Abstract
Concentrations of trace elements in drinking water affect its safety and acceptability for use. Potentially toxic element (PTE) contaminations are considered extremely hazardous because of toxicity, persistence, and bioaccumulative behaviour. Many areas in the Southern African Development Community are data poor and have poor accessibility. The results of our previous research identified the presence of fossil waters in southern Limpopo National Park. Groundwater and river water are the only sources of drinking water for the villages in the study area. The current study focuses on the understanding of trace element distribution and health perspectives of PTEs (Hg, U, Sr, B, and Mn) in the groundwater and surface water samples (rivers and lakes) collected within the buffer zone of the Limpopo National Park, Southern Mozambique. Two sampling campaigns (October 2016–March 2017) were carried out during the end of the wet season and the end of the dry season to analyse the differences. The results improved our knowledge of the occurrence of trace elements in drinking water in an area where water resources play a fundamental role—because of their scarcity—and where the climate is harsh. ICP-MS results provided information on concentration ranges, highlighting the exceedance of the permissible maximum limit of mercury imposed by the World Health Organization on several groundwater samples. In the buffer zone of Limpopo Park, the highest levels of risk seem to be associated with the presence of Hg and U in drinking water. The use of risk assessment markers such as non-cancer risk value (hazard quotient [HQ]) revealed the exceedance of HQ values for Hg and U. The HQ values are higher in the wet season than the dry season, and most of the exceedance has been found in groundwater. HQ values are higher in exposed children than exposed adults. The water of Lake Massingir seems to be safer than any other source, but people do not currently use it because of the distance between the lake and their villages. Proactive control and research on alternative solutions for the water needs of the population and on creation of water distribution are recommended. In the current study, drinking water was the only route of exposure that was evaluated. Therefore, it would be appropriate to investigate the concentrations of PTEs in crops, livestock, and any other potential pathways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The present study is a part of the research activity of the SECOSUD Phase II project (‘Conservation and equitable use of biological diversity in the Southern African Development Community’) founded by the Italian Ministry of Foreign Affairs in the Southern African Development Community (SADC). The project operates in the framework of the three Rio Conventions of the United Nations on the environment: the Convention on Biological Diversity (CBD), the United Nations Convention to Combat Desertification (UNCCD), and the United Nations Framework Convention on Climate Change (UNFCCC). SECOSUD II aims at consolidating the capacities of decision-makers responsible for land planning and management of natural resources as well as promoting their use to support the harmonisation of land management processes at the national, regional, and international levels. The final objective is to boost biodiversity conservation and sustainable economic development in the SADC region, consistent with the CBD goals and in synergy with the goals of UNCCD and UNFCCC.
Groundwater plays a critical role in most rural areas of Mozambique because it is the primary source of water resources for human use. According to the national census carried out in 2007, about 60% of the population in Mozambique use groundwater for domestic water supply, and this percentage has likely been increasing in recent years.
From 2008 to 2012, the Government of the Netherlands and UNICEF operated a water and sanitation programme with a budget of US$ 42.82 million, 37.4% of which was earmarked for the construction of 2000 new boreholes. This amounted to one-fifth of the new water points needed in the country to meet the Millennium Development Goals and was estimated to consume nearly 40% of Mozambique’s domestic drilling capacity (RWSN 2015). According to the Direcção Nacional de Águas (DNA 2006), there were over 17.000 boreholes throughout Mozambique in 2001. Pavelic et al. (2012), in their review of 15 countries of sub-Saharan Africa, claim that shallow open wells are the primary tool (46.8%) to reach these underground waters, but the water is also extracted through hand pumps mounted on boreholes (14.1%). The main factor restricting groundwater development is the lack of hydrogeological information. To date, groundwater is used only sparsely for irrigation, mostly on a small scale for subsistence farming. Tapped water (10.4%) is also partly produced from groundwater. Only 17.1% of households use surface water from rivers and lakes (Pavelic et al. 2012). Mozambique is hugely dependent on imported water flows in terms of both quantity and quality because of its position at the downstream end of transboundary river basins. Furthermore, water supply through rainwater is particularly limited in the southern part of Mozambique because both spatial and temporal distribution of rainfall is critical, and rain is restricted to a short portion of the year. Mozambique, with an area of 309,496 miles2 (801,563 km2), remains fairly sparsely populated with 29 people per square kilometre, ranked 178th global position (World Population Review 2019), but its growth rate is 2.5% annually. The population is soaring, requiring the creation of severe water resource management and a clear information framework. The information available on the quality of groundwater in the aquifers of Mozambique is very poor. Some studies, including a recent work conducted by our research group (Barbieri et al. 2019), highlight salinity problems in the principal aquifer (20–80 m depth) in the south of the country because of natural seawater intrusion, marine deposits, and fossil water, forming areas with brackish groundwater even many kilometres away from the coast. This work provides additional information on the potentially toxic elements (PTEs) in water, and the risk they pose to human health. The work’s results about salinity problems agree with those of Pavelic et al.’s (2012) review, which also draws a correlation between localised groundwater salinisation occurring in the Chokwe (Gaza province) area and the extensive irrigation (using surface water) conducted since the 1930s. High conductivity values make these waters unsuitable for many crops (Barbieri et al. 2019) and for livestock watering. These values are well above the WHO standards of 1500 µS/cm for drinking, rendering the waters inadequate to meet the drinking water needs for villages in this rural area. Pollution by heavy metals is rarely measured or recorded, even though this information is crucial because elevated levels of total dissolved solids (TDS) and electrical conductivity (EC) increases the mobilisation of trace metals (Acosta et al. 2011). Paalman et al. (1994) explain that two mechanisms are primarily responsible for this process: (1) complexation capacity of salt-derived anions with heavy metals, and (2) competition for salt-derived cations with positively charged heavy metal species for sorption sites in the solid phase. The Groundwater Resource Information Project (GRIP LIMPOPO 2002) data set, a project aimed at collecting groundwater quality and quantity data to improve development and management, reports that there is no data about trace elements in groundwater in our study area. The trace elements’-mediated pollution in groundwater is a severe global concern because of their persistence, accumulation, and environmental toxicity.
The present study evaluates the contamination status of heavy metals and other trace elements in surface water and groundwater inside the buffer zone of the LNP, Southern Mozambique. It explores the differences between the dry season (DS) and wet season (WS) and evaluates the human health risks of some PTEs such as Hg, U, Sr, B, and Mn in drink-use water resources. The human health risk assessment examines the groundwater and surface water to humans via direct consumption of drinking water, and the human health effects have been established using the average daily dose (ADD) and the hazard quotient (HQ).
The study area
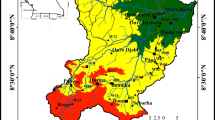
Limpopo National Park (LNP) is part of a Transfrontier Conservation Area (Great Limpopo Transfrontier Conservation Area, TFCA) established to manage conservation areas that border each other across international boundaries (Fig. 1). These areas were designed to include ecological corridors, private game reserves, communal natural resource management areas, and hunting concession areas. Their first mission is to create a globally preferred prime ecotourism destination. To do this, it is necessary to remove all social barriers within TFCAs so that animals can roam freely within the local ecosystem (GLTP 2019). Conservation-induced displacement has forced people living in the LNP’s core area to relocate to the buffer zone. The buffer zone is, therefore, an area designated for human settlements that were present within a park before its establishment. The government is encouraging people to move out of the park by providing incentives such as specific services (agricultural and community infrastructure) and other ‘livelihood’ opportunities (Van Wyk 2004). The villages in this area currently consist of subsistence farming families, whose productivity is strictly influenced by climate variability. Almost all the annual rainfall occurs within 6 months (from October to March) across several isolated rain days and locations. There is a great separation during the year between the rainy season (or WS) and the DS (from April to September, DS). The WS coincides with the warm period of the year. The evaporation rate is very high and exceeds the rainfall rate (IWMI/ARC 2003). Based on the Köppen classification, the climate in this area is semi-arid, dry, and hot (BSh group) (FAO-SAFR 2004, citing Köppen 1918) (Fig. 2), with a mean annual temperature exceeding 18 °C (Fig. 3) and an annual rainfall of about 500 mm (Fig. 4) (Ministerio do Turismo 2003). Some 35,000 people live within the LNP’s buffer zone, clustered in 44 villages along the Limpopo River and Elephant River (Direcão National de Areas de Conservacão 2003). LNP is located along the Limpopo River and Elephant River, which form the eastern and southern park boundaries, respectively.

modified from Peace Parks Foundation 2001)
Limpopo National Park and the larger Great Limpopo Transfrontier Conservation area. The red rectangle highlights the study area (image
Mozambique climate zones (image from Africa Groundwater Atlas 2019)
Mozambique mean annual temperature (image from Africa Groundwater Atlas 2019)
Mozambique mean annual rainfall (image from Africa Groundwater Atlas 2019)
The geology of the study area is characterised by unconsolidated quaternary sedimentary rocks and sedimentary rocks of the Cretaceous–Tertiary age. The most common soil classifications in the study area are arenosols and cambisols (Africa Groundwater Atlas 2019; Panagos et al. 2012).
Through an overview of recent production in Mozambique, we can identify three scales of production (Lehto and Goncalves 2008):
-
Large scale: Natural gas and aluminium, but both from imported raw materials;
-
Medium scale: Construction materials such as gravel, clay, limestone, and ilmenite; and
-
Small scale: Sea salt, gemstones, coal, bauxite, gold, diatomite, bentonite, and other clays.
The study area is located in Southern Mozambique, which is mostly covered by Phanerozoic rocks. The economically most interesting mineral resources in this area are the gas occurrences in the Pande and Temane fields in Inhambane as well as the heavy mineral sands in the paleo-dunes in Gaza and Zambézia provinces. Moreover, the recent demand for construction materials has revitalised many rhyolite quarries and gravel, sand, and clay pits. This demand is high where cities are expanding, especially in the Maputo Province. Alluvial, micro-sized diamonds have been found in the Limpopo and Singédzi rivers in Gaza Province, probably transported by the rivers from the South African Kaapvaal Craton (Lehto and Goncalves 2008). In the southern part of Gaza Province, which is the area where our study was conducted, sand and gravel deposits occur along the Elephants River and the main Limpopo River. We noticed that there are many sand pits in the Chilembene-Chókwè and Massingir areas. Rehabilitation of the Massingir dam (414026E/7355195N) has consumed a large quantity of sand and gravel, which has been extensively extracted from the river valley (Elephants River) at several locations (Lehto and Goncalves 2008). The Massingir Dam is the second largest in the country; it holds a reservoir with storage capacity of 2.8 billion cubic metres of water.
The sampling sites of our study area are inside the buffer zone of the LNP in Southern Mozambique (Fig. 5), and the study area is the same as described in our previous work (Barbieri et al. 2019) to which we refer the reader for a comprehensive view of the geological and hydrological background and an overview of climate and geographic information.
Sampling sites. Based on Barbieri et al. (2019)
Methodology
This study is based on two surveys carried out in October 2016 and March 2017. October is the end of the DS. In this month, we collected seven surface water samples (lake and rivers) and nine water samples from village hand pump wells and boreholes (depth < 30 m). March is the end of the WS. Six water samples from village hand pump wells were collected along with three surface water samples. To locate the sampling sites, we used a global positioning system. In our previous work, we identified non-potable water sources. We accounted for them in this work because they are used daily by the local population. A WTW multi 3410 portable probe allowed us to measure chemical–physical parameters such as temperature (± 0.1 °C) of the water, pH (± 0.01 unit), and EC (± 1 µS/cm). We calibrated the probe before any sampling activity to collect the most accurate data. The following equation (Eq. 1) was used to measure the TDS indirectly:
where EC is the electrical conductivity, and 0.64 is the conversion factor defined by Moharir et al. (2002).
The dissolved trace elements, including lithium, beryllium, boron, aluminium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, gallium, arsenic, rubidium, strontium, molybdenum, cadmium, caesium, barium, mercury, lead, and uranium, were measured at the Geochemistry Laboratory of Sapienza University in Rome with an inductively coupled plasma mass spectrometry ICP-MS (X Series 2 Thermo Fisher Scientific). We analysed, after each tenth sample, blank and two standard reference materials (SRM1640a) purchased from the National Institute of Standards and Technology (United States) as water samples. The parameters for water analyses by ICP-MS (X Series II Thermo Fisher Scientific Inc.) are reported in Supplementary File S1. The measurement of uncertainties for all constraints was measured and controlled by using a regular laboratory replica of samples and by confirming the precision/calibration of the instrument over regular runs with standard solutions. The recoveries of analytes of interest were in the range of 98.4–106.3%, and the recoveries of the SRM1640a were in the range of 91.2–111.6%. We used the addition of 206Pb, 207Pb, and 208Pb isotopes to measure the concentration of lead.
In October 2016, a total of 16 samples were collected, seven surface water (rivers and lakes) and nine groundwater (boreholes and hand pump). October coincides with the end of the dry season, in which precipitations are almost absent and vegetation is dry. In March 2017, a total of nine samples were collected, three surface waters and six groundwaters. March coincides with the end of the wet season. These 2 months of the year were chosen for sample collection to investigate the chemical differences between the waters in the two seasons of the year. Field activity and sampling expeditions were conducted following the guidelines supplied by the United Nations Environment Programme and the World Health Organization (UNEP/WHO 1996). To choose sampling points, we analysed the distribution of the villages in the southern area of the buffer zone and analysed which communities host the highest number of people. All the measures necessary to carry out quality sampling were followed. All hand pump or boreholes sampled were in use at the time, but as a precautionary measure, they were purged several times to avoid falsification of the data caused by water stagnation. The entire instrumentation used for sampling (e.g. flasks and bottles) was previously washed with distilled water. In the case of hand pumps, the water was collected in a basin and subsequently sampled, and in the case of wells, the water was collected using a bucket. Water drawn using a syringe was filtered (0.45 µm) and acidified (three drops of nitric acid HNO3 with pH < 2 in 15 ml flasks) in the field to preserve and reduce precipitation of trace elements. The samples were then stored in refrigerated bags at approximately 4 °C until dispatch after a week to the Geochemistry Laboratory of Sapienza University in Rome. The results from the analyses were compared with the limits for trace elements in drinkable water established by the World Health Organization (WHO 2011).
Health risk assessment
The relative toxicity values used in the analysis were taken from the US Department of Energy’s RAIS (Risk Assessment Information System) compilation (U.S. Department of Energy 2019). They are used by regulatory authorities such as the US EPA (Environmental Protection Agency) to describe the risk category of a chemical substance. To evaluate the possible risks associated with the consumption of contaminated water, different health risk assessment parameters were calculated.
Health risk assessment for PTEs was evaluated for the exposure pathway. Hg, U, B, Sr, and Mn were selected for quantitative risk assessment because they were present at high concentration in the water samples. Groundwater and surface water to humans via direct consumption of drinking water was the pathway investigated in this study. The exposed subjects were divided into two groups: adults and children. Individuals with an exposure duration of 17 years, an average body weight of 29 kg, and an intake rate/unit time of 2 L/day were considered for children. The value of body weight is derived from the average body weight of a person from 0 to 17 years. ADD (mg/kg/day) and HQ are calculated for each PTE in drinking water (Ali et al. 2019). The ADD of an element represents the ADD received by individuals via the consumption of drinking water in a study area. The ADD is used to quantify the oral exposure dosage for deleterious substances and is defined by the following equation (Eq. 2) (USEPA 1986):
where
-
C is the concentration of the target pollutant in the environmental compartment (mg/L);
-
IR is the intake rate/unit time (2 L/day for children and 3 L/day for adults);
-
ED is the exposure duration (17 years for children and 58 years for adults);
-
EF is the exposure frequency (365 days/year);
-
BW is the average body weight of the receptor (29 kg for children and 61 kg for adults); and
-
AT is the average time/years, which is equal to average life expectancy and is multiplied by 365 to change the factors from years to days.
The symbol, unit of measurement, value, and reference of the above parameters are given in Table 1.
Life expectancy and average body weight were taken from Tchamo et al. (2016). Non-carcinogenic harm is the toxic risk sustained because of exposure (Islam et al. 2018), and the extent of harm is indicated in terms of HQ. The HQ is the ratio of the potential exposure to a substance and the level at which no adverse effects are expected. It is primarily used by the US EPA to assess the health risks of air toxics. Exposure to water PTEs was evaluated for non-cancer risk such as HQ. The quotient of the ADD was divided by the oral reference dose (RfD) of the respective PTEs as determined by Eq. (3) (USEPA 1989):
The oral RfD is an estimate of continuous oral exposure to the human population (including sensitive subgroups) that is not likely to indicate appreciable risk of harmful effects over a lifetime. The oral RfD of Hg, U, B, Sr, and Mn is 1.60E-04, 2.00E-04, 2.00E-01, 6.00E-01, and 2.40E-02 (mg/kg)/day, respectively (U.S. Department of Energy 2019). An HQ less than or equal to 1 indicates that adverse effects are not likely to occur and thus can be considered to have negligible hazard. HQs greater than 1 are not statistical probabilities of harm occurring. Instead, they are a simple statement of whether (and by how much) an exposure concentration exceeds the reference concentration.
Results and discussion
pH, EC, TDS, and trace elements distribution
All the groundwater samples collected from the different study area locations were clear and colourless. C01–C10 samples were groundwater, and C11–C17 samples were surface water. Almost all the samples had pH levels in the typical range for drinking water (about 6.5–8.5) recommended by the WHO, but all three surface water samples taken at the end of the WS had pH values above 8.5. Sample C17 (Lake Massingir) had a pH value of 9.3 at the end of the DS. The increase in the pH value in groundwater in the WS is, we believe, not related to the dissolution of carbonates. Strontium and barium concentrations decrease in the WS, so the change in pH cannot be due to the dissolution of carbonates. Regarding surface waters, the situation is different, and the increase in pH value in the WS could be due to the dissolution of carbonates. The EC and TDS values of surface water at the end of the DS varied from 405 to 2460 µS/cm and from 259 to 1574 mg/L, respectively. The same parameters at the end of the WS varied from 388 to 2346 µS/cm and from 248 to 1501 mg/L, respectively. The EC and TDS values of groundwater at the end of the DS varied from 1534 to 5960 µS/cm and from 982 to 3814 mg/L, respectively. At the end of the WS, they varied from 1522 to 4974 µS/cm and from 974 to 3183 mg/L. Therefore, there were no seasonal variations in surface water for the parameters of EC and TDS. Instead, the maximum value of both parameters decreased during the WS in groundwater.
Chemical–physical parameters and concentration of trace elements measured in water samples are summarised in Supplementary File S2. All the analysed samples respected the limits imposed by the WHO for the trace elements of interest, except for mercury. Mercury was found above the limit in six samples, all of which were groundwaters, with a marked difference between the DS and WS (average values of 2.19 and 7.49 µg/L, respectively). Other elements whose average concentrations in groundwater increased from the DS to the WS were as follows: B, Al, Mn, Fe, Ni, Cu, Zn, Pb, and U. The average concentrations of some elements remained almost the same, such as Li, Be, V, Cr, Co, Ga, As, Rb, Mo, and Cs. Sr and Ba were the only two elements whose concentrations were lower at the end of the WS. Figures 6 and 7 use bar plots to provide a visual understanding of the differences between dry and WS in groundwater and surface water, respectively. Regarding average concentrations of Al and Fe in surface waters, there was a large variation between the DS and WS (Al 6.59 and 712.11 µg/L and Fe 2.37 and 1208 µg/L, respectively). This increase in iron and aluminium concentrations in the rainy season could be due to the surface water runoff, specifically the effect of the clay mineral washout. Moreover, these differences in surface waters could be traced back to seasonal human productive activities, such as gravel and sand pits, which could be some kilometres away from the sampling point, upstream of the waterway. The concentrations of Be and Cd at DS were found to be below the detection limit (< D.L.), just like Ga and Cd at WS.
The correlation matrix highlights positive or negative correlations between element concentrations. The groundwater correlation matrix at the DS (Supplementary File S3) showed a positive correlation between EC and Ga (0.886), EC and Pb (0.823), U and V (0.843), Co and Rb (0.813), Co and Cs (0.867), Co and Hg (0.851), Ga and Rb (0.850), Ga and Sr (0.873), and Ga and Hg (0.859). There were no significant inverse correlation relationships. Correlation ratios in surface waters were different between the two seasons (Supplementary Files S4 and S5). The only correlation ratio present in both seasons was positive correlations between Ni and Cu and between Pb and Zn. A correlation matrix for groundwater was not carried out in the rainy season because of the insufficient number of samples to make it useful.
The presence of U in groundwater may be associated with geogenic sources such as the leakage of U from adjoining basements with granite-rich rock formations, but it is often associated with anthropogenic activities such as the widespread use of pesticides and fertilisers and direct disposal of industrial waste into waterways. The high concentration of U in the study region may be due to geogenic or extensive use of pesticides and direct disposal of industrial waste into waterways within the upper Limpopo River Basin. In the study area, agriculture is used exclusively for subsistence without the use of pesticides. The situation is different some tens of kilometres upstream from the LNP and within the same river basin in South Africa. Further investigation is required to evaluate the source and mobilisation of U in drinking water. Mercury is a local, regional, and global pollutant; whereas the research has focused on Hg pollution at the global scale, it is important to emphasise that Hg also has regional and local dimensions. Primary anthropogenic Hg emissions greatly exceed natural geogenic sources, resulting in increases in Hg reservoirs and subsequent secondary Hg emissions that facilitate its global distribution. The result of the emitted Hg is primarily recalcitrant soil pools and deep ocean waters and sediments. We found a slight correlation between Hg, B, Rb, Zn, Cr, Al, Pb, Ga, and EC, but further investigations are required.
Health risk assessment
The results of the health risk assessment of human exposure to trace elements in drinking water from the buffer zone of the Limpopo National Park are given in Supplementary File S6.
In the current study, the non-cancer risk values for B, Sr, and Mn were inside the safe level for all the sampling sites. However, the results showed exceedance of HQ values for Hg and U. The HQ values were higher in the WS than the DS, and most of the exceedance concerned groundwater. The highest HQ values for mercury and uranium in children were 4.17 (C01, Munhamane Village) and 7.44 (C05, Massingir Hospital), respectively. As adults, the highest HQ values for mercury and uranium were 2.97 (C01, Munhamane Village) and 5.31 (C05, Massingir Hospital). These values are extremely high, so we can hypothesise negative effects on populations, especially on the most sensitive subjects. Surface waters are much safer, especially during the DS. The water of the Lake Massingir exposes the population to potential toxic elements to a lesser extent than groundwater does.
Conclusions and recommendations
The human body can be exposed to trace elements through several exposure pathways, mostly dependent on pollution mediums such as water, air, food, and soil. However, among these pathways, exposure via drinking contaminated water is a critical pathway for trace elements to enter the human body (Caussy et al. 2003; Muchuweti et al. 2006). The current study revealed groundwater pollution prevailing in several areas along major rivers within the buffer zone of the Limpopo National Park. Most of the trace elements analysed (B, Al, Mn, Fe, Hg, U, Pb, Cu, Co, As, Ni, Zn) had higher concentrations in groundwater during the wet season than the dry season as indication that the soluble forms of the metals are either present in the environment or produced after chemical reaction. This could be due to the variation of the geochemical balance in the two seasons, influenced by different conditions of chemical–physical parameters such as the pH value and the electrical conductivity. It is therefore evident that meteoric waters influence partially the underground water system due to high rates of evapotranspiration and runoff. Coal mining upstream of the Limpopo catchment area in South Africa could affect the distribution and concentration of trace metals in superficial water, as suggested by the correlation value that emerged from the analyses carried out in this study between vanadium and uranium (0.84).
The countless gravel and sand pits have probably influenced the chemical characteristics of surface waters in the area, and seasonal differences in the chemical composition of surface waters suggest a seasonal nature of mining activity.
The high concentrations of boron, mercury, copper, zinc, and uranium in groundwater can be traced back to a geogenic origin, and their seasonal difference could be due to a greater and lesser mobility of the elements, influenced by the variation of chemical–physical parameters.
Health risks due to underground water consumption increase during the rainy season. Therefore, we can set up a system that can capture meteoric water to be used for drinking purposes and thus reduce consumption of groundwater, but this may be unfeasible because of precipitation rates. This proposal should be evaluated based on its advantages and disadvantages, and economic viability. Another possible option could be to create a water supply system starting from the waters of Lake Massingir. We recommend further analysis to assess the feasibility of the project and the drafting of an environmental impact assessment to evaluate the short-term and long-term ecological changing of the lake.
The subjects most at risk are children, for whom HQ values exceed the maximum safety limit. The daily use of contaminated resources such as drinking water can lead to the development of serious diseases. In this study, drinking pathway was investigated, so the results did not consider many other exposure routes such as food. Moreover, it is necessary to consider the high sensitivity of subjects such as infants and pregnant women and extreme hygienic health, nutritional, and climatic conditions. It is also advisable to study the distribution of pathologies potentially related to chronic oral exposure to mercury and uranium.
References
Acosta, J. A., Jansen, B., Kalbitz, K., Faz, A., & Martínez-Martínez, S. (2011). Salinity increases mobility of heavy metals in soils. Chemosphere, 85(8), 1318–1324.
Africa Groundwater Atlas (2019). Climate. British Geological Survey. [http://earthwise.bgs.ac.uk/index.php/Climate]. Retreived 09 Nov 2019.
Ali, W., Aslam, M. W., Feng, C., Junaid, M., Ali, K., Li, S., et al. (2019). Unraveling prevalence and public health risks of arsenic, uranium and co-occurring trace metals in groundwater along riverine ecosystem in Sindh and Punjab, Pakistan. Environmental Geochemistry and Health, 41(5), 2223–2238. https://doi.org/10.1007/s10653-019-00278-7.
Barbieri, M., Ricolfi, L., Vitale, S., Muteto, P. V., Nigro, A., & Sappa, G. (2019). Assessment of groundwater quality in the buffer zone of Limpopo National Park, Gaza Province, Southern Mozambique. Environmental Science and Pollution Research, 26(1), 62–77.
Caussy, D., Gochfeld, M., Gurzau, E., Neagu, C., & Ruedel, H. (2003). Lessons from case studies of metals: Investigating exposure, bioavailability, and risk. Ecotoxicology and Environmental Safety, 56, 45–51.
Direcão Nacional de Areas de Conservacão (2003) Limpopo National Park Management and Development Plan, (Maputo, Ministry of Tourism, 2003).
DNA (2006). Direcção Nacional de Águas. Estratégia nacional de gestão de recursos hídricos.
FAO-SAFR (2004) Drought impact mitigation and prevention in the Limpopo River Basin: A situation analysis. Sub-Regional Office for Southern Africa/Harare. Rome.
GLTP. Great Limpopo Transfrontier Park. About GLTP. [http://www.greatlimpopo.org/about-gltp/]. Retreived 15 April 2019.
Islam, M. A., Romic´, D., Akber, M. A., & Romic´, M. (2018). Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environmental Geochemistry and Health, 40, 59–85.
IWMI/ARC (2003) Limpopo Basin Profile. CGIAR Challenge program on water and food. Pretoria.
Lehto, T., & Goncalves, R. (2008). Mineral resources potential in Mozambique. Geological Survey of Finland Special Paper, 48, 307–321.
Limpopo Groundwater Resource Information Project (GRIP) (GRIP LIMPOPO) (2002). An initiative of the Department of Water Affairs (DWA) to collate groundwater information and establish a ground-water information system for the Limpopo Province. Online v.2.0. [http://griplimpopo.co.za/]. Retreived 15 April 2019.
Ministerio do Turismo. (2003). Limpopo National Park: Management and Development Plan. Maputo: Ministério do turismo.
Moharir, A., Ramteke, D. S., Moghe, C. A., Wate, S. R., & Sarin, R. (2002). Surface and groundwater quality assessment in Bina region. Indian Journal of Environmental Protection, 22(9), 961–969.
Muchuweti, M., Birkett, J., Chinyanga, E., Zvauya, R., Scrimshaw, M. D., & Lester, J. (2006). Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agriculture, Ecosystems & Environment, 112, 41–48.
Paalman, M. A. A., van der Weijden, C. H., & Loch, J. P. G. (1994). Sorption of cadmium on suspended matter under estuarine conditions: competition and complexation with major seawater ions. Water, Air, and Soil Pollution, 73, 49–60.
Panagos, P., Van Liedekerke, M., Jones, A., & Montanarella, L. (2012). European soil data centre: Response to European policy support and public data requirements. Land Use Policy, 29(2), 329–338.
Pavelic, P., Giordano, M., Keraita, B. N., Ramesh, V., & Rao, T. (2012). Groundwater availability and use in Sub-Saharan Africa: a review of 15 countries. International Water Management Institute (IWMI).
Peace Parks Foundation. (2001). Great Limpopo (map). Peace Parks Foundation, Stellenbosch, South Africa. [https://www.peaceparks.org/tfcas/great-limpopo/].
RWSN (2015). Strengthening the Domestic Drilling Industry Lessons from the Mozambique Drillers Association. RWSN Water and Sanitation Programme.
Tchamo, M. E., Santos, M. A. D. M. D., Almeida, M. B. D., & Leandro, C. G. (2016). Physical fitness and Birth Weight in young men from Maputo City, Mozambique. Revista Brasileira de Medicina do Esporte, 22(1), 66–70.
UNEP, WHO (1996). Water Quality Monitoring.
U.S. Department of Energy (2019). RAIS: Risk Assessment Information System, https://rais.ornl.gov/index.html.
USEPA. (1986). Guidelines for the health risk assessment of chemical mixtures. Federal Register, 51, 34014–34025.
USEPA (1989). Risk assessment guidance for superfund. Volume I: Human health evaluation manual (Part A). EPA/540/1-89/002.
Van Wyk, A.W., 2004: Personal communication.
World Health Organization (WHO) (2011). Guidelines for Drinking-water Quality, 4th edition.
World Population Review (2019). [http://worldpopulationreview.com/countries/mozambique-population/]. Retreived 10 Oct 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary File S1
Parameters for water analyses by ICP-MS (X Series II Thermo Fisher Scientific Inc.) (DOCX 13 kb)
Supplementary File S2
Chemical–physical parameters and concentration of trace elements (DOCX 41 kb)
Supplementary File S3
Groundwater correlation matrix in the dry season (DOCX 19 kb)
Supplementary File S4
Correlation ratios in surface waters in the dry season (DOCX 17 kb)
Supplementary File S5
Correlation ratios in surface waters in the wet season (DOCX 17 kb)
Supplementary File S6
The results of health risk assessment of human exposure to trace elements in drinking water from the buffer zone of the Limpopo National Park. (DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Ricolfi, L., Barbieri, M., Muteto, P.V. et al. Potential toxic elements in groundwater and their health risk assessment in drinking water of Limpopo National Park, Gaza Province, Southern Mozambique. Environ Geochem Health 42, 2733–2745 (2020). https://doi.org/10.1007/s10653-019-00507-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00507-z