Abstract
The chronic exposure of benthic organisms to metals in sediments can lead to the development of tolerance mechanisms, thus diminishing their responsiveness. This study aims to evaluate the accumulation profiles of V, Cr, Co, Ni, As, Cd, Pb and Hg and antioxidant system responses of two benthic organisms (Cerastoderma edule, Bivalvia; Nephtys hombergii, Polychaeta). This approach will provide clarifications about the ability of each species to signalise metal contamination. Organisms of both species were collected at the Tagus estuary, in two sites with distinct contamination degrees (ALC, slightly contaminated; BAR, highly contaminated). Accordingly, C. edule accumulated higher concentrations of As, Pb and Hg at BAR compared to ALC. However, antioxidant responses of C. edule were almost unaltered at BAR and no peroxidative damage occurred, suggesting adjustment mechanisms to the presence of metals. In contrast, N. hombergii showed a minor propensity to metal accumulation, only signalising spatial differences for As and Pb and accumulating lower concentrations of metals than C. edule. The differences in metal accumulation observed between species might be due to their distinctive foraging behaviour and/or the ability of N. hombergii to minimise the metal uptake. Despite that, the accumulation of As and Pb was on the basis of the polychaete antioxidant defences inhibition at BAR, including CAT, SOD, GR and GPx. The integrated biomarker response index (IBRv2) confirmed that N. hombergii was more affected by metal exposure than C. edule. In the light of current findings, in field-based studies, the information of C. edule as a bioindicator should be complemented by that provided by another benthic species, since tolerance mechanisms to metals can hinder a correct diagnosis of sediment contamination and of the system’s health. Overall, the present study contributed to improve the lack of fundamental knowledge of two widespread and common estuarine species, providing insights of the metal accumulation profiles under a scenario of chronic contamination. Finally, this work provided useful information that can be applied in the interpretation of future environmental monitoring studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine organisms inhabiting highly contaminated environments, such as estuaries, are exposed to multiple stress agents and might develop an ability to cope with those stressors (Amiard-Triquet et al. 2011). In turn, this ability may allow a prevalence of the most well-adjusted specimens (Meyer and Di Giulio 2003), which could lead to a decrease in the biodiversity due to the extinction of the less adjusted ones (Lotze et al. 2006; Worm et al. 2006; Amiard-Triquet et al. 2011). Both genetic or physiological strategies could be on the basis of the organism adjustment to environmental contaminants (Amiard-Triquet et al. 2011). The first, also named as resistance, often confer a fitness increase at the population level, being inherited by the following generations (Meyer and Di Giulio 2003). On the other hand, physiological strategies (or tolerance) increase fitness at the individual level, reflecting biochemical, metabolic or even behavioural alterations. Ultimately, these responses enable the organisms to maintain their homeostasis when exposed to contaminants (Meyer and Di Giulio 2003; Geracitano et al. 2004).
Metals are recognized as long-lasting pollutants in estuarine systems, frequently potentiating the occurrence of biological adjustments in the exposed organisms. Hence, aquatic organisms inhabiting metal-contaminated estuaries may present several physiological responses that can be associated with the toxicokinetics and/or toxicodynamics processes. Regarding the toxicokinetics, the evaluation of metal accumulated levels in organisms represents the temporal integration of uptake, transport, transformation, accumulation, half-life periods and excretion. In turn, toxicodynamics is related to the toxic effects of metals at the organ, cellular, and molecular levels (Nordberg et al. 2014). To perform a realistic and suitable evaluation of the environmental health status of metal contaminated areas based on measurable biological variables (biomarkers), it seems crucial to address both processes mentioned above. Furthermore, the relevance and validity of a given risk assessment strategy also depend on the selection of appropriate sentinel species, thereby raising a challenging question concerning the adequacy of more resistant and better adjusted species. In fact, at impacted environments, the evaluation of tolerant organisms can limit sub-lethal alterations, diminishing their responsiveness to stressors and, therefore, disabling the discrimination of different contamination degrees.
Since metals present a strong association with the sediment, metals bound to the sediments and in the interstitial water may directly and/or indirectly affect benthic organisms. Additionally, since both feeding and behaviour of an organism directly affect its contact with sediment contaminants, it is essential to evaluate a range of organisms that have different routes of exposure (King et al. 2004). Therefore, bivalves and polychaetes exhibiting distinctive feeding and living habits are frequently selected to evaluate the biological effects of the sediment metal contamination (Courtney and Clements 2002; Elliott and Quintino 2007; Carvalho et al. 2011; Pereira et al. 2012; Freitas et al. 2012; Maranho et al. 2015).
In what concerns to the strategies adopted by bivalves and polychaetes, and regarding metals toxicokinetics, those directly related to the uptake, detoxification and storage seem of utmost relevance. Besides being modulated by environmental, biological and chemical conditions (Wang 2001; Wang and Rainbow 2005), the metal uptake rates can also be reduced as a response to the metal exposure per se, which results in metal-tolerant individuals (Wang and Rainbow 2005). Tolerance may be driven both by changes in metal uptake rates and alterations in rates of excretion and/or storage detoxification (Mason and Jenkins 1995; Wang and Rainbow 2005).
Metal-tolerant bivalves and polychaetes might as well have developed strategies regarding metals’ toxicodynamics. There are several studies linking metal exposure to the production of reactive oxygen species (ROS), which, in turn, may result in cellular damage, namely protein oxidation, lipid peroxidation and DNA damage (e.g. Valko et al. 2005; Zhang et al. 2010; Freitas et al. 2012). To counteract the excessive production of ROS bivalves and polychaetes can use a range of low molecular weight enzymatic (e.g. CAT and SOD) and non-enzymatic (e.g. GSH) players (Regoli 1998; Geracitano et al. 2004; Alves de Almeida et al. 2007; Ahmad et al. 2011, 2012; Freitas et al. 2012). These components interact in a sophisticated network, which main priority is the ROS detoxification and, consequently, the prevention of the cellular injuries referred above (Regoli and Giuliani 2014). Bearing this in mind, a question arises: are the antioxidant system defences somehow able to contribute to the tolerance of bivalves and polychaetes at metal-contaminated environments? A few studies suggested that the antioxidant system may play an important role in the tolerance skills of the bivalves Scrobicularia plana (Ahmad et al. 2012) and Mytilus galloprovincialis (Box et al. 2007) under contaminated areas.
To the authors’ knowledge, there is a significant lack of information regarding the involvement of antioxidant systems modulation on the tolerance mechanisms of marine invertebrates inhabiting metal-contaminated areas, particularly concerning the polychaetes’ group. Moreover, there are only a few studies comparing species from different taxonomic groups with distinct ecological functions in the environment (e.g. Pérez et al. 2004; Solé et al. 2009; Freitas et al. 2012). For instance, the common cockle Cerastoderma edule (Linnaeus, 1758) and the polychaete worm Nephtys hombergii (Savigny in Lamarck, 1818) are two benthic species with a wide geographical distribution (Rodrigues et al. 2006; Silva et al. 2006). The bivalve C. edule is an active suspension filter-feeder (Dabouineau and Ponsero 2009) while N. hombergii is a predator that excavates burrows in the sediment on the hunt for food (Budd and Hughes 2005). Thus, both species present a distinct foraging behaviour, which is known to strongly influence metal accumulation patterns (Farag et al. 1998).
When planning monitoring studies at metal contaminated aquatic environments, it is important to be aware of the accumulation and physiological responses profiles of the candidate species or taxa, to select the most suitable bioindicators. Thus, the main aims of the present study were: (i) to evaluate the metal accumulation and antioxidant system profiles of two species that are representative of their taxonomic groups, namely C. edule (Bivalvia) and N. hombergii (Polychaeta), from a metal-contaminated area and a reference area in Tagus estuary; (ii) to infer about the development of tolerance mechanisms in the organisms under metal exposure, addressing the repercussion on the value of each species as bioindicators of metal contamination. Keeping in view the role of antioxidants modulation in metal tolerance, organisms collected were analysed for metal bioaccumulation, and a battery of biochemical assays concerning the antioxidant system was performed.
Materials and methods
Study area characterization
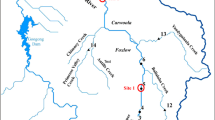
The present study was conducted in the Tagus estuary (Fig. 1), which is located in the most populated area of Portugal (Lisbon metropolitan area) and is one of the largest estuaries in Europe with a broad shallow bay covering an area of about 320 km2. The Tagus estuary has an extremely high socio-economic value supporting several industries and human population. It has been under intensive anthropogenic pressure, not only due to effluents from about 2.5 million inhabitants (Chainho et al. 2010), but also by contamination from several chemical, petrochemical, metallurgic, shipbuilding, cement manufacture industries and agriculture fertilizers/pesticides (Duarte et al. 2008). These impacts resulted in a high metal accumulation in sediments and organisms (e.g. Cr, Ni, Cd, Pb and Hg; among other contaminants), particularly in a confined area—Barreiro (Canário et al. 2005; Neto et al. 2011; Caçador et al. 2012). The Tagus estuary also comprises an important Natural Reserve area with low anthropogenic impacts, which is located in the northern part of the estuary in its south margin, including Alcochete area (Fig. 1).
Sampling procedures
A field campaign was performed in February 2013. Two sampling areas were selected taking into account the different metal contamination levels previously described for the sediments (Canário et al. 2005; França et al. 2005; Vale et al. 2008; Neto et al. 2011). The most contaminated area was located at Barreiro city margins (BAR), while Alcochete (ALC) was selected as a reference site since it presents minor contamination levels (Canário et al. 2005; Vale et al. 2008) (Fig. 1). At each sampling area, surface sediments were collected in triplicate with a Van Veen grab (0.05 m2). From each grab, a sub-sample of sediment for metals determination was taken with a 2.5–15 cm corer, placed into plastic bags and transported to the laboratory. At the field, C. edule and N. hombergii specimens were separated from the sediment by sieving it through a 0.5 mm2 mesh sieve and transported to the laboratory.
Water physico-chemical parameters (pH, salinity and temperature) were measured in situ in both sampling areas with an YSI 650 multi-parameter probe (Yellow Springs, USA).
At the laboratory, organisms were carefully washed to remove the excess of mud and measured. C. edule specimens presented a width (mean ± standard deviation) of 1.64 ± 1.01 cm (ALC) and 1.56 ± 0.99 cm (BAR), while N. hombergii specimens showed a length (mean ± standard deviation) of 20.6 ± 10.9 mm (ALC) and 29.0 ± 7.8 mm (BAR). Organisms were divided into two sets of samples: one for metal determination and other for oxidative stress evaluation. The organisms allocated to the subset for metals determination were depurated in plastic containers with water from the respective sampling areas and with constant aeration during 48 h (Boening 1999). After the depuration period, organisms of both species are expected to be clean, minimizing thus the bias caused by adsorbed particles to the tissues. For both sets of samples, C. edule (soft tissues) and N. hombergii (whole organism), were preserved until further procedures at −20 and −80 °C for metal determination and oxidative stress evaluation, respectively.
Analytical procedures
Determination of metal levels in sediments and biological samples
Prior to the analysis, each sediment sample was oven-dried at 40 °C to constant weight. Total Hg concentrations were determined in both dried sediment and lyophilized biological samples (C. edule and N. hombergii) by atomic absorption spectrometry, with no pre-treatment, using a silicon UV diode detector Leco AMA-254, after pyrolysis of each sample in a combustion tube at 750 °C under an oxygen atmosphere and collection on a gold amalgamator (Costley et al. 2000). Total Hg levels were expressed as microgram per gram of dry weight (µg g−1).
For determination of V, Cr, Co, Ni, As, Cd and Pb, sediment samples (about 100 mg) were completely mineralized with HF (40 %) and Aqua Regia (HCl 36 %:HNO3 60 %; 3:1) in closed Teflon bombs (100 °C for 1 h), evaporated to near dryness (DigiPrepHotBlock – SCP Science), and re-dissolved with 1 mL of doubled-distilled HNO3 (prepared from 65 % pro analysis) and 5 mL of ultra-pure water. Then, the samples were heated for 20 min at 75 °C and diluted to 50 mL with ultra-pure water (Caetano et al. 2007). Freeze dried biological samples were digested with a mixture of HNO3 (doubled distilled from 65 %) and H2O2 (suprapure, 30 %) at 60 °C for 12 h, at 100 °C for 1 h and at 80 °C for 1 h according to the method described in Ferreira et al. (1990). Procedural blanks were prepared using the same analytical procedure and reagents but without sample. The metal quantification was made by an ICP-MS in a Thermo Elemental—X Series and the results were expressed as microgram per gram of dry weight (µg g−1).
The accuracy of the analytical procedures was verified through the analysis of certified reference materials, 1646a (estuarine sediment), Pacs-2 (marine sediment), Mess-3 (marine sediment), 1566a (oyster tissue), BCR 278 (mussel tissue) and IAEA 452 (scallop tissue).
Biochemical analyses in C. edule and N. hombergii
Biological samples (C. edule soft tissues and N. hombergii whole tissues) were homogenized using a Potter–Elvehjem homogenizer, in chilled phosphate buffer (0.1 M, pH 7.4) in a 1:10 ratio [tissue mass (mg):buffer volume (mL)]. Eight individuals of C. edule were homogenized for each site. In the particular case of N. hombergii, organisms were homogenized as pools (2 individuals per pool, in a total of 5 pools), due to the small sample mass. The resulted homogenate was then divided into two aliquots for lipid peroxidation (LPO) measurement and post-mitochondrial supernatant (PMS) preparation. The PMS preparation was obtained by centrifugation in a refrigerated centrifuge (Eppendorf 5415R) at 13,400 g for 20 min at 4 °C. Aliquots of PMS were then divided into microtubes and stored at −80 °C until further analyses.
Catalase (CAT) activity was assayed in PMS (at 25 °C) by Claiborne (1985) method, with slight modifications. Briefly, the assay mixture consisted of 0.195 mL phosphate buffer (0.05 M, pH 7.0) with hydrogen peroxide (H2O2; 0.010 M) and 0.005 mL of PMS in a final volume of 0.2 mL. Change in absorbance was measured in appropriated UV-transparent microplates (UV-Star® flat-bottom microplates, Greiner Bio-One GmbH, Germany), recorded in a SpectraMax 190 microplate reader at 240 nm and CAT activity was calculated in terms of μmol H2O2 consumed min−1 mg protein−1 using a molar extinction coefficient of 43.5 M−1 cm−1.
Superoxide dismutase (SOD) was assayed in PMS (at 25 ºC) with a Ransod kit (Randox Laboratories Ltd., UK). The method employs xanthine and xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT), forming a red formazan dye determined at 505 nm in a SpectraMax 190 microplate reader. Then, SOD activity is measured by the degree of inhibition of this reaction, considering that one SOD unit causes a 50 % inhibition of the INT reduction rate, under the conditions of the assay. Results were expressed as SOD units mg protein−1.
Glutathione peroxidase (GPx) activity was determined in PMS (at 25 °C) according to the method described by Mohandas et al. (1984) and modified by Athar and Iqbal (1998). The assay mixture consisted of 0.09 mL phosphate buffer (0.05 M, pH 7.0), 0.03 mL ethylenediaminetetraacetic acid (EDTA; 0.010 M), 0.03 mL sodium azide (0.010 M), 0.03 mL glutathione reductase (GR; 2.4 U mL−1), 0.03 mL reduced glutathione (GSH; 0.010 M), 0.03 mL nicotinamide adenine dinucleotide phosphate-oxidase (NADPH; 0.0015 M), 0.03 mL H2O2 (0.0025 M) and 0.03 mL of PMS in a total volume of 0.3 mL. Oxidation of NADPH to NADP+ was recorded at 340 nm in a SpectraMax 190 microplate reader and GPx activity was calculated in terms of nmol NADPH oxidized min−1 mg protein−1 using a molar extinction coefficient of 6.22 × 103 M−1 cm−1.
Glutathione reductase (GR) activity was assayed in PMS (at 25 °C) by the method of Cribb et al. (1989) with some modifications. Briefly, the assay mixture contained 0.050 mL of PMS fraction and 0.250 mL of reaction medium consisted of phosphate buffer (0.05 M, pH 7.0), NADPH (0.0002 M), glutathione disulfide (GSSG; 0.001 M) and diethylenetriaminepentaacetic acid (DTPA; 0.0005 M). The enzyme activity was determined by measuring the oxidation of NADPH at 340 nm in a SpectraMax 190 microplate reader and calculated as nmol NADPH oxidized min−1 mg protein−1 using a molar extinction coefficient of 6.22 × 103 M−1 cm−1.
Glutathione-S-transferase (GST) activity was determined in PMS (at 25 °C) using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate according to the method of Habig et al. (1974). The assay mixture consisted in 0.2 mL of phosphate buffer (0.2 M, pH 7.9), CDNB (0.060 M) and GSH (0.010 M). The reaction was initiated by the addition of 0.1 mL of PMS and the increase in absorbance was recorded at 340 nm in a SpectraMax 190 microplate reader. GST activity was calculated as nmol CDNB conjugate formed min−1 mg protein−1 using a molar extinction coefficient of 9.6 × 103 M−1 cm−1.
Total glutathione (GSHt) content in PMS was precipitated with trichloroacetic acid (TCA; 12 %) for 1 h (4 °C) and then centrifuged at 12,000 g for 5 min at 4 °C. GSHt content was determined in the resulting supernatant (deproteinated PMS) (at 25 °C) adopting the enzymatic recycling method using GR excess, whereby the sulfhydryl group of GSH reacts with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; Ellman’s reagent) producing a yellow colored 5-thio-2-nitrobenzoic acid (TNB) (Tietze 1969; Baker et al. 1990). The rate of TNB production is directly proportional to this recycling reaction, which is in turn directly proportional to the GSH concentration in the sample. The assay mixture consisted in 0.2 mL sodium phosphate buffer (0.143 M, pH 8), EDTA (0.0063 M), DTNB (0.001 M) and NADPH (0.00034 M), added to 0.04 mL of deproteinated PMS. The reaction was initiated with 0.04 mL of GR (8.5 U mL−1). Formation of TNB was measured in a SpectraMax 190 microplate reader at 415 nm. It should be noted that GSSG is converted to GSH by GR in this system, which consequently measures total GSH. The results were expressed as nmol TNB formed min−1 mg protein−1 using a molar extinction coefficient of 14.1 × 103 M−1 cm−1.
As estimation of LPO, TBARS quantification was carried out in the previously prepared homogenate according to the procedure of Ohkawa et al. (1979) and Bird and Draper (1984) and adapted by Wilhelm Filho et al. (2001a; 2001b). Briefly, 0.005 mL of butylatedhydroxytoluene (BHT; 4 % in methanol) and 0.045 mL of phosphate buffer (0.05 M, pH 7.4) were added to 0.05 mL of homogenate and mixed well to prevent oxidation. To this aliquot, 1 mL of TCA (12 %), 0.9 mL of Tris–HCl (0.060 M, pH 7.4 and 0.0001 M DTPA) and 1 mL of thiobarbituric acid (TBA; 0.73 %) were added and well mixed. This mixture was heated for 1 h in a water bath set at 100 ºC and then cooled to room temperature, transferred into 2-mL microtubes and centrifuged at 15,700 g for 5 min. The absorbance of each sample was measured at 535 nm in a SpectraMax 190 microplate reader. The rate of LPO was expressed in nmol of thiobarbituric acid reactive substances (TBARS) formed mg protein−1 using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.
Total protein contents were determined according to the Biuret method (Gornall et al. 1949), using bovine serum albumin (E. Merck-Darmstadt, Germany) as a standard.
In the particular case of N. hombergii, it was not possible to measure GST activity as well as GSHt content, since those parameters presented values under the detection limit of the methodology.
Data analysis
Statistica 8.0 software was used for the data analysis. Data were first tested for normality and homogeneity of variances and transformed by ln (x) whenever normality or homogeneity was not met. Differences were considered significant at p < 0.05. A one-way analysis of variance (ANOVA) was applied to compare study areas (ALC versus BAR) for metal concentrations (in sediment and in biological tissues) and oxidative stress biomarkers in individuals.
To compare the total metal content in sediments from the two sampling areas, the Metal Pollution Index (MPI) was obtained according to the following equation (AMA 1992; Usero et al. 1996):
where, Cf1 is the concentration of the first metal, Cf2 is the concentration of the second metal and Cfn is the concentration of the nth metal.
To compare metal accumulation patterns between both species, two ratios (Ratio 1 and Ratio 2) were calculated according to the following formulas:
Ratio 1 was calculated both for sediments and the indicator species. [Metal] is the mean concentration of a certain metal in the sediment or in organisms (C. edule and N. hombergii) from BAR or ALC. Values above 1 indicate that the sediment/organisms from BAR presented/accumulated higher levels of a certain metal than sediment/organisms from ALC. Values below 1 indicate that the sediment/organisms from ALC presented/accumulated higher metal levels than sediment/individuals from BAR.
where, [Metal] is the mean concentration of a metal in each of the indicator species. Values above 1 indicate that C. edule accumulated higher levels of a specific metal than N. hombergii, while values below 1 show a higher metal accumulation in N. hombergii than in C. edule. Ratio 2 was calculated for both sites separately.
Biomarkers described in “Biochemical analyses in C. edule and N. hombergii ” section (CAT, SOD, GPx, GR, GST, GSHt and LPO) were combined into a stress index termed “integrated biomarker response version 2” (IBRv2) described by Sanchez et al. (2013). The IBRv2 is an improvement of the IBR calculation, previously described by Beliaeff and Burgeot (2002), since it allows the simultaneous integration of both up- and down-regulated biomarkers. This index was calculated for both species (C. edule and N. hombergii). Briefly, the mean of the individual biomarkers data (Xi) was compared to the mean reference value (X0; mean value of each biomarker at the reference site—ALC) previously estimated for each biomarker; then, a log transformation was applied to reduce variance Yi = log (Xi/X0); in a next step, the general mean (µ) and the standard deviation (σ) of Yi were computed as described by Beliaeff and Burgeot (2002), and Yi is standardized Zi = (Yi − µ)/σ. To create a basal line to represent the biomarker variation, the mean of standardized biomarker response (Zi) and the mean of reference biomarker data (Z0) were used to define a biomarker deviation index A = Zi − Z0. To obtain IBRv2, the absolute values of A parameters calculated for each biomarker were summed IBRv2 = ∑|A|. Finally, A parameters were depicted in a star plot to represent the deviation of each investigated biomarker in relation to reference values. The area up to 0 reflects biomarker induction, and the area down to 0 indicates a biomarker inhibition.
Results
Water and sediment characteristics
Water physico-chemical parameters, measured in ALC and BAR sites (Fig. 1) showed similar values between the two locals, namely pH of 8.9 ± 0.3 (ALC) and 8.4 ± 0.2 (BAR), salinity of 25.9 ± 0.1 (ALC) and 24.4 ± 0.1 (BAR) and temperature of 12.9 ± 0.1 °C (at both ALC and BAR).
In general, BAR presented higher values of V, As, Cd, Pb and Hg than ALC site, resulting in a greater metal pollution index (MPI) in the first site (Table 1). Moreover, both ALC and BAR sites presented values of Ni, As, Pb and Hg above the effects range low (ERL). It is unlikely that sediment metal levels below the ERL threshold can exert toxicity. Moreover, only at BAR site the Hg levels were above the effects range median (ERM) suggesting the probable occurrence of toxicity associated with this element.
Metal levels in C. edule and N. hombergii
Individuals of C. edule from BAR accumulated significantly higher levels of As, Pb and Hg than those collected in ALC (Table 2). N. hombergii from BAR showed significantly higher levels of As and Pb compared to the polychaetes collected in ALC (Table 2).
The quotient between metal levels in organisms and sediment from BAR and ALC was calculated for both species and sediment (Fig. 2a). In general, the sediment ratio was higher than 1, confirming the higher metal contamination at BAR. Regarding C. edule, ratios were higher than 1 for V, Co, As, Pb and Hg, while ratios lower than 1 were observed for Cr, Ni and Cd, which was, in general, in agreement with the sediment ratios. Ratios of N. hombergii were greater than 1 for Cr, As and Pb. Otherwise, ratios lower than 1 were found for V, Co, Ni, Cd and Hg in N. hombergii.
Comparison of metal accumulation patterns between C. edule and N. hombergii. Ratio 1 (a): ratio of metal accumulation levels between sites for sediments and the indicator species; values above 1—sediment/organisms from BAR presented/accumulated higher levels of a certain metal than sediment/organisms from ALC and values below 1—sediment/organisms from ALC presented/accumulated higher metal levels than sediment/organisms from BAR. Ratio 2 (b): ratio of metal accumulation levels between species for each site; values above 1—C. edule accumulated higher levels of metal than N. hombergii and values below 1—N. hombergii accumulated higher levels of metal than C. edule. The metal As did not show accumulation differences between species, for both sites, neither did Co at ALC
The metal accumulation profiles of both species in each local showed that, in general, C. edule accumulates higher levels of metals than N. hombergii (Fig. 2b). Those differences can range from 2-fold (e.g. Cd or Hg) to 11-fold for Ni in C. edule relatively to N. hombergii. Co and As are the exceptions to this pattern, presenting higher accumulation levels in N. hombergii than in C. edule and similar levels between the organisms, respectively.
Biochemical analyses in C. edule and N. hombergii
Cerastoderma edule showed significant differences in oxidative stress responses between sites only for the GPx activity, which was greater in BAR than ALC site (Table 3). Nephtys hombergii from BAR showed significant lower activities of CAT, SOD, GR and GPx, than those collected in ALC (Table 3). The oxidative damage, measured as LPO levels, showed no differences between sites for both species (Table 3). In general, C. edule presented higher levels of the antioxidant enzymatic activities (with the exception of GPx) than N. hombergii, for the same sites.
The IBRv2 results showed that N. hombergii (Fig. 3b) was the most sensitive bioindicator species when compared with C. edule (Fig. 3a), since the first presented a higher IBRv2 value (IBRv2 = 6.43 and IBRv2 = 3.62, respectively). Through the observation of the IBRv2 star plots, it was possible to observe that for N. hombergii (Fig. 3b), CAT, SOD, GR and GPx activities were the most discriminating factors between sites, since BAR individuals (black line) demonstrated lower activities of those enzymes than the reference (grey dashed line). Besides, that, a slight increase of LPO levels was also evident in individuals from BAR. In C. edule (Fig. 3a), CAT, GR, GSHt and especially GPx were the most important factors, concerning spatial discrimination, since BAR individuals (black line) demonstrated higher activities of those than the reference (grey dashed line).
Integrated biomarker response (IBRv2) values of C. edule (a) and N. hombergii (b) collected at the Tagus estuary and associated star plots. Black line corresponds to IBRv2 index of individuals from contaminated site (BAR) and is represented in relation to the reference site (ALC; 0; grey dashed line). Values above 0 reflect induction of the biomarker; while below 0 indicate reduction of the biomarker
Discussion
Metal accumulation kinetics not always reflect sediment contamination
The evaluation of the physico-chemical characteristics of sediments from estuarine sites confirmed the distinction between the two zones, particularly regarding metal contamination, namely ALC and BAR areas. In general, the first presented lower sediment metal concentrations than the latter, resulting in a minor metal pollution index (MPI), identifying BAR as a metal-contaminated area. In fact, Hg levels in sediments of BAR were above the ERM thus, toxicity related with this element is likely to occur (Long et al. 1995). Moreover, levels of Ni, As and Pb in sediments of BAR were above their respective ERL, suggesting a probable risk to organisms. Despite sediments of ALC presented levels of Ni, As, Cd, Pb and Hg above their ERL, a higher toxicity risk is expected to be occurring at BAR, since metal levels in sediments were higher than in ALC. Our results are in agreement with several previous studies that reported similar levels of Ni, As, Cd, Pb and Hg in the sediments from the same estuarine areas (Vale et al. 2008; Canário et al. 2010; Neto et al. 2011).
The present study revealed different metal accumulation kinetics for C. edule and N. hombergii. C. edule accumulation patterns better mirrored the sediment contamination by metals when compared with N. hombergii. The polychaete accumulated significantly lower concentrations of metal, regardless the site. These results may, in part, be explained by the distinct foraging behaviour presented by both species. N. hombergii is a predator and, consequently, more likely to uptake metals adsorbed in its preys (Robinson et al. 2003) than in the water or sediment particles, as expected to occur in suspension filter-feeders as C. edule (Bergayou et al. 2009).
A very distinct pattern regarding the accumulation of Hg was observed for both species. Only C. edule showed a significantly higher accumulation of Hg at the highest contaminated site (BAR). The inability of N. hombergii to discriminate sites regarding Hg accumulation is a remarkable result, especially considering the elevated persistence and bioaccumulation propensity of Hg, as well as the higher levels observed in the sediments from BAR. These dissimilar responses of C. edule and N. hombergii to the sediment metal contamination may also indicate a tolerant behaviour by the polychaetes living in the BAR area. N. hombergii might be able to perform a selection of the particles regarding their metal contamination (based on the taste) in order to avoid them (Mason and Jenkins 1995; Rainbow et al. 2004). Another possibility is the regulation of metals’ intake as observed by King et al. (2004) for the polychaetes Australonereis ehlersi and Aglaophamus australiensis that were able to minimise the uptake of Zn from the sediment and water compartments, although the opposite behaviour occurred for Cu. These mechanisms allowed the organisms to tolerate high concentrations of metals in the environment (Berthet et al. 2003; King et al. 2004).
Cerastoderma edule populations were able to survive and inhabit at BAR, despite the elevated metal bioaccumulation, which also suggests some tolerance mechanisms acquired by the bivalve. This might be due to the ability of the compartmentalization of metals as metal-sensitive fractions (i.e. organelles and heat-sensitive proteins) and biological detoxification of metals (i.e. metallothioneins and metal-rich granules) (Wallace et al. 2003). Such compartmentalization of the metal ions will reduce their bioavailability to body tissues and consequent toxicity to the organisms. Such process has been previously described as a metal tolerance mechanism in bivalves (Wallace et al. 2003; Vijver et al. 2004; Freitas et al. 2012).
Metal accumulation in C. edule and N. hombergii can be influenced by several geo- and physico-chemical parameters as well as biological factors as globally described for other benthic species (Sanchiz et al. 2001; King et al. 2004; Baudrimont et al. 2005). The biology of the selected species, including different feeding behaviours, respiration, mobility and living habits, are important variables to take into account concerning metal uptake and accumulation (King et al. 2004). Nevertheless, it is interesting to notice that the species, which whole body is in a direct contact with the sediment (N. hombergii), presents significantly lower metal levels than the bivalve (C. edule), protected by the shells, suggesting a certain impermeability of the integument that can hinder the uptake of metals.
Relationship between oxidative stress profiles and metal bioaccumulation levels in C. edule and N. hombergii
The balance between the pro-oxidant challenge promoted by ROS and the organism’s ability to detoxify the reactive intermediates, namely through the antioxidant defence system, is crucial to the evolution of severe cellular damage, either in DNA, proteins or lipids (Muniz et al. 2008). Regarding the toxicity mechanisms of metals, one of the most important is the enhancement of the intracellular ROS levels, through Fenton-like reactions (Ercal et al. 2001; Frenzilli et al. 2001). Besides, some metals, namely Cd, Pb and Hg, due to their electron-sharing affinities, can form covalent attachments with the enzymes from the antioxidant system, inactivating them (Ercal et al. 2001).
The evaluation of oxidative stress profiles in N. hombergii suggested that metals, namely As and Pb, could be acting by inhibiting the antioxidant enzymes. Such inhibition occurred at the highest contaminated site (BAR) in comparison to reference site (ALC) and, thus, antioxidant enzymes levels were able to perform site discrimination. Since N. hombergii from BAR accumulated significantly higher concentrations of As and Pb than individuals from ALC, those metals might be the trigger of the CAT, SOD, GR and GPx inhibitions. In particular, CAT, SOD and GPx were described as Pb targets, by the formation of complexes with their substrates or by promoting an enzymatic synthesis inhibition (see Ercal et al. 2001). Most of the previous studies reported an enhancement of antioxidant defences of polychaetes after metal exposure (Geracitano et al. 2004; Pérez et al. 2004; Díaz-Jaramillo et al. 2013; Gomes et al. 2013). On the contrary, Sandrini et al. (2008) found a relationship between the exposure of the polychaete Laeonereis acuta to Cd and the decrease of CAT activity, which is in line with current data. Also, Nusetti et al. (2001) found that after 7 days of exposure to sublethal Cu concentrations, GR activity in the polychaete Eurythoe complanata decreased. Despite the antioxidant defences inhibition, the polychaete N. hombergii was able to prevent the induction of LPO, probably either by other enzymatic and non-enzymatic antioxidant players not measured in the present study or different detoxifying mechanisms. Regardless the absence of LPO, other cellular injuries as DNA oxidative damage or protein oxidation should not be disregarded.
As previously stated, C. edule was the species that better reflected the sediment metal contamination. This species showed higher metal accumulation levels but seems to be more adjusted than N. hombergii regarding the modulation of the antioxidant defences. This tolerance is evident since C. edule antioxidant defence parameters were almost unaltered between the two assessed sites, ALC and BAR. However, minor increases in CAT and GR activities and significantly higher GPx were found at BAR. Such responses might indicate a proactive action by the cockle antioxidant system to prevent the increase of damage, as LPO, that was not detected. Similar results were observed by Freitas et al. (2012), who found that none of the C. edule biochemical parameters studied, namely CAT, SOD and GPx, reflected the contamination gradient. Moreover, Zhang et al. (2010) did not find significant alterations concerning the antioxidant defences (CAT, SOD and GST) of the bivalve Chlamys farreri after an exposure to Cu. Contrastingly, a few studies revealed different patterns, namely an enhancement of CAT and SOD activities in M. galloprovincialis in polluted areas (Box et al. 2007) and the increase of CAT and LPO in C. edule under metal exposure (Bergayou et al. 2009).
In a recent work, Regoli and Giuliani (2014) stated that constantly higher levels of antioxidants activity in exposed organisms (than in reference ones) reflect their need to maintain a more elevated protection toward the environmental pro-oxidant challenge. Additionally, a comparable antioxidant efficiency between polluted and reference populations would indicate the occurrence of tolerant or counteractive mechanisms (Regoli and Giuliani 2014), which seems to be in line with C. edule responses. On the other hand, depressed defences indicate the inability of organisms to counteract the toxicity of ROS (Regoli and Giuliani 2014) and the occurrence of oxidative damage can be expected. Regardless the absence of LPO in N. hombergii from BAR, the polychaete antioxidant defences were depressed, thus the oxidative damage at different cellular structures, namely DNA and proteins, are plausible to be occurring.
Insights into environmental health assessment using C. edule and N. hombergii: a pros and cons perspective
When planning environmental health assessment studies using bioindicator species, it is important to have a comprehensive knowledge of the ecological and biochemical behaviour of the candidate sentinel species. In particular, the metal accumulation patterns and the oxidative stress profiles comprise two relevant topics, if we intend to investigate tolerance mechanisms in the selected organisms. In the present study, both species showed distinctive patterns. Even if both organisms were able to develop mechanisms that allow their survival in the contaminated area of the estuary (BAR), considering their presence in both areas (Piló et al. 2015), they seem to present different degrees of adaptation/acclimatisation. C. edule clearly reflected the metal contamination in the sediments from both areas of study. Thus, if one study aims to assess a direct relationship between the metals in the sediment and the bioaccumulation levels on the organism, the common cockle C. edule should be a good candidate. On the other hand, if the aim of the study is to evaluate early biochemical effects in the exposed organisms, the polychaete N. hombergii seems more appropriate. In particular, if we consider the antioxidant system responses, this species seems more responsive.
The clear interspecific variability in their responses can result from their different habits, physiology and life styles. The cockle is a filter-feeder while the polychaete lives burrowed in the sediment thus, they will better inform about water- and sediment-bound chemicals, respectively (Pérez et al. 2004). Freitas et al. (2012) also found differences between the bivalve C. edule and the polychaete Diopatra neapolitana, in what concerns to the metal accumulation and compartmentalization as well as oxidative stress parameters. In that study, the authors stated that the bivalve handled the metal chelation and precipitation in a more successful way while the polychaete was the best bioindicator to assess the oxidative stress status, which is in agreement with the current study.
Nevertheless, considering the numerous factors modulating metals bioaccumulation and antioxidant defences and taking into account the diversity of bivalves and polychaetes’ habits, biology, life styles, the extrapolation of the results of C. edule and N. hombergii to their respective taxonomic groups (i.e. Bivalvia and Polychaeta) cannot be performed. Ultimately, when possible, both bioindicators should be adopted and integrated to allow for a more comprehensive approach, particularly if the study aims to assess the general environmental and ecotoxicological health status of a system and their communities, as the macrobenthos at the estuaries.
Conclusions
Overall, the current findings highlighted distinctive patterns of the two potential bioindicator species from Bivalvia and Polychaeta taxonomic groups regarding metal accumulation and antioxidant system responses, possibly related with their distinct foraging behaviour. Under a metal contamination scenario, C. edule better reflected the sediment metal contamination patterns, accumulating higher concentrations of As, Pb and Hg. Differently, N. hombergii showed a proficient behaviour in the reduction of metal uptake, which provided tolerance to metals. N. hombergii antioxidant system responses were more informative than the ones of C. edule. Nevertheless, the polychaete defences were inhibited in the most contaminated site, probably due to the higher accumulation of As and Pb.
In general, the accumulation of metal levels in C. edule was faithful to sediment contamination. The value of C. edule as a bioindicator species seems to be diminished by tolerance mechanisms to metal contamination, which in turn decreased its responsiveness. Thus, it is of utmost importance to complement the information of C. edule with that of another benthic species, namely N. hombergii, to better evaluate the sediment quality and the system health.
The present study contributed to improve the lack of fundamental knowledge concerning C. edule and N. hombergii metal accumulation patterns and antioxidant system responses under environmentally metal exposure. Hence, this work provided useful information that can be adopted, supporting the interpretation of future environmental monitoring studies data.
References
Agencia de Medio Ambiente de Andalucia, Spain (AMA) (1992) Determining the pesticide content in waters and the metal content in living organisms. AMA, Seville, pp 55–67
Ahmad I, Mohmood I, Mieiro CL et al (2011) Lipid peroxidation vs. antioxidant modulation in the bivalve Scrobicularia plana in response to environmental mercury—organ specificities and age effect. Aquat Toxicol 103:150–158. doi:10.1016/j.aquatox.2011.02.017
Ahmad I, Mohmood I, Coelho JP et al (2012) Role of non-enzymatic antioxidants on the bivalves’ adaptation to environmental mercury: organ-specificities and age effect in Scrobicularia plana inhabiting a contaminated lagoon. Environ Pollut 163:218–225. doi:10.1016/j.envpol.2011.12.023
Almeida EA, Bainy ACD, Loureiro APM et al (2007) Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comp Biochem Physiol 146:588–600. doi:10.1016/j.cbpa.2006.02.040
Amiard-Triquet C, Rainbow PS, Romeo M (2011) Tolerance to environmental contaminants. CRC Press, Environ Ecol Risk Assess. doi:10.1201/b10519
Athar M, Iqbal M (1998) Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis 19:1133–1139. doi:10.1093/carcin/19.6.1133
Baker M, Cerniglia G, Zaman A (1990) Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190:360–365. doi:10.1016/0003-2697(90)90208-Q
Baudrimont M, Schäfer J, Marie V et al (2005) Geochemical survey and metal bioaccumulation of three bivalve species (Crassostrea gigas, Cerastoderma edule and Ruditapes philippinarum) in the Nord Médoc salt marshes (Gironde estuary, France). Sci Total Environ 337:265–280. doi:10.1016/j.scitotenv.2004.07.009
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322. doi:10.1002/etc.5620210629
Bergayou H, Mouneyrac C, Pellerin J, Moukrim A (2009) Oxidative stress responses in bivalves (Scrobicularia plana, Cerastoderma edule) from the Oued Souss estuary (Morocco). Ecotoxicol Environ Saf 72:765–769. doi:10.1016/j.ecoenv.2008.09.012
Berthet B, Mouneyrac C, Amiard JC et al (2003) Accumulation and soluble binding of cadmium, copper, and zinc in the polychaete Hediste diversicolor from coastal sites with different trace metal bioavailabilities. Arch Environ Contam Toxicol 45:468–478. doi:10.1007/s00244-003-0135-0
Bird R, Draper H (1984) Comparative studies on different methods of malonaldehyde determination. Methods Enzimol 105:299–305. doi:10.1016/S0076-6879(84)05038-2
Boening DW (1999) An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ Monit Assess 55:459–470. doi:10.1023/A:1005995217901
Box A, Sureda A, Galgani F et al (2007) Assessment of environmental pollution at Balearic Islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis. Comp Biochem Physiol 146:531–539. doi:10.1016/j.cbpc.2007.06.006
Budd G, Hughes J (2005) Nephtys hombergii: a catworm. In: Mar. Biol. Assoc. United Kingdom. http://www.marlin.ac.uk/species/detail/1710. Accessed 10 Jan 2016
Caçador I, Costa JL, Duarte B et al (2012) Macroinvertebrates and fishes as biomonitors of heavy metal concentration in the Seixal Bay (Tagus estuary): which species perform better? Ecol Indic 19:184–190. doi:10.1016/j.ecolind.2011.09.007
Caetano M, Fonseca N, Cesário R, Vale C (2007) Mobility of Pb in salt marshes recorded by total content and stable isotopic signature. Sci Total Environ 380:84–92. doi:10.1016/j.scitotenv.2006.11.026
Canário J, Vale C, Caetano M (2005) Distribution of monomethylmercury and mercury in surface sediments of the Tagus Estuary (Portugal). Mar Pollut Bull 50:1142–1145. doi:10.1016/j.marpolbul.2005.06.052
Canário J, Vale C, Poissant L et al (2010) Mercury in sediments and vegetation in a moderately contaminated salt marsh (Tagus Estuary, Portugal). J Environ Sci 22:1151–1157. doi:10.1016/S1001-0742(09)60231-X
Carvalho S, Pereira P, Pereira F et al (2011) Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Mar Environ Res 71:97–110. doi:10.1016/j.marenvres.2010.11.005
Chainho P, Silva G, Lane MF et al (2010) Long-term trends in intertidal and subtidal benthic communities in response to water quality improvement measures. Estuaries Coasts 33:1314–1326. doi:10.1007/s12237-010-9321-2
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) Handbook of methods in oxygen radical research. CRC Press Inc, Boca Raton, pp 283–284
Costley CT, Mossop KF, Dean JR et al (2000) Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal Chim Acta 405:179–183. doi:10.1016/S0003-2670(99)00742-4
Courtney LA, Clements WH (2002) Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshw Biol 47:1766–1778. doi:10.1046/j.1365-2427.2002.00896.x
Cribb A, Leeder J, Spielberg S (1989) Use of a microplate reader in an assay of glutathione reductase using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 183:195–196. doi:10.1016/0003-2697(89)90188-7
Dabouineau L, Ponsero A (2009) Synthesis on biology of common European cockle Cerastoderma edule. Université Catholique de l’Ouest, p 23
Díaz-Jaramillo M, da Rocha AM, Chiang G et al (2013) Biochemical and behavioral responses in the estuarine polychaete Perinereis gualpensis (Nereididae) after in situ exposure to polluted sediments. Ecotoxicol Environ Saf 89:182–188. doi:10.1016/j.ecoenv.2012.11.026
Duarte B, Reboreda R, Caçador I (2008) Seasonal variation of extracellular enzymatic activity (EEA) and its influence on metal speciation in a polluted salt marsh. Chemosphere 73:1056–1063. doi:10.1016/j.chemosphere.2008.07.072
Elliott M, Quintino V (2007) The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar Pollut Bull 54:640–645. doi:10.1016/j.marpolbul.2007.02.003
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539. doi:10.2174/1568026013394831
Farag AM, Woodward DF, Goldstein JN et al (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene river basin, Idaho. Arch Environ Con Tox 127:119–127. doi:10.1007/s002449900295
Ferreira AM, Cortesão C, Castro OG, Vale C (1990) Accumulation of metals and organochlorines in tissues of the oyster Crassostrea angulata from the Sado Estuary, Portugal. Sci Total Environ 97:627–639. doi:10.1016/0048-9697(90)90266-W
França S, Vinagre C, Caçador I, Cabral HN (2005) Heavy metal concentrations in sediment, benthic invertebrates and fish in three salt marsh areas subjected to different pollution loads in the Tagus Estuary (Portugal). Mar Pollut Bull 50:998–1003. doi:10.1016/j.marpolbul.2005.06.040
Freitas R, Costa E, Velez C et al (2012) Looking for suitable biomarkers in benthic macroinvertebrates inhabiting coastal areas with low metal contamination: comparison between the bivalve Cerastoderma edule and the polychaete Diopatra neapolitana. Ecotoxicol Environ Saf 75:109–118. doi:10.1016/j.ecoenv.2011.08.019
Frenzilli G, Nigro M, Scarcelli V et al (2001) DNA integrity and total oxyradical scavenging capacity in the Mediterranean mussel, Mytilus galloprovincialis: a field study in a highly eutrophicated coastal lagoon. Aquat Toxicol 53:19–32. doi:10.1016/S0166-445X(00)00159-4
Geracitano LA, Bocchetti R, Monserrat JM et al (2004) Oxidative stress responses in two populations of Laeonereis acuta (Polychaeta, Nereididae) after acute and chronic exposure to copper. Mar Environ Res 58:1–17. doi:10.1016/j.marenvres.2003.09.001
Gomes T, Gonzalez-Rey M, Rodríguez-Romero A et al (2013) Biomarkers in Nereis diversicolor (Polychaeta: Nereididae) as management tools for environmental assessment on the southwest Iberian coast. Sci Mar 77:69–78
Gornall A, Bardawill C, David M (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
King CK, Dowse MC, Simpson SL, Jolley DF (2004) An assessment of five Australian polychaetes and bivalves for use in whole-sediment toxicity tests: toxicity and accumulation of copper and zinc from water and sediment. Arch Environ Contam Toxicol 47:314–323. doi:10.1007/s00244-004-3122-1
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97. doi:10.1007/BF02472006
Lotze HK, Lenihan HS, Bourque BJ et al (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–1809. doi:10.1126/science.1128035
Maranho LA, DelValls TA, Martín-Díaz ML (2015) Assessing potential risks of wastewater discharges to benthic biota: an integrated approach to biomarker responses in clams (Ruditapes philippinarum) exposed under controlled conditions. Mar Pollut Bull. doi:10.1016/j.marpolbul.2015.01.009
Mason A, Jenkins K (1995) Metal detoxication in aquatic organisms. In: Tessier A, Turner D (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York, pp 479–608
Meyer JN, Di Giulio RT (2003) Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl 13:490–503. doi:10.2307/3099913
Mohandas J, Marshall J, Duggin G et al (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807. doi:10.1016/0006-2952(84)90353-8
Muniz J, McCauley L, Scherer J et al (2008) Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol Appl Pharmacol 227:97–107. doi:10.1016/j.taap.2007.10.027
Neto AF, Costa JL, Costa MJ et al (2011) Accumulation of metals in Anguilla anguilla from the Tagus estuary and relationship to environmental contamination. J Appl Ichthyol 27:1265–1271. doi:10.1111/j.1439-0426.2011.01814.x
Nordberg GF, Fowler BA, Nordberg M (2014) Handbook on the toxicology of metals, 4th edn. Academic Press, Amsterdam
Nusetti O, Esclapés M, Salazar G et al (2001) Biomarkers of oxidative stress in the polychaete (Amphinomidae) under short term copper exposure. Bull Environ Contam Toxicol 66:576–581. doi:10.1007/s00128-001-0047-5
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Pereira P, Carvalho S, Pereira F et al (2012) Environmental quality assessment combining sediment metal levels, biomarkers and macrobenthic communities: application to the Óbidos coastal lagoon (Portugal). Environ Monit Assess 184:7141–7151. doi:10.1007/s10661-011-2486-8
Pérez E, Blasco J, Solé M (2004) Biomarker responses to pollution in two invertebrate species: Scrobicularia plana and Nereis diversicolor from the Cádiz bay (SW Spain). Mar Environ Res 58:275–279. doi:10.1016/j.marenvres.2004.03.071
Piló D, Pereira F, Carriço A et al (2015) Temporal variability of biodiversity patterns and trophic structure of estuarine macrobenthic assemblages along a gradient of metal contamination. Estuar Coast Shelf Sci. doi:10.1016/j.ecss.2015.06.018
Rainbow PS, Geffard A, Jeantet AY et al (2004) Enhanced food-chain transfer of copper from a diet of copper-tolerant estuarine worms. Mar Ecol Prog Ser 271:183–191. doi:10.3354/meps271183
Regoli F (1998) Trace metals and antioxidant enzymes in gills and digestive gland of the mediterranean mussel Mytilus galloprovincialis. Arch Environ Contam Toxicol 34:48–63. doi:10.1007/s002449900285
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117. doi:10.1016/j.marenvres.2013.07.006
Robinson KA, Baird DJ, Wrona FJ (2003) Surface metal adsorption on zooplankton carapaces: implications for exposure and effects in consumer organisms. Environ Pollut 122:159–167. doi:10.1016/S0269-7491(02)00302-0
Rodrigues AM, Meireles S, Pereira T et al (2006) Spatial patterns of benthic macroinvertebrates in intertidal areas of a Southern European estuary: the Tagus, Portugal. Hydrobiologia 555:99–113. doi:10.1007/s10750-005-1109-1
Sanchez W, Burgeot T, Porcher JM (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725. doi:10.1007/s11356-012-1359-1
Sanchiz C, Garcίa-Carrascosa AM, Pastor A (2001) Relationships between sediment physico-chemical characteristics and heavy metal bioaccumulation in Mediterranean soft-bottom macrophytes. Aquat Bot 69:63–73. doi:10.1016/S0304-3770(00)00120-0
Sandrini JZ, Lima JV, Regoli F et al (2008) Antioxidant responses in the nereidid Laeonereis acuta (Annelida, Polychaeta) after cadmium exposure. Ecotoxicol Environ Saf 70:115–120. doi:10.1016/j.ecoenv.2007.03.004
Silva G, Costa JL, de Almeida PR, Costa MJ (2006) Structure and dynamics of a benthic invertebrate community in an intertidal area of the Tagus estuary, western Portugal: a six year data series. Hydrobiologia 555:115–128. doi:10.1007/s10750-005-1110-8
Solé M, Kopecka-Pilarczyk J, Blasco J (2009) Pollution biomarkers in two estuarine invertebrates, Nereis diversicolor and Scrobicularia plana, from a Marsh ecosystem in SW Spain. Environ Int 35:523–531. doi:10.1016/j.envint.2008.09.013
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Usero J, González-Regalado E, Gracia I (1996) Trace metals in the bivalve mollusc Chamelea gallina from the Atlantic coast of southern Spain. Mar Pollut Bull 32:305–310. doi:10.1016/0025-326X(95)00209-6
Vale C, Canário J, Caetano M et al (2008) Estimation of the anthropogenic fraction of elements in surface sediments of the Tagus Estuary (Portugal). Mar Pollut Bull 56:1364–1367. doi:10.1016/j.marpolbul.2008.04.006
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. doi:10.2174/0929867053764635
Vijver MG, Van Gestel CAM, Lanno RP et al (2004) Internal metal sequestration and its ecotoxicological relevance: a review. Environ Sci Technol 38:4705–4712. doi:10.1021/es040354g
Wallace WG, Lee BG, Luoma SN (2003) Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar Ecol Prog Ser 249:183–197. doi:10.3354/meps249183
Wang WX (2001) Comparison of metal uptake rate and absorption efficiency in marine bivalves. Environ Toxicol Chem 20:1367–1373. doi:10.1002/etc.5620200628
Wang WX, Rainbow PS (2005) Influence of metal exposure history on trace metal uptake and accumulation by marine invertebrates. Ecotoxicol Environ Saf 61:145–159. doi:10.1016/j.ecoenv.2005.01.008
Wilhelm Filho D, Torres MA, Tribess TB et al (2001a) Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Brazilian J Med Biol Res 34:719–726. doi:10.1590/S0100-879X2001000600004
Wilhelm Filho D, Tribess T, Gaspari C et al (2001b) Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 203:149–158. doi:10.1016/S0044-8486(01)00599-3
Worm B, Barbier EB, Beaumont N et al (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790. doi:10.1126/science.1132294
Zhang Y, Song J, Yuan H et al (2010) Biomarker responses in the bivalve (Chlamys farreri) to exposure of the environmentally relevant concentrations of lead, mercury, copper. Environ Toxicol Pharmacol 30:19–25. doi:10.1016/j.etap.2010.03.008
Acknowledgments
This work was supported by CESAM (UID/AMB/50017) and “Fundação para a Ciência e a Tecnologia” (FCT/MEC; Government of Portugal) through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020, through the Research Project PTDC/AAC-AMB/121037/2010, the Investigation Fellowship (BI/CESAM/PTDC/AAC-AMB/121037/2010; Ana Marques), and the Post-doctoral fellowships SFRH/BPD/69563/2010 (Patrícia Pereira) and SFRH/BPD/88947/2012 (Sofia Guilherme).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Marques, A., Piló, D., Araújo, O. et al. Propensity to metal accumulation and oxidative stress responses of two benthic species (Cerastoderma edule and Nephtys hombergii): are tolerance processes limiting their responsiveness?. Ecotoxicology 25, 664–676 (2016). https://doi.org/10.1007/s10646-016-1625-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1625-y