Abstract
The focus of the present study was to assess the quality of different drinking water sources, impacts of poor water quality on human health, and to apportion pollution source(s) of the district Bajaur, Pakistan. Drinking water samples (n = 331) were randomly collected from springs, hand pumps, open wells, and tube wells and analyzed for physicochemical parameters including toxic elements, and bacteriological contamination (i.e., Escherichia coli). Furthermore, a questionnaire survey was conducted to record the cases of waterborne diseases in the study area. The results showed that total suspended solids and bacteriological contamination exceeded the permissible limits of the WHO in all four of the water sources. Among the potentially toxic elements, Cd, Pb, and Mn were above the permissible limits of the WHO in some samples. The hazard index for spring water was found to exceed the toxicity level (i.e., HI > 1) set by US EPA for both adults and children, while the sources from hand pumps, open wells, and tube wells were within the safe limit. The order for the overall safety level for water quality in the study area was tube wells > open wells > hand pumps > springs. The pollution source apportionment statistics revealed that both geogenic and anthropogenic activities are the sources of drinking water contamination. The results of the questionnaire survey indicated that reports of waterborne diseases were highest in respondents who took their drinking water from springs, whereas reports of diseases were moderate in respondents taking their water from open wells and hand pumps and lowest in respondents taking their water from tube wells. Based on the findings of the study, the tube well source of water is recommended for drinking water purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Good quality drinking water is an essential requirement for life and play a vital role in human health and development (Qasemi et al. 2018; He and Wu 2019; Delpla et al. 2020). However, the continuously growing global population and agricultural production, urbanization, and industrialization have exerted pressure on water resources (Tian et al. 2019; He and Wu 2019). Anthropogenic activities and geogenic oxidation of mineralized rock contribute potentially toxic elements (PTEs) to water resources. Bacteriological contamination, especially Escherichia coli (E. coli), also affects the quality of drinking water (Radfard et al. 2019). This contamination of drinking water may pose serious health risks upon consumption (Cairncross and Feachem 2018; Nawaz and Ali 2018). According to a recent estimate, 2.3 billion people are suffering from waterborne diseases all over the world (Ahmed et al. 2020). The release of PTEs to the aquatic environment degrades the quality of water resources (Divahar et al. 2019; Li et al. 2019). According to the WHO (2011), water pollution contributes to 70% of different diseases and 20% of cancers on a global basis. The sources of surface water, i.e., springs, streams, lakes, and rivers, are more vulnerable to PTEs and microbial contamination than groundwater sources (Ding et al. 2015; Abdalla and Khalil 2018). Therefore, for majority of the world population, groundwater is the primary source of drinking water (Adimalla and Qian 2019; Ji et al. 2020). However, a number of studies have reported that both anthropogenic and geogenic activities are a significant sources of PTEs contamination in groundwater (Panneerselvam et al. 2020; Ricolfi et al. 2020; Soujanya Kamble et al. 2020). The PTEs ccontamination can adversely affect human health, economic development, and social cohesion in a community (Begum et al. 2015; Adimalla and Qian 2019).
Due to the significant toxicity, persistence, and bioaccumulative nature of PTEs, monitoring of these elements has become a priority for environmental and health agencies (Hussain et al. 2020; Ricolfi et al. 2020). However, monitoring is difficult in developing countries, due to lack of professional expertise, management systems and financial resources (Amin et al. 2019). Pakistan is a developing country and stands 80th among the list of 122 countries for providing safe drinking water. Therefore, a significant proportion of the population of 21 million population does not have access to safe and clean drinking water (Ahmed et al. 2020). In Pakistan, drinking water from unprotected springs and wells contributes to the high rate of morbidity and mortality, and approximately 70% of the water resources of the country have been affected by organic, inorganic, and bacteriological contamination (Malik et al. 2009). As a result, 20–40% of the population of Pakistan is suffering from various waterborne diseases (Muhammad et al. 2018). Most of the inhabitants in the countryside use contaminated water for drinking and other household purposes. As a result, waterborne diseases, such as hepatitis (A, B, and C), cholera, typhoid, dysentery, and diarrhea, are common among the population in Pakistan (Bosan et al. 2010; Daud et al. 2017). Ahmed et al. (2020) stated that microbial contamination is the main source of waterborne diseases in Sindh province of Pakistan.
The study area (district Bajaur) of Pakistan is located on the countryside in an arid and semi-arid climatic zone. It is located in the Khyber Pakhtunkhwa province adjacent to Afghanistan, and the region suffers from many developmental challenges and conflict. Therefore, little attention has been given regarding water quality and other quality of life parameters. The local population has been using contaminated water for many decades, and as a result, waterborne diseases are endemic in the study area. To the best of our knowledge, this is the first study from the region to assess the quality of different drinking water sources and explore the associated health risks. Hence, a detail study was undertaken to understand the distribution of contamination and determine the potential human health risks for adults and children from exposure to PTEs and microbial pathogens. Therefore, it is hoped that the present study can be used as a reference in evaluation and decision-making processes, regarding management and policies to improve water quality in the study area. Consequently, the focus of this research was to comparatively (1) study the different water sources, i.e., springs, hand pumps, open wells, and tube wells for physicochemical parameters, and levels of PTEs and E. coli, (2) conduct a human health risk assessment (HRA) for adults and children consuming PTEs in drinking water of the study area, (3) identify potential pollution source(s) for each source of drinking water, and (4) conduct a questionnaire survey of the incidence of waterborne diseases in the region to see if there are correlations with the levels of contamination in drinking water.
Materials and Methods
Study Area
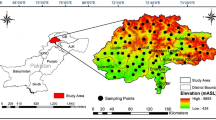
The study area is located in a rural tribal district in western Pakistan, which is located between latitude 34°30′–34°58′N and longitude 71°11–71°48′E (Fig. 1). The district is spread over an area of 1290 km2 with a 1.1 million population. The district is connected with district Dir lower (Hindukush ranges) in the north, Malakand area (river Panjkora) in the east, district Mohmand in the south, and the Kunar valley of Afghanistan to the west (Fig. 1). The climatic condition of area is arid to semi-arid, having extreme summer and winter seasons with an average annual rainfall of ~ 800 mm. The topography of the study area consists of hilly, mountainous, and plain areas, and the geology is comprised of different types of rock formations, including Mesozoic intrusive and metamorphic rocks, Permian and Triassic rocks, undivided Paleozoic rocks, lower Paleozoic rock sand undivided Precambrian rocks (Ullah et al. 2017). The detailed geology of the study area is shown in Fig. 2a.
A digital elevation model divides the study area into three regions, with low, intermediate, and high elevation. The lowest elevation is 480 m, while the highest elevation is 2761 m above sea level. In the high elevation region, mountains are covered by snow in the winter season, which feeds the surface waters and springs during the rest of the year. The hilly and mountainous area is distributed in the east and west of the district Bajaur, accounting for approximately 60% of the total area. Such areas are mainly composed of metamorphic and carbonate sedimentary rocks. In this area, water fed by springs are the main sources of drinking water, which mainly discharges to the surface in pores and fracture lines of the rocks. Such water generally has poor water quality, but due to the low income of the people in the region, it is used for drinking and other purposes. The plain area occupies the remaining 40% of the area of the region, and the geology is mainly composed of carbonate sedimentary and intermediate volcanic rocks, as shown in Figs. 2b and c. Groundwater accessed by wells is the main source of drinking water in this area. The depth of deep aquifers is approximately 300–400 m, and this deep groundwater accessed by tube wells has the best water quality. More shallow groundwater is accessed by hand pumps and open (dug) wells.
Flow Direction Mapping
A flow direction map of the study area was generated from a (sink-free) Digital Elevation Model (DEM). The flow direction of groundwater was predicted by evaluating every central pixel from input blocks of 3 × 3 pixels, each time comparing the value of the central pixel with the value of the 8 neighboring pixels. The researcher can choose whether to calculate the flow direction from the central pixel by either the steepest slope method, that is finding the steepest downhill slope of a central pixel relative to one of the 8 neighbors pixels, or alternatively, by the lowest height method, which is simply finding the neighboring pixel that has the smallest value of all 8 neighboring pixels (this value should also be smaller than the value of the central pixel). In this case, the steepest slope method was used, and so the elevation differences were calculated for each block of 3 × 3 input pixels between the central pixel and the 8 neighboring pixels. To compensate for distances, the elevation difference values of the 4 corner neighboring pixels were divided by 1.4, whereas the elevation difference values of the 2 horizontal neighbors and the 2 vertical neighbors were divided by 1. Then, the neighbor with the largest downhill slope steepness value was found. The position of this neighbor determines the flow direction of the current central pixel. The generated flow direction map is shown in Fig. 2d, e, f.
Collection and Analysis of Samples
From the entire region, a total of 331 water samples were collected, with 96 samples from springs, 75 samples from groundwater accessed by hand pumps, 88 from groundwater accessed by open wells, and 72 from groundwater accessed by tube wells. Before sampling, the water was pumped for more than 5 min from each source except for the spring water. Prior to sample collection, two sets of polyethylene bottles (250 ml) were washed with a 10% nitric acid (HNO3) solution, rinsed, and rinsed twice with deionized water. Water collected in one set of bottles was acidified with a few drops of HNO3 for analysis of PTEs concentration, whereas the other set of samples was not acidified and was used for physicochemical parameters analysis. The collected samples were packed, labeled, and transported to the Department of Environmental Sciences at the University of Peshawar, Pakistan, for further analysis.
Among the physicochemical parameters, temperature, pH, electrical conductivity (EC), turbidity, and dissolved oxygen (DO) were analyzed in situ (during the samples collection), and the parameters determined in situ analysis (except for temperature) were repeated in the laboratory. Temperature was determined using an EA 722AA-100 thermister probe (Winland Electronics), pH by a Seven2GoTM pH meter (Mettler Toledo), EC using a Consort C931 conductivity meter (Topac), and turbidity using a Jenway 6035 turbidity meter (Keison Products). These measurements were repeated three time for each parameter, and the mean values were calculated. The samples were analyzed for other physicochemical parameters using the standard methods described by the American Public Health Association (APHA 1989). The total suspended solids (TSS) and total dissolved solids (TDS) were analyzed using gravimetric methods. The hardness was analyzed by the titration method, where EDTA titrant (0.01 M) was prepared and standardized against calcium chloride solution, using a buffer solution and Erichrome Black T indicator. Each sample was titrated against the EDTA titrant to the end point and hardness calculated using Eq. (1).
where A stands for ml of the EDTA solution used and B = mg CaCO3 equivalent to 1.00 mL EDTA titrant (APHA 1989).
The levels of dissolved oxygen (DO) were determined using an InLab® OptiOx-5 m instrument (Mettler Toledo). The concentration of nitrate was determined using a UV–visible spectrophotometer (Hach model DR 5000). The major cations (i.e., Ca, Mg, Na, and K) and PTEs (i.e., Cd, Pb, Mn, Cr, Zn, and Co) were determined using an Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) supplied by PerkinElmer (Optima 8300). Briefly, the ICP-OES was standardized for the major elements and the PTEs through their respective standard solutions and blanks before running the samples. The accuracy of the instrument was greater than 98% with a correlation coefficient of R2 > 0.99. Each water sample was analyzed in triplicate, and the mean value and the standard deviation around the mean was calculated. Standard solutions and laboratory blanks were retested and used before analysis of each parameter. For analysis by ICP-OES, the internationally recognized standards were used for calibration. The data on mean concentrations were analyzed statistically using Statistix 10 software and Origin 2019 software was used for graphical plotting.
The counts of E. coli in water samples were determined by the membrane filter method, where 100-mL samples were filtered through sterile cellulose nitrate ester membrane filters (0.45 µm), placed on selective media m-fecal coliform agar plates, and incubated at 37–45 °C. After 24 h, the most probable numbers of E. coli were counted using micrometer plates (Xu et al. 2018).
Health Risk Assessment
The first step in the health risk assessment for consumption of PTEs in drinking water was a calculations of the chronic daily intake (CDI) of PTEs in water. The CDI calculation in Eq. (2) includes data on the ratio of the PTE concentration (Cmetal) multiplied by the average oral daily intake (Di) to the average body weight (BW). The calculation in Eq. (3) is the potential noncarcinogenic health risk index (HRI) calculated from the ratio of the CDI to the Reference Dose (RfD) for each element. Equation (4) is the combined Hazard Index (HI) obtained by adding the HRI values for multiple PTEs. The values of HRI or HI > 1 were considered as indicating a significant noncarcinogenic health risk (USEPA 2011; Muhammad and Nafees 2018).
where The Di of water is 2.0 and 1.0 L per person per day for adults and children, respectively, and BW is 72.0 kg for adults and 32.7 kg for children (Nawab et al. 2018; Muhammad et al. 2020). The RfD is the reference dose defined as “the maximum permissible risk to the human population by conserving a sensitive group during a lifetime” (Wei et al. 2015). The RfD values are 5 × 10–4 for Cd, 1.5 for Cr, 3.6 × 10–2 for Pb, 1.4 × 10–1 for Mn, and 3.0 × 10–1 for Zn respectively (Nawab et al. 2017).
Source Apportionment
Principal component analysis (PCA) and absolute principal component multiple linear regression analysis (APCS-MLR) statistics were used to identify the contamination source(s) in the four drinking water sources. The PCA was used to simplify the numeric matrix of the dataset by consolidating most information from the original dataset into several principal components through Varimax rotation with Kaiser Normalization, with each component explaining part of the variance of the whole dataset. Thus, the principal components are considered as indicating sources of pollution (Khan et al. 2018). Then, the APCS-MLR was applied by combining multiple linear regressions with the denormalized principal component score values is described previously (Zhou et al. 2007; Su et al. 2011). After confirming the number and identity of the possible source(s) influencing drinking water quality for the four different sources through PCA, then source apportionment was computed using APCS-MLR method. All of the statistical dataset analyses were determined using IBM SPSS 20 (Chicago, IL) software.
Questionnaire Survey
To get insight from the public regarding the impacts of water pollution on human health, a structured questionnaire survey was conducted in the study district. In the whole district, 3435 households were selected randomly, in which the consumers from all the four types of water sources were engaged in the survey. The questionnaire consisted of both open- and close-ended questions to get a full spectrum of information about significant waterborne diseases. Information, such as age, monthly income, education, smoking and nonsmoking habits, body weight, source of drinking water, occupation, waterborne diseases, and human health risks data, were collected and recorded. All of the survey respondents within the local communities were directly interviewed in their local language by the researchers. Furthermore, key informant interviews were arranged with the area medical experts (i.e., medical officers, paramedics, and technologists) from the community health centers, basic health units, township (tehsil) headquarter hospitals, and district headquarter hospital in order to collect information related to the waterborne diseases. The disease(s) record of respondents were randomly selected, keeping the water source as an independent variable, to determine whether there were correlations between the diseases and the water source. In the study area and throughout the country of Pakistan, the health institutions or any government or private institutes are ethically bound to provide help regarding community welfare programs and to share their data whenever required.
Results and Discussions
Physicochemical Characteristics
The physicochemical characteristics of the four water sources showed that the lowest water quality was in spring water compared with groundwater accessed by hand pumps, open wells, and especially tube wells. The overall water quality of the four water sources was in the order of tube wells > open wells > hand pumps > springs (Figs. 3, 4). For the springs and hand pumps water sources, the pH was slightly acidic which is correlated with the high concentrations of dissolved PTEs (Cd, Pb, and Mn). The acidic pH of water favors high concentrations of PTEs present in free-ion form in these water sources (Muhammad et al. 2020). The open wells and tube wells had much lower concentrations of PTEs and a circum-neutral pH. The TSS values exceeded the maximum permissible limit (MPL) of the WHO (2011) in all the four water sources. Among these, spring water had the highest mean concentration (1.57 mg L−1) of TSS, while mean TSS was lowest (0.41 mg L−1) in water from tube wells. The higher concentration of TSS in spring water was related to the high turbidity in the spring water (Fig. 3a). The higher turbidity and TSS contents have unpleasant effects and provide shelter to pathogens that causes various diseases (Jehan et al. 2019). The present study results were consistent with Arain et al. (2014) for comparing the water quality from springs and groundwater (tube wells and hand pumps).
Other physicochemical parameters, such as temperature, TDS, hardness, nitrate, DO, and major cations (Ca, Mg, Na, and K) shown in Fig. 3a, were within their permissible limits and therefore these parameters are not discussed in detail. The counts of E. coli were above the MPL (0 in 100 mL sample) identified by the WHO (2011) in the water from springs, hand pumps, and open wells, but these counts were below the MPL in water from the tube wells (Fig. 3b). The high counts of E. coli indicates the potential presence of pathogenic bacteria in the water sources, which can cause various gastrointestinal illnesses in humans, as well as typhoid fever and hepatitis (Khan et al. 2013; Amin et al. 2019; Ahmed et al. 2020). The excessive amounts of E. coli in the water sources might be due to improper disposal of organic wastes and/or seepage from septic tanks or latrines. Moreover, in the rainy season, manure from animals is transported into upstream areas and fecal bacteria may enter into the springs, and shallow ground water accessed by hand pumps and open wells.
Among data of the PTEs, Cd, Pb, and Mn concentrations in many water samples exceeded the MPL of the WHO (2011) for all water sources, except for water from the tube wells, while concentrations of Cr, Zn, and Co were all below the MPL (Fig. 4). The concentrations of PTEs were comparatively high in springs and low in tube wells. The high concentration of Cd in drinking water may causes renal dysfunction, anemia, bronchitis, and emphysema (Horiguchi et al. 2013). The concentration of Pb was also high in some samples, which may be associated with low IQ, neurological damage, kidney damage, abdominal pain, anemia, and behavioral disturbances (Steenland and Boffetta 2000; Järup 2003; Balamurugan et al. 2020). Similarly, Mn concentrations were high in water from springs and hand pumps, which may cause the disease known as Manganism and neurological problems (Dieter et al. 2005). Anthropogenic sources of PTEs in spring water could be due to contamination from house hold wastes, open drainage channels, unprotected tertiary drains, and agriculture runoff (Daud et al. 2017; Adimalla and Qian 2019; Ji et al. 2020; Owens et al. 2020). However, as mentioned previously, the high pH of the sources of spring water promotes the dissolution of PTEs from geogenic sources into the drinking water. Compared with other studies, the concentrations of PTEs in the study area were higher than those reported in drinking water originating from mafic and ultramafic rocks in a region in northern Pakistan (Shah et al. 2012).
Health Risk Assessment
In the study area, the highest CDI values were calculated for Zn for both adults and children, in all the four water sources. The CDI values (adults and children) of PTEs were in the order of springs > hand pumps > open wells > tube wells, as documented in the Supplementary Information (Tables S-1 and S-2). The CDI values were further evaluated for the calculation of HRI (noncarcinogenic risk) in both adults and children. The HRI results showed that the toxicity of individual PTEs is below a value of 1 for both adults and children, but children (Fig. 5b) have comparatively higher values of HRI than adults (Fig. 5a) group. Among the four water sources, water from springs showed the highest noncarcinogenic risks relative to water from hand pumps and open wells, while water from tube wells water showed the lowest noncarcinogenic risks. The overall noncarcinogenic risk in the four water sources was in the order of springs > hand pumps > open wells > tube wells. Furthermore, the HRI results for all the PTEs were in the order of Mn > Pb > Cr > Cd > Co > Fe > Cu > Zn in both adults and children (Fig. 5).
The HI results for water from springs were greater than a value of 1 for both adults and children (Fig. 6), which indicates noncarcinogenic health risks for populations obtaining their drinking water from this source (Xiao et al. 2019). Among the other three water sources, the HI calculated for water from the hand pumps was quite close to the toxicity limit (especially in children), indicating possible future health risks if the contamination sources are not properly managed. The HI results for water from open wells and tube wells were in the safe limit (HI < 1). The overall HI risk in the four water sources was in the decreasing order of springs > hand pumps > open wells > tube wells. Other studies (Shah et al. 2012) have reported similar findings for surface and groundwater in a region in northern Pakistan.
Source Apportionment
The PCA results for the spring water revealed that four factors (F1…F4) explained a total cumulative variance of 85.9% (Table 1). Among these factors, F1 contributed 60.5% to the total variance, with high loadings for the parameters of temperature (0.871), E. coli (0.867), Cd (0.935), Cr (0.954), Pb (0.938), Co (0.954), Fe (0.952), Mn (0.956), Cu (0.956), and Zn (0.940). Previous studies have shown that the levels of Cd, Pb, Cr, Mn, and Cu are influenced by weathering of mafic and ultramafic rocks in the region and local mining (Shah et al. 2012), while the E. coli levels are influenced by various anthropogenic activities, such as improper disposal of waste, seepage from septic tanks, and animal wastes (Khan et al. 2013). The PCA results for water from springs indicate that loadings to F1 are due to both geogenic and anthropogenic contributions. The factor 2 (F2) contributes 12.0% to the total variance, with high loading for the parameters of EC (0.832), TDS (0.865), turbidity (0.728), Ca (0.801), and Mg (0.857). The results suggest that F2 may be influenced by schistose rocks having sulfide beams, feldspar, talc, and chlorite (Khan et al. 2013). The factor 3 (F3) and factor 4 (F4) make very low contributions to the PCA ordination (< 10%), which can be discounted.
For the PCA of data from the hand pump water source, the total cumulative variance of six factors were 85.4%, in which F1 contributed 43.0% to the total variance with high loadings for the parameters of temperature (0.677), EC (0.910), TDS (0.916), turbidity (0.965), Mg (0.907), Cd (0.853), Cr (0.841), Pb (0.860), Co (0.878), Cu (0.731), and Zn (0.802), as shown in Table 1. The levels of Cd, Cr, Pb, Co, Cu, and Zn could be influenced by the dissolution of these elements from mafic and ultramafic rocks (Shah et al. 2012), but also from leaching of metals from older water distribution systems (Xiao et al. 2019). Additionally, the EC, TDS, turbidity, and Mg may be influenced by natural sources, i.e., weathering of exposed rocks, surface run-off, and agricultural activities (Rasool et al. 2016). The F2 contributed 12.7% to the total variance, with high loadings for the parameters of pH (0.896) and E. coli (0.673). Bacteriological contamination is commonly associated with reduced pH. The F2 results indicate that shallow groundwater sources of drinking water accessed by hand pumps may be influenced by leaching of contaminants from agricultural activates (Khan et al. 2013). The F3, F4, F5, and F6 were discounted due to low contributions to the PCA.
The PCA analysis of data from the open wells water source also generated six factors, with a total cumulative variance of 85.0%, in which F1 contributed 22.5% to the total variance, with high loadings for the parameters of temperature (0.677), EC (0.910), TDS (0.916), turbidity (0.965), Mg (0.907), Cd (0.853), Cr (0.841), Pb (0.860), Co (0.878), Cu (0.731), and Zn (0.802), as shown in Table 2. The Cd, Pb, Cr, Cu, and Zn levels could have been influenced by dissolution from mafic and ultramafic rocks bedrocks (Shah et al. 2012). The EC, TDS, turbidity, and Mg levels are likely influenced by anthropogenic activities (seepage from septic tanks and run-off), as well as rocks weathering (Rasool et al. 2016). These result showed that the parameters contributing to F1 may be influenced by both anthropogenic and geogenic contributions. The F2 contributed 15.1%, with high loadings to the pH (0.896) and E. coli (0.673). The bacteriological contamination, also discussed above for waters accessed using hand pumps are probably influenced by inputs into shallow sources of groundwater from human and animal fecal wastes. The F3, F4, F5, and F6 have very low contributions (< 10%) and were discounted.
The PCA analysis of data from the tube wells revealed seven factors with the total cumulative variance of 78.0%, in which F1 contribute 34.6% to the total variance with high loadings for the parameters of temperature (0.727), turbidity (0.907), TDS (0.907), Cr (0.687), Pb (0.794), and Co (0.893), as summarized in Table 2. The TDS, hardness, Ca, and Mg levels were probably influenced by the geology of limestone and calcareous schists, and Pb, Cr, and Cu levels could be influenced by the mafic and ultramafic bedrocks in the area (Shah et al. 2012). The result indicates that the parameters contributing to F1 may be from geogenic contribution only. The F2 contributed 12.3% to the total variance, with high loadings for the parameters of Fe (0.891), Mn (0.769), Cu (0.393), and Zn (0.908). These results indicate that leaching of metals from old water distribution systems may be contaminating the drinking water (Khan et al. 2013). The F3, F4, F5, F6, and F7 were discounted due to their low contributions (< 10%). The overall results of PCA analysis indicate that both geogenic and anthropogenic sources contributes to the contamination of drinking water in the region.
The APCS-MLR results revealed that the spring water was influenced by both anthropogenic and geogenic activities. Weathering of the bedrocks contributed 43%, while anthropogenic factors, such as septic seepage, surface run-off, and waste disposal contributed 44%, and some nonclassified sources contributed 12% to the total pollution (Fig. 7a). For water accessed by hand pumps, most of the sites were contaminated by both geogenic and anthropogenic pollution. In the geogenic pollution, the bedrocks contributed 26% to the pollution, mixed pollution in the form of soil weathering and run-off contributed 23%, bacteriological contamination contributed 18%, and the remainder of the contributions were from nonclassified sources (13% and 11%), as shown in Fig. 7b. In the open well water, the major portion of contamination (34%) was from a mix of both geogenic and anthropogenic sources. Moreover, the anthropogenic pollution in the form of bacteriological contamination contributed 25% to the total pollution, whereas the nonclassified pollution contributed 14% and 13% (Fig. 7c). In the case of the water accessed by tube wells, the major pollution contributor was the geogenic source, which contributed 50%, whereas the anthropogenic source (water distribution system) contributed only 17% and nonclassified sources contributed 10% (Fig. 7d). Pollution sources contributing < 10% that are not associated with any major source can generally be discounted. Once again, APCS-MLR analysis indicated that all four water sources have been contaminated from both the geogenic and anthropogenic sources. Similar findings of geogenic and anthropogenic pollution sources have been reported by Khan et al. (2018).
Health Survey
During the questionnaire survey, various symptoms related to gastrointestinal illness (i.e., diarrhea, dysentery, vomiting), as well as hepatitis A, B, and C and kidney problems, etc. were recorded from respondents in the study area. In this study, the risk of waterborne diseases were highest in the rural townships (tehsils) i.e., Warh Mamund, Loi Mamund, Barang, and Chamarkand (62–68% incidence) compared with the more urban townships (tehsils) i.e., Khar, Nawagai, and the southern part of Salarzai (Table 3). The rural areas are located near the Afghanistan boarder where spring water is the main sources of drinking water. High incidences of waterborne diseases have been previously reported among rural populations in other regions (Daud et al. 2017; Owens et al. 2020). Across all sites, populations that accessed drinking water from springs had a higher risk of illnesses compared with populations that used groundwater for drinking. Areas with poor infrastructure for sanitation are prone to microbiological contamination through open drainage channels, unprotected tertiary drains, leakage from septic systems and latrines, and contaminated soil. Similarly, the incidence of illnesses were 42–47% greater in townships (tehsils) accessing water using hand pumps and open wells in the townships of Salarzai (upper) and Utmankhel relative to the lower incidence of illness in areas using water from tube wells (Table 3). These findings are consistent with the results from the data for the physicochemical parameters and PTEs concentrations, which also showed high contamination of water from springs and comparatively low contamination in water from tube wells. Khan et al. (2013) reported similar results of low incidences of waterborne diseases in an areas located in northwestern Pakistan serviced by tube wells (i.e., deep aquifers) relative to a high incidence of waterborne diseases in areas where drinking water was accessed by hand pumps (i.e., from shallow groundwater).
Conclusions
The main conclusions from this comparative study of four water sources in the district Bajaur of western Pakistan are these: (1) sources of drinking water from springs have acidic pH, higher turbidity and TSS, and elevated levels of PTEs relative to drinking water accessed from tube wells; (2) E. coli counts exceeded the MPL in water accessed from springs, hand pumps, and open wells water, but counts were below the MPL in water accessed from tube wells. Up to 78% of the water samples collected from this region of Pakistan were of poor quality and not suitable for drinking purpose; (3) the water quality of tube wells source is better than water accessed from springs, hand pumps, and open wells; (4) among the four water sources, springs showed comparatively the highest noncarcinogenic risks (HRI) related to concentrations of PTEs, whereas tube wells showed the lowest risks and were within the safe limits. However, the total hazard index (HI) for water accessed by hand pumps was quite close to the toxicity limit (especially in children) and monitoring and management of contamination sources should be a priority; and (v) both anthropogenic and geogenic sources contribute to the poor water quality in the study area. Based on the findings, the water accessed from deep aquifers using tube wells is safe and should be used as a source of drinking water. Capital projects to access drinking water from deep aquifers should be a priority in the areas of western Pakistan where springs are the source of drinking water. Furthermore, pollution abatement measures and improvements to water distribution systems are needed across the entire region to reduce contamination by bacterial pathogens and toxic metals.
References
Abdalla F, Khalil R (2018) Potential effects of groundwater and surface water contamination in an urban area, Qus City, Upper Egypt. J Afr Earth Sci 141:164–178. https://doi.org/10.1016/j.jafrearsci.2018.02.016
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol Environ Saf 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Ahmed J, Wong LP, Chua YP et al (2020) Quantitative microbial risk assessment of drinking water quality to predict the risk of waterborne diseases in primary-school children. Int J Environ Res Public Health 17:1–16. https://doi.org/10.3390/ijerph17082774
Amin R, Zaidi MB, Bashir S et al (2019) Microbial contamination levels in the drinking water and associated health risks in Karachi, Pakistan. J Water Sanit Hyg Dev 9:319–328. https://doi.org/10.2166/washdev.2019.147
APHA (1989) Standar methods for the examination of water and wastewater
Arain MB, Ullah I, Niaz A et al (2014) Evaluation of water quality parameters in drinking water of district Bannu, Pakistan: multivariate study. Sustain Water Qual Ecol 3:114–123. https://doi.org/10.1016/j.swaqe.2014.12.005
Balamurugan P, Kumar PS, Shankar K et al (2020) Non-carcinogenic risk assessment of groundwater in southern part of Salem district in Tamilnadu, India. J Chil Chem Soc 65:4697–4707. https://doi.org/10.4067/S0717-97072020000104697
Begum S, Shah MT, Muhammad S, Khan S (2015) Role of mafic and ultramafic rocks in drinking water quality and its potential health risk assessment, Northern Pakistan. J Water Health 13:1130–1142. https://doi.org/10.2166/wh.2015.066
Bosan A, Qureshi H, Bile KM et al (2010) A review of hepatitis viral infections in Pakistan. J Pak Med Assoc 60:1045–1058
Cairncross S, Feachem R (2018) Environmental health engineering in the tropics. Routledge. https://www.taylorfrancis.com/books/environmental-health-engineering-tropics-sandy-cairncross-richard-feachem/10.4324/9781315883946
Daud MK, Nafees M, Ali S et al (2017) Drinking water quality status and contamination in Pakistan. Biomed Res Int. https://doi.org/10.1155/2017/7908183
Delpla I, Proulx F, Rodríguez MJ (2020) A methodology to prioritize spatio-temporal monitoring of drinking water quality considering population vulnerability. J Environ Manag. https://doi.org/10.1016/j.jenvman.2019.109869
Dieter HH, Bayer TA, Multhaup G (2005) Environmental copper and manganese in the pathophysiology of neurologic diseases (Alzheimer’s disease and manganism). Acta Hydrochim Hydrobiol 33:72–78. https://doi.org/10.1002/aheh.200400556
Ding J, Jiang Y, Fu L et al (2015) Impacts of land use on surface water quality in a subtropical river basin: a case study of the dongjiang river basin, Southeastern China. Water (Switzerland) 7:4427–4445. https://doi.org/10.3390/w7084427
Divahar R, Aravind Raj PS, Sangeetha SP, Mohanakavitha T (2019) Impact of industrial wastewater disposal on surface water bodies in kalingarayan canal, Erode district. Indian J Ecol 46:823–827
He S, Wu J (2019) Relationships of groundwater quality and associated health risks with land use/land cover patterns: a case study in a loess area, Northwest China. Hum Ecol Risk Assess 25:354–373. https://doi.org/10.1080/10807039.2019.1570463
Horiguchi H, Oguma E, Sasaki S et al (2013) Age-relevant renal effects of cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ Int 56:1–9. https://doi.org/10.1016/J.ENVINT.2013.03.001
Hussain R, Khattak SA, Sattar S et al (2020) Evaluation of the contaminated soil and its impacts on Tobacco (Nicotiana tabacum L.) crops in Swabi, Pakistan. J Himal Earth Sci 53:34–48
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Jehan S, Khan S, Khattak SA et al (2019) Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Measurement 139:249–257. https://doi.org/10.1016/j.measurement.2019.02.090
Ji Y, Wu J, Wang Y et al (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expo Health 12:469–485. https://doi.org/10.1007/s12403-020-00357-6
Khan S, Shahnaz M, Jehan N et al (2013) Drinking water quality and human health risk in Charsadda district, Pakistan. J Clean Prod 60:93–101
Khan HM, Muhammad N, Nafees M et al (2018) Spatial distribution and pollution source (s) apportionment in adjacent tributaries of River Panjkora. J Biodivers Environ Sci 12:391–403
Li P, Tian R, Liu R (2019) Solute geochemistry and multivariate analysis of water quality in the Guohua phosphorite mine, Guizhou Province, China. Expo Heal 11:81–94. https://doi.org/10.1007/s12403-018-0277-y
Malik AH, Khan ZM, Mahmood Q et al (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168:1–12
Muhammad N, Nafees M (2018) Geo-chemical investigation and health risk assessment of potential toxic elements in industrial wastewater irrigated soil: a geo-statistical approach. J Biodivers Environ Sci 12:367–380
Muhammad N, Nafees M, Hussain R et al (2018) Pollution and energy reduction strategy in soft drink industries. Environ Sci Pollut Res 25:28153–28159. https://doi.org/10.1007/s11356-018-2861-x
Nawaz S, Ali Y (2018) Factors affecting the performance of water treatment plants in Pakistan. Water Conserv Sci Eng 3:191–203. https://doi.org/10.1007/s41101-018-0051-9
Muhammad N, Nafees M, Khan MH et al (2020) Effect of biochars on bioaccumulation and human health risks of potentially toxic elements in wheat (Triticum aestivum L.) cultivated on industrially contaminated soil. Environ Pollut 260:1–12. https://doi.org/10.1016/j.envpol.2019.113887
Nawab J, Khan S, Khan MA et al (2017) Potentially toxic metals and biological contamination in drinking water sources in chromite mining-impacted areas of pakistan: a comparative study. Expo Health 9:275–287
Nawab J, Farooqi S, Xiaoping W et al (2018) Levels, dietary intake, and health risk of potentially toxic metals in vegetables, fruits, and cereal crops in Pakistan. Environ Sci Pollut Res 25:5558–5571. https://doi.org/10.1007/s11356-017-0764-x
Owens CEL, Angles ML, Cox PT et al (2020) Implementation of quantitative microbial risk assessment (QMRA) for public drinking water supplies: systematic review. Water Res 174:115614
Panneerselvam B, Paramasivam SK, Karuppannan S et al (2020) A GIS-based evaluation of hydrochemical characterisation of groundwater in hard rock region, South Tamil Nadu India. Arab J Geosci. https://doi.org/10.1007/s12517-020-05813-w
Peavy HS, Rowe DR, Tchobanoglous G (1985) Environmental engineering. McGraw-Hill, New York
Qasemi M, Farhang M, Biglari H et al (2018) Health risk assessments due to nitrate levels in drinking water in villages of Azadshahr, northeastern Iran. Environ Earth Sci. https://doi.org/10.1007/s12665-018-7973-6
Radfard M, Yunesian M, Nabizadeh R et al (2019) Drinking water quality and arsenic health risk assessment in Sistan and Baluchestan, Southeastern Province. Iran Hum Ecol Risk Assess 25:949–965. https://doi.org/10.1080/10807039.2018.1458210
Rasool A, Farooqi A, Xiao T et al (2016) Elevated levels of arsenic and trace metals in drinking water of Tehsil Mailsi, Punjab, Pakistan. J Geochem Explor 169:89–99. https://doi.org/10.1016/j.gexplo.2016.07.013
Ricolfi L, Barbieri M, Muteto PV et al (2020) Potential toxic elements in groundwater and their health risk assessment in drinking water of Limpopo National Park, Gaza Province Southern Mozambique. Environ Geochem Health. https://doi.org/10.1007/s10653-019-00507-z
Shah MT, Ara J, Muhammad S et al (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67. https://doi.org/10.1016/j.gexplo.2012.04.008
Soujanya Kamble B, Saxena PR, Kurakalva RM, Shankar K (2020) Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city, India. SN Appl Sci 2:1–22. https://doi.org/10.1007/s42452-020-2199-0
Steenland K, Boffetta P (2000) Lead and cancer in humans: Where are we now? Am J Ind Med 38:295–299. https://doi.org/10.1002/1097-0274(200009)38:3%3c295::AID-AJIM8%3e3.0.CO;2-L
Su S, Li D, Zhang Q et al (2011) Temporal trend and source apportionment of water pollution in different functional zones of Qiantang River, China. Water Res 45:1781–1795. https://doi.org/10.1016/j.watres.2010.11.030
Tian M, Li W, Ruan M et al (2019) Water quality pollutants and health risk assessment for four different drinking water sources. In: E3S web of conferences. EDP Sciences
Ullah Z, Naz A, Saddique U et al (2017) Potentially toxic elements concentrations and human health risk assessment of food crops in Bajaur Agency, Pakistan. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6824-1
USEPA (2011) Integrated risk information system. In: Integrated risk information system. https://www.epa.gov/iris. Accessed 9 Feb 2019
Wei X, Gao B, Wang P et al (2015) Pollution characteristics and health risk assessment of heavy metals in street dusts from different functional areas in Beijing, China. Ecotoxicol Environ Saf 112:186–192. https://doi.org/10.1016/j.ecoenv.2014.11.005
WHO (2011) Guidelines for drinking-water quality-4th edn. In: iasaude.pt. http://www.who.int. Accessed 21 Apr 2020
Xiao J, Wang L, Deng L, Jin Z (2019) Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci Total Environ 650:2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322
Xu L, Zhang C, Xu P, Wang XC (2018) Mechanisms of ultraviolet disinfection and chlorination of Escherichia coli: culturability, membrane permeability, metabolism, and genetic damage. J Environ Sci (China) 65:356–366. https://doi.org/10.1016/j.jes.2017.07.006
Zhou F, Guo H, Liu L (2007) Quantitative identification and source apportionment of anthropogenic heavy metals in marine sediment of Hong Kong. Environ Geol 53:295–305. https://doi.org/10.1007/s00254-007-0644-7
Acknowledgments
This research was financially supported by Higher Education Commission Pakistan under International Research Support Initiative Program (Grant No: 1-8/HEC/HRD/2018/8939, PIN: IRSIP 41 BMS83). The authors acknowledge Centralized Resource Laboratory and Department of Environmental Sciences, University of Peshawar, Pakistan for providing instrumental facilities. They are thankful to Health Department of the district Bajaur and the local community for their help during the questionnaire survey.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in the present study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, M.H., Nafees, M., Muhammad, N. et al. Assessment of Drinking Water Sources for Water Quality, Human Health Risks, and Pollution Sources: A Case Study of the District Bajaur, Pakistan. Arch Environ Contam Toxicol 80, 41–54 (2021). https://doi.org/10.1007/s00244-020-00801-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00801-3