborne versus dietary routes of exposure. Environ Toxicol Chem 24:2954–2964
Garcia Sampaio FG, Boijink CL, Oba ET, Santos LRB, Kalinin AL, Rantin FT (2008) Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp Biochem Physiol 147C:43–51
Garcia-Santos S, Fontainhas-Fernandes A, Wilson JM (2006) Cadmium tolerance in the Nile tilapia (Oreochromis niloticus) following acute exposure: assessment of some ionoregulatory parameters. Environ Toxicol 21:33–46
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol 133C:287–303
Grosell M, McDonald MD, Wood CM, Walsh PJ (2004) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) I: hydromineral balance and plasma nitrogenous waste products. Aquat Toxicol 68:249–262
Heath AG (1995) Water pollution and fish physiology, 2nd edition. CRC press, New York, pp 359
Hidalgo MC, Exposito A, Palma JM, Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193
Hollis L, McGeer JC, McDonald DG, Wood CM (2000) Protective effects of calcium against chronic waterborne cadmium exposure to juvenile rainbow trout. Environ Toxicol Chem 19:2725–2734
Jorgensen SW (2010) A derivative of encyclopedia of ecology. Ecotoxicology. Academic Press, London, p 390
Kanak EG, Dogan Z, Eroglu A, Atli G, Canli M (2014) Effects of fish size on the response of antioxidant systems ofOreochromis niloticus following metal exposures. Fish Physiol Biochem 40(4):1083–1091
Lartillot S, Kedziora P, Athias A (1988) Purification and characterization of a new fungal catalase. Prep Biochem 18:241–246
Livingstone DR, Lips F, Martinez PG, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol 112:265–276
Lowry OH, Rosebrough NJ, Farra NJ, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Monserrat JM, Martinez PE, Geracitano LA, Amado LL, Martins CMG, Pinho GLL, Chaves ISC, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol 146C:221–234
Monteiro SM, Mancera JM, Fontainhas-Fernandes A, Sousa M (2005) Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp Biochem Physiol 141 C:375–383
Morgan IJ, Henry RP, Wood CM (1997) The mechanism of acute silver nitrate toxicity in freshwater rainbow trout (Oncorhynchus mykiss) is inhibition of gill Na+ and Cl− transport. Aquat Toxicol 38:145–163
Morgan TP, Guadagnolo CM, Grosell M, Wood CM (2005) Effects of water hardness on the physiological responses to chronic waterborne silver exposure in early life stages of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 74:333–350
Orun I, Selamoglu Talas Z, Ozdemir I, Alkan A, Erdogan K (2008) Antioxidative role of selenium on sometissues of (Cd2+, Cr3+): induced rainbow trout. Ecotoxicol Environ Saf 71:71–75
Parvez S, Sayeed I, Raisuddin S (2006) Decreased gill ATPase activities in the freshwater fish Channa punctata (Bloch) exposed to a diluted paper mill effluent. Ecotoxicol Environ Saf 65:62–66
Richard JG, Playle RC (1999) Protective effects of calcium against the physiological effects of exposure to a combination of cadmium and copper in rainbow trout (Oncorhynchus mykiss). Can J Zool 77:1035–1047
Rogers JT, Richards JG, Wood CM (2003) Ionoregulatory disruption as the acute toxic mechanism for lead in the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 64:215–234
Saglam D, Atli G, Canli M (2013) Investigations on the osmoregulation of freshwater fish (Oreochromis niloticus) following exposures to metals (Cd, Cu) in differing hardness. Ecotoxicol Environ Saf 92:79–86
Saglam D, Atli G, Doan Z, Baysoy E, Gurle C, Eroglu A, Canli M (2014) Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turkish J Fish Aquat Sci 14(1):43–52
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher JM, Ait-Aissa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharm 19:177–183
Sancho E, Fernandez-Vega C, Ferrando MD, Andreu-Moliner E (2003) Eel ATPase activity as biomarker of thiobencarb exposure. Ecotoxicol Environ Saf 56:434–441
Thaker J, Chhaya J, Nuzhat S, Mittal R, Mansuri AP, Kundu R (1996) Effects of chromium (VI) on some ion-dependent ATPases in gills, kidney and intestine of a coastal teleost Periophthalmus dipes. Toxicology 112:237–244
USEPA (2007) Aquatic life ambient freshwater quality criteria for copper, EPA-822-R-07-001. USEPA, Washington
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol 100 C(1):173–176
Wood CM, Farrel AP, Brauner CJ (2012a) Fish physiology: homeostasis and toxicology of essential metals, vol, 31 A. Academic Press, London, p 497
Wood CM, Farrel AP, Brauner CJ (2012b) Fish physiology: homeostasis and toxicology of essential metals, vol 31 B. Academic Press, London, p 507
Zikic V, Stajn AS, Ognjanovic BI, Pavlovic SZ, Saicic ZS (1996) Activities of superoxide dismutase and catalase in erythrocytes and transaminases in the plasma of carps (Cyprinus carpio L.) exposed to cadmium. Physiol Res Acad Sci Bohemoslov 46 (5):391–396
Acknowledgments
This study was supported by a grant (FBA-2014-2592) from the research fund of Cukurova University (Turkey).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canli, E.G., Atli, G. & Canli, M. Responses of the Antioxidant and Osmoregulation Systems of Fish Erythrocyte Following Copper Exposures in Differing Calcium Levels. Bull Environ Contam Toxicol 97, 601–608 (2016). https://doi.org/10.1007/s00128-016-1931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1931-3