Abstract
Water quality and quantity are the major environmental concerns of developing countries like Pakistan. Safe drinking water is one of the major prerequisites for a healthy life. The current study assessed the contamination of drinking water including physicochemical parameters (pH, TDS, EC, TH, Cl1−, NO3 1−, PO4 3−, SO4 2−, Mn, Cd, Pb, Ni, Cr) and bacteriological contamination (Fecal coliform) in chromite mining-impacted areas of Pakistan. However, the main focus of the study was to identify the potential health risk of these parameters to the local people of the study area. Atomic Absorption Spectrometer (Perkin Elmer, AAS-PEA-700) was used to analyze the concentrations of potentially toxic metals. The concentrations of Cd, Pb, Ni, and Cr ranged from below detection limit (BDL)-7.20, BDL-14.4, BDL-78.3 and 0.20–69.0 and exceeded their respective limits in 31, 11, 17, and 36% samples of drinking water set by World Health Organization, while the concentration of Ni (63% samples) also exceeded the safe limit set by Pakistan Environmental Protection Agency. Furthermore, the chronic daily intake (CDI) and health risk index (HRI) of potentially toxic metals through consumption of drinking water were found in the order of Mn > Ni > Cr > Cd > Pb and Cd > Ni > Mn > Pb > Cr, respectively. The HRI values of selected toxic metals in mining-impacted sites were found within the safe limit (HRI <1). The results showed that both geogenic and anthropogenic activities were the main sources of drinking water contamination in the study area.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is one of the fundamental constituents in our environment needed for all living organisms including human beings (Shah et al. 2012; Bell 1998). Therefore, it is necessary to make sure the adequate supply of water for living beings in order to perform their functions properly. The physical, chemical, and biological properties of water are the important factors for causing diseases in human beings (Nawab et al. 2016). The contamination of water with potentially toxic heavy metals including cadmium (Cd), chromium (Cr), nickel (Ni), lead (Pb), manganese (Mn), zinc (Zn), cobalt (Co), and copper (Cu) and different anions such as chloride (Cl−1), nitrate (NO −13 ), nitrites (NO2 −), phosphate (PO4 3−), and sulfate (SO4 2−) is a global phenomenon and environmental issue for both developing and developed countries (Khan et al. 2013a, Gul et al. 2015). Furthermore, the ecosystem resource is primarily contaminated from natural sources including ore deposits and geogenic activities and anthropogenic activities such as mining, smelting, industries, waste water irrigation, and intensive agriculture practices (Ullah et al. 2013; Ettler et al. 2012; Nawab et al. 2015a, 2016). Contamination of water with animals and human feces is also considered the important pathway for transmission of pathogens to human body (Abu-Amr and Yassin 2008; Nawab et al. 2016; Khan et al. 2013b, 2015). These are continuously degrading the quality of water and making it unfit for drinking, agricultural, domestic, and industrial uses (Shah et al. 2012).
Toxic metals such as Cd, Mn, Pb, Ni, Cr, and mercury (Hg) are responsible for arising high toxicity in human and aquatic ecological life due to their long-time existence and bioaccumulative nature. The adverse effects of these potential toxic metals include headache, abnormal pain, hypertension, nerve damages, irritability, kidney and liver problems, intellectual disabilities, sideroblastic anemia, fatal cardiac arrest, and carcinogenic (Pekey et al. 2004; Shah et al. 2012; Kavcar et al. 2009). High concentrations of anions such as NO −13 , PO4 3−, and SO4 2−, are also causing adverse health problems including methemoglobinemia (blue baby syndrome) and laxative action (Jordao et al. 2002; Khan et al. 2013a). Biological contaminants in drinking water cause different health problems including dysentery, typhoid fever, intestinal infection, hepatitis, cholera, diarrhea, and other illness. Usually, coliform bacteria are used for the indication of other pathogenic contaminations that cause severe human health problems, but they are certainly not considered causing diseases (Muhammad et al. 2016; Khan et al. 2013b; Emmanuel et al. 2009).
The rapid urbanization along with the poor development with scarce water reservoirs and poor sanitation practices leads to increase water-related diseases (Lehloesa and Muyima, 2000). Globally, women play a key role in conservation and management of water resources and provision of safe drinking water. Pakistan faces several troubles in terms of water pollution, availability, water quality, water usage and deaths originated by water-borne diseases like other developing countries (Khan et al. 2013a). In current scenario, developing countries like Pakistan face a lot of problems regarding water quantity and quality. About 70% of its water resources are contaminated with microbial, organic, and inorganic pollutants (Malik et al. 2009). In hilly areas of Pakistan, a majority of the people depends on surface and ground water (springs) sources for drinking purposes. On the basis of geology of the study area having mafic and ultramafic rocks, chromite mining, and open dumping of mining wastes, high concentrations of toxic heavy metals are expected in drinking water of the area. Open defecation and grazing of animals are also the common practices in hilly areas of Pakistan which can easily contaminate the majority of water sources (ground and surface water) with biological organisms. Furthermore, well-published and detailed data are not available regarding the drinking water quality situation and the prevailing water-borne diseases in chromite mining northern areas of Pakistan. The main objective of this study was to investigate the health risk associated with potentially toxic metals and bacterial contamination in both ground and surface water sources in chromite mining-affected areas of Pakistan. This study compares the drinking water contamination of different sites with each other and also with international standards, and furthermore, it also highlights the possible impacts on the local people’s health using the water from available sources (surface and ground).
Materials and Methods
Study Area
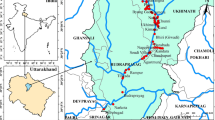
The study area is composed of mining-impacted sites (MIS) located in two districts such as Kohistan (MIS1) and Shangla (MIS2) and two agencies including Malakand (MIS3) and Mohmand (MIS4) (Fig. 1).
MIS1 lies between 34.52 to 33.52 degree latitude N and 72.56 to 33.13 degree longitude E and surrounded on south by Battagram District, on northeast by Gilgit-Baltistan Province, on southeast by Mansehra District, and on the west by Shangla and Swat Districts (DCR 1998a). MIS2 lies between 34.52 to 33.13 degree latitude N and 72.56 to 73.02 degree longitude E and bounded by Buner District on south, Kohistan District on north side, Swat District on west side, and Battagram District on east side (DCR 1998b). MIS3 is located in latitude of 34°30′ 0 north and longitude of 71°45′ 0 east in the northern mountainous area of Pakistan. This agency is surrounded by mountains which make boundary with the north of Swat District, the west of Bajaur Agency, the northern side link with Dir District, and the south is Mardan and Peshawar Districts (SDPI, 2012). The climate of the agency falls under semi-climatic zone with the lowest precipitation occurring in summer on the eastern side, while more precipitation occurs in winter on western side (Zabihullah et al. 2006). However, MIS4 is located in latitude of 34°22′20″ north and longitude of 71°27′26″ east and bounded by Bajaur Agency on north, south to the Khyber Agency, east Malakand and Charsadda Districts, and south east to the Peshawar District. The climate of Mohmand Agency is cooler in winter and hotter in summer, while most precipitation occurs in winter season (Shah et al. 2012).
Water Sampling
The surface and groundwater samples (n = 109) used for drinking purposes were collected from each site (MIS1, MIS2, MIS3, and MIS4) in two clean polythene bottles. The polythene bottles were washed with double deionized water before sampling. In order to reduce the growth of microbes and the formation of colonies, a few drop of HNO3 were added to one water sample for potential toxic metal analysis, while the non-acidified samples were transferred immediately for bacteriological analysis to the University of Swat. Sampling was carried out from various sources of groundwater such as wells, springs, and surface water including rivers, streams, and perennial rivers. In order to minimize the contamination, sterilized bottles were used. Physical parameters including pH, TDS, and electrical conductivity (EC) were measured on the spot by using water checker U-10.
Laboratory Analysis
After collection, water samples were immediately transported to the laboratory to obtain more accurate data, because chemical and biological activities may change the sample's composition. Hardness and chloride (Cl−) content were determined by volumetric analysis (Titration method) as given in APHA (1992), while NO3 1−, PO4 3−, and SO4 2− using UV–visible spectrophotometer (HACH 2800). Bacterial analysis was carried out through Del Agua potable water kit using colony-forming unit (CFU/100 mL). The concentrations of potentially toxic metal were determined using graphite furnace atomic absorption spectrophotometer (AAS) (Perkin Elmer USA, ASS-PEA-700). For quality assurance, each sample was analyzed in triplicate, and after every 10 samples, a blank and three standards (i.e., 2.5, 5, and 10 μg/L for heavy metals) of respective metals were analyzed on atomic absorption. The reproducibility was found to be at 95% confidence level. Therefore, average value of each water sample was used for further interpretation. The standard of all five parameters was prepared form diluting 1000 mg/L of certified standard solution (Fluka Kamica, Buchs, Switzerland) of each corresponding metals and run according to the standard procedure.
Health Risks Assessment
Chronic Daily Intake (CDI)
There are several pathways such as physical contact, food chain, and inhalation, through which potentially toxic metals can enter into the human body (dermal contact, inhalation, and ingestion), but the most important pathway for heavy metals is the oral intake of contaminated food/water to the body; thus, the accumulation of toxic metals then started in the body (ATSDR 1993b). The CDI through water ingestion was calculated by the modified equation from (US EPA 1999; Chrostowski 1994)
where C, DI, and BW represent the concentration of HM in water (μg/L), average daily intake rate (2 L/day), and body weight (72 kg) and 1 L/day for child body weight (32.7 kg) (Nawab et al. 2016).
Health Risk Indexes (HRIs)
In order to evaluate the health risks, the following equation can be used to calculate HRI (Khan et al. 2008):
where the values of reference dose for oral toxicity for Cr, Pb, Cd, Mn, and Ni are 1.5E+03, 3.6E+01, 5.0E−01, 1.4E+02, and 2.0E+01 (μg/kg day) (Shah et al. 2012; Nawab et al. 2016; USEPA 2005). The value of HRI is considered safe for the consumer if it is less than one (HRI >1).
Statistical Analysis
The ranges, means, and standard deviations were calculated using Excel 2010 (Microsoft Office). The statistical analyses such as one-way ANOVA and inter-elemental correlation were calculated by the SPSS software version 21. The graphs were prepared using the Sigma plot, while the Arc Geographic Information System was used for the preparation of the location map of the study area.
Results and Discussion
Physicochemical Parameters
The values of physiochemical parameters in drinking water of the study area (MIS1, MIS2, MIS3, and MIS4) are summarized in Table 1. The pH of water is considered as the most important water quality parameter. The mean value of pH was in the order of MIS1 > MIS2 > MIS4 > MIS3. In the study area, the pH values in drinking water samples ranged from 6.90 to 8.40, as shown in Table 1. The minimum pH value (6.90) was observed in MIS3, while the maximum pH value (8.40) was observed in MIS1 and MIS2. The pH of MIS2 and MIS1 was appeared slightly alkaline but still within its permissible limit. The pH values in all four areas were observed within permissible limit set by WHO (2006) and Pak-EPA (2008). Similarly, the TDS values in the study area (MIS1, MIS2 MIS3, and MIS4) ranged from 17 to 362, 104 to 121, 141 to 369, and 142 to 388 mg/L in the same four locations. The minimum TDS value (17 mg/L) was observed in the water samples of MIS1, while the maximum value (388 mg/L) was observed in MIS4. The mean values of TDS in the study area were found in the order of MIS4 > MIS3 > MIS1 > MIS2. The concentrations of TDS in the study area were within the permissible limit of WHO (2006) and Pak-EPA (2008), but the values were still higher than those reported by Dhakate and Singh (2008) in Orissa, India. The values of EC in the same four locations (MIS1, MIS2, MIS3, and MIS4) were in the range of 31–845, 195–223, 309–825, and 308–835 µS/cm. The minimum EC (31 µS/cm) and maximum value (845 µS/cm) were observed in the water samples collected from MIS1. EC values in the study area were observed greater than those reported by Khan et al. (2013a) in the districts Charsadda, Pakistan, and (Dhakate and Singh 2008) Orissa, India.
The values of total hardness (TH) ranged from 350 to 900, 333 to 954, 750 to 1450, and 700 to 1550 mg/L in the same four locations (MIS1, MIS2, MIS3, and MIS4), respectively, of the study area. The minimum concentration (333 mg/L) was observed in MIS2 area, while maximum concentration (1550 mg/L) was observed in MIS4 area. The concentrations of TH in MIS3 and MIS4 were observed greater than the permissible limit of WHO (2006) and Pak-EPA (2008), while 20% in MIS1 and MIS2, the values of TH were found within the permissible limit set by WHO (2006) and Pak-EPA (2008), while 80% exceeded the limit. This high concentration of TH in water samples of MIS3 and MIS4 areas may be due the high contents of anions (carbonate, bicarbonate, chloride, and sulfate) and major cations (magnesium and calcium) (Das et al. 2013 ).
The concentrations of anions are important in drinking water, because their excessive or low intake can cause adverse effects on human health (Khan et al. 2013a). For this purpose, the analyses of some anions such as Cl1−, NO3 1−, PO4 3−, and SO4 2−were carried out. The mean concentrations of anions in the study area were found in the order of Cl1−> SO4 2−> NO3 1−> PO4 3−. The concentrations of Cl in the water samples collected from the study area were ranged from BDL-380, BDL-354, BDL-332.52, and BDL-332 mg/L in MIS1, MIS2, MIS3, and MIS4, respectively. Minimum mean value of Cl1− (113 mg/L) was observed in MIS1 area, while maximum (204 mg/L) was found in water samples collected from MIS2 area. The values of NO3 1− in the same four locations (MIS1, MIS2, MIS3, and MIS4) ranged from 0.08 to 10.43, 1.03 to 7.83, 0.44 to 9.99, and 1.40 to 9.44 mg/L, respectively, as shown in Table 1. The minimum concentration of NO3 1− (0.44 mg/L) was observed in MIS3 area, while maximum concentration (10.4 mg/L) was observed in MIS1 area. Furthermore, the concentrations of PO4 3− were ranged from 1.10 to 3.82, 0.09 to 3.31, 1.00 to 2.65, and 1.00 to 2.78 mg/L in the same four locations (MIS1, MIS2, MIS3, and MIS4), respectively. The minimum value of PO4 3− (1.00 mg/L) was found in both MIS3 and MIS4 areas, while the maximum value (3.82 mg/L) was also observed in water samples collected from MIS4 area. Similarly, SO4 2− concentration ranged from 8.94 to 18.4, 1.45 to 16.0, 12.5 to 22.0, and 14.5 to 25.7 mg/L in MIS1, MIS2, MIS3, and MIS4, respectively, as shown in Table 1. The minimum SO4 2− value (1.45 mg/L) was recorded in MIS2, while maximum value (25.7 mg/L) was observed in the water samples collected from MIS4 area. The values of major anions (Cl1− and NO3 1−) were observed within their respective permissible limits set by WHO (2006) and Pak-EPA (2008). So far, no permissible limits have been set for SO4 2− and PO4 3−in drinking water by the regularity authorities. The concentrations of Cl1−, NO3 1−, and SO4 2− were observed lower, while Cl greater than those reported by Khan et al. (2013b) in the district Charsadda, Pakistan.
Fecal Coliform Bacteria
The presence of fecal coliform bacteria in the study area ranged from BDL-60.0, BDL-32.0, BDL-97.0, and BDL-89.0 colony-forming unit per 100 mL (CFU/100 mL) was observed in MIS1, MIS2, MIS3, MIS4, respectively (Fig. 2). The mean minimum number of fecal coliform bacteria (8.45 CFU/100 mL) was observed in the water samples collected from MIS2, while the mean maximum number of fecal coliform bacteria (18.25 CFU/100 mL) was observed in the water samples of MIS3. In the study area, 78% of both surface and groundwater sources were contaminated with fecal coliform bacteria when compared with WHO (2006) and Pak-EPA (2008) safe limits, that is, (0 CFU/100 mL) of water. This high contamination of fecal coliform bacteria may be from open defecation, low-quality septic tanks, and human and animal feces (Nawab et al. 2016).
Potentially Toxic Heavy Metals
The concentrations of potentially toxic metals in water samples collected from the study area are summarized in Fig. 3a. The concentrations of toxic metals were found in the order of Mn > Ni > Cr > Pb > Cd in the study area. The values of Mn in MIS1, MIS2, MIS3, and MIS4 ranged from 210 to 293, BDL-221, 14 to 399, and 1070 to 396 µg/L, respectively, as shown in Table 2. Minimum value below detection limit (BDL) was observed in the water samples of MIS2, while the maximum value of Mn (399 µg/L) was observed in MIS3 area. Mn concentration was observed within permissible limit set by WHO (2006) and Pak-EPA (2008). The concentration of Mn was observed greater than those reported by Khan et al. (2013a) in drinking water of Swat, northern Pakistan.
Similarly, Cd concentration ranged from 0.40 to 6.10, 1.00 to 7.20, 0.70 to 6.00, and BDL-5.00 µg/L in the same four locations (MIS1, MIS2, MIS3, and MIS4), respectively. The maximum value (7.20 µg/L) was found in MIS2. Cd concentration in 31% water samples exceeded the permissible limit set by WHO (2006), while all the Cd concentrations were within the safe limit of Pak-EPA (2008). Cd is carcinogenic to human being and categorized as Group 1 by International Agency for Research on Cancer. Cd also causes cancer to lungs and the research has shown positive correlation for kidney and prostate cancers (IARC 2012). High Cd intake can also causes kidney, pulmonary, and skeletal damages (Godt et al. 2006; Nordberg et al. 2002)
Similarly, the concentration of Pb in the same four locations (MIS1, MIS2, MIS3, and MIS4) ranged from 1.00 to 13.8, BDL-13.2, 0.70 to 13.8, and 1.00 to 13.0 µg/L, respectively, as shown in Table 2. Minimum concentration of Pb (BDL) was recorded in some water samples collected from MIS2, while maximum (14.4 µg/L) was recorded in MIS1. However, Pb concentration in 11% samples exceeded the acceptable limit set by WHO (2006), while all the samples of Pb were within the safe limits of Pak-EPA (2008). Pb exposure can cause a wide range of health problems in both children and adults, such as renal failure, coma, small effects on metabolism, and intelligence to convulsions and even death (Papanikolaou et al. 2005). According to International Agency for Research on Cancer evaluation, inorganic Pb compounds are possibly carcinogenic to humans (Group 2A). Figure 4 represents the spatial distribution of heavy metals in drinking water sources in MIS1, MIS2, MIS3, MIS4, and reference sites, respectively. The results revealed that almost all the sites were contaminated with toxic metals when compared with reference sites. The Cd and Pb concentrations in the study area were observed greater than those reported by Khan et al. (2013b) in drinking water of district Charsadda, Pakistan.
The concentrations of Ni ranged from 18.0 to 77.0, BDL-73.8, 21.0 to 76.0, and 14.0 to 78.3 µg/L in the same four locations (MIS1, MIS2, MIS3, and MIS4), respectively. The minimum value of Ni (BDL) was observed in MIS2, while the maximum value (78.0 µg/L) was recorded in drinking water samples collected from MIS4. Ni concentration in 17% samples was observed greater than the permissible limit of WHO (2006), while 63% water samples exceeded the safe limit of Pak-EPA (2008). Human are exposed to Ni through several pathways including contaminated food and water ingestion, inhalation, and dermal absorption (IARC 2012; ATSDR 2005). According to International Agency for Research on Cancer, Ni compounds are classified as Group 1 carcinogen to humans. Ni and its compounds can cause lung cancers and Para nasal sinuses (IARC 2012).
The concentrations of Cr in water samples ranged from 12.0 to 67.8, 0.20 to 68.3, 13.0 to 69.0, 18.0 to 62.2 µg/L in MIS1, MIS2, MIS3, and MIS4, respectively, as shown in Table 2. The minimum value of Cr (0.20 µg/L) was found in MIS2, while maximum value (69.0 µg/L) was found in the water samples collected from MIS3. The concentration of Cr in 36% samples was observed greater than the permissible limit of WHO (2006) and Pak-EPA (2008), while 64% samples were observed within permissible limit. IARC also classified Cr as Group 1 carcinogen for human and causes cancer of lungs. Similarly, positive correlation has also been observed between Cr compounds and cancer of nose and nasal sinuses (IARC 2012). The mean Ni and Cr concentrations in the study area were observed less than those reported by Khan et al. (2013b) in drinking water of district Swat, Pakistan.
The results revealed that these high concentrations of Cr, Ni, Cd, and Pb may be due to the open dumping of mining wastes, mafic and ultramafic rocks, and Pb/Zn sulfide deposits (Nawab et al. 2015a, b, c). On the other side, from several years in these areas, low-scale chromite mining has been occurred, which is responsible for these high concentrations of heavy metals in water sources.
Human Health Risk Assessment
Chronic Daily Intake of Metals
Figure 3b summarizes the overall chronic daily intake value of toxic metals. The CDIs values of selected potentially toxic metals in the study area were found in the order of Mn > Ni > Cr > Cd > Pb. The CDI values of Mn for adults in drinking water of MIS1, MIS2, MIS3, and MIS4 ranged from 0.58 to 8.13, BDL-0.61, 3.16 to 11.1, and 2.97 to 11.0 µg/kg/day, while for children 0.64 to 8.96, BDL-0.67, 3.48 to 12.2, and 3.27 to 12.1 µg/kg/day, respectively, as shown in Table 3. The highest mean CDI of Mn (12.2 µg/kg/day) was found for children in MIS3 area, while lowest (BDL µg/kg/day) was found for adults in MIS2. The Cd CDIs for adults and children were ranged from 0.01 to 0.16, 0.02 to 0.20, 0.01 to 0.16, BDL-0.13 and 0.01 to 0.18, 0.03 to 0.22 0.02 to 0.18, BDL-0.15 µg/kg/day, in MIS1, MIS2, MIS3, and MIS4, respectively. The highest mean Cd CDI (1.34 µg/kg/day) was found for children in water samples collected from MIS3, while the lowest mean Cd CDI (BDL µg/kg/day) was observed for both adults and children of MIS4 (Table 3). Similarly, the CDIs values of Pb for adults in the same four locations (MIS1, MIS2, MIS3, and MIS4) ranged from 0.02 to 0.39, BDL-0.37, 0.01 to 0.38, and 0.02 to 0.36 µg/kg/day respectively, while for children the values were ranged from 0.03 to 0.43, BDL-0.41, 0.02 to 0.42, and 0.03 to 0.39 µg/kg/day. The highest mean CDI for Pb (0.43 µg/kg/day) was observed for children in MIS1, while the lowest mean Pb CDI (BDL µg/kg/day) was observed for both adults and children in MIS2. The CDIs of Ni for adults and children were in the range of 0.50–2.13, BDL-2.05, 0.58–2.11, 0.38–2.17 and 0.55–2.35, BDL-2.25, 0.64–2.32, 0.42–2.39 µg/kg/day, respectively in MIS1, MIS2, MIS3, and MIS4, as shown in Table 3. The maximum mean Ni CDI (2.39 µg/kg/day) was recorded for children in MIS4, while the minimum mean value (BDL µg/kg/day) was observed for adults and children in MIS2. Similarly, the Cr CDIs for adults and children in same four locations (MIS1, MIS2, MIS3 and MIS4) ranged from 0.33 to 1.88, BDL-1.89, 0.36 to 1.91, 0.50 to 1.74, and 0.36 to 2.07, BDL-2.08, 0.39 to 2.11, 0.55 to 1.92 µg/kg/day, respectively. The highest mean CDI value of Cr (2.11 µg/kg/day) was found for children in MIS3, while the lowest mean Cr CDI (BDL µg/kg/day) was found for both adults and children in MIS2 (Table 3). In the study area, the maximum CDI value was found for Mn (12.2 µg/kg/day). The CDI values in the study area appeared lesser than those reported by Khan et al. (2013b) in drinking water of district Swat, Pakistan.
Health Risk Index of Metals
The overall HRI values of heavy metals in the study area are shown in Fig. 3c. The HRI values of selected potentially toxic metals in the study area were found in the order of Cd > Ni > Mn > Pb > Cr. The HRI of Mn through consumption of potentially toxic metals contaminated water for adults and children were 2.65E−02, 1.00E−03, 5.45E−02, 5.72E−02 and 2.92E−02, 1.07E−03, 6.00E−02, 6.30E−02 in MIS1, MIS2, MIS3, and MIS4, respectively (Table 4). However, the HRI of Cd for adults and children were 1.50E−01, 0.18E−01, 1.60E−01, 1.40E−01 and 1.60E−01, 0.19E−01, 1.90E−01, 1.60E−01, respectively, in the same four locations. Similarly, Pb HRI for adults and children were 5.55E−03, 3.88E−02, 5.00E−03, 5.00E−03 and 6.11E−03, 4.44E−02, 5.27E−03, 5.55E−03, respectively, in the same four locations. The HRI value of Ni in the same four locations for adults and children 7.85E−02, 2.75E−02, 7.15E−02, 7.90E−02 and 8.55E−02, 3.05E−02, 7.90E−02, 8.65E−02, respectively, through consumption of heavy metal-contaminated water (Table 4). Similarly, the HRI of Cr for adults were 8.80E−04, 6.00E−04, 8.47E−04, and 8.33E−04, while for children were 9.67E−04, 6.60E−04, 9.27E−04, and 9.13E−04, respectively, in MIS1, MIS2, MIS3, and MIS4, as shown in Table 4. In the study area, the HRI values of selected toxic metals were observed within safe limit (HRI <1) when compared with the limits of USEPA (1999).
Statistical Analysis
One-Way ANOVA
The statistical comparison of potentially toxic metals was carried out through one-way ANOVA. The result showed that the concentrations of physical parameters such as pH, EC, TH, NO3 1−, PO4 3− and SO4 2− were significantly higher (p = 0.00) in drinking water of the four districts, as compared with the reference sites (Table 5), while the concentrations of HMs such as Mn and Cr were significantly higher (p = 0.00) in drinking water of the study area as compared with reference location (Table 5). However, insignificant variations were found for TDS, total hardness, Cl1−, Cd, Pb, and Ni in drinking water of the study area.
aDegree of freedom, bFactor bold values indicate significane at level of 0.001 Inter-Metals Correlation
Table 6 summarizes the inter-metals correlation of the selected parameters in the study area. Correlation analysis provides the most valuable information about the source and pathway of the selected water parameters (Nawab et al. 2015a, b, c). In drinking water of the study area, both positive and negative correlations were found for some pairs. Positive correlation was found for EC–TDS (r = 0.364), TH–TDS (r = 0.224), TH–EC (r = 0.728), NO −13 –TDS (r = 0.583), NO −13 –EC (r = 0.622), NO3 1−–TH (r = 0.513), PO4 3−–Cl1− (r = 0.302), SO4 2−–TDS (r = 0.2.18), SO4 2−–EC (r = 0.710), SO4 2−–TH (r = 0.519), SO4 2−–NO3 1− (r = 0.366), Mn–TDS (r = 0.283), Mn–EC (r = 0.721), Mn–TH (r = 0.545), Mn–NO3 1− (r = 509), Mn–SO4 2− (r = 0.608), Ni–TDS (r = 0.200), Ni–EC (r = 0.300), Ni–TH (r = 0.238), Ni–SO4 2− (r = 0.435), Ni–Mn (r = 0.449), Cr–TDS (r = 0.237), Cr–EC (r = 0.269), Cr–SO4 2− (r = 0.252), Cr–Mn (r = 0.228), and Cr–Ni (r = 0.282), while negative correlation was found for EC–pH (r = −0.554), TH–pH (r = 0.349), NO −13 –pH (r = 332), PO4 3−–EC (r = −0.200), SO4 2−–pH (r = −0.319), SO4 2−–Cl1− (r = −0.227), Mn–pH (r = −0.348), and Mn–Cl1− (r = −0.209), which showed that the source and pathway of these parameters are different.
Conclusion
The results indicated that the concentration of TH was higher than the acceptable limit of WHO, while 78% water samples were contaminated with fecal coliform bacteria and exceeded the acceptable limit (0.00 CFU/100 mL). However, the concentrations of potentially toxic metals including Cd, Pb, Ni, and Cr were exceeded the permissible limit of WHO (2006) and Pak-EPA (2008). Regarding the health risk assessment, the CDI and HRI indicated that the drinking water sources were considered safe for the consumption of human beings because the HRI were less than one. Finally, it was concluded that the drinking water of the study area was contaminated due to mining of chromite ores weathering/leaching of mafic and ultramafic rocks, open defecation, and animal wastes. Due to these processes, some potential toxic metals such as Cr, Pb, Cd, and Ni and fecal coliform bacteria can be posed potential health risk to the local people; therefore, some prevention and mitigation measurements are needed to minimize the future possible health risk in the local population associated with the ingestion of drinking water.
References
Abu-Amr SS, Yassin MM (2008) Microbial contamination of the drinking water distribution system and its impact on human health in Khan Yunis Governorate, Gaza strip. J R Inst Public Health 122(11):1275–1283. doi:10.1016/j.puhe.2008.02.009
APHA (1992) Standard Methods for the examination of water and wastewater, 18th edn. American Public Health Association and Water Environmental Foundation EPS Group, Inc., Maryland, pp 2–55, 129–131
ATSDR (1993b) Toxicological Profile for Arsenic. Clement International Corp. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, ATSDR/TP-92/02
ATSDR (2005) Toxicological profile for nickel. Agency for Toxic Substances and Disease Registry. US Public Health Service
Bell FG (1998) Environmental geology: principles and practice. Blackwell, New York
Chrostowski PC (1994) Exposure assessment principles. In: Patrick DR (ed) Toxic air pollution handbook. Van Nostrand Reinhold, New York, pp 133–163
Das S, Patnaik SC, Sahu HK, Chakraborty A, Sudarshan M, Thatoi HN (2013) Heavy metal contamination, physico-chemical and microbial evaluation of water samples collected from chromite mine environment of Sukinda, India. Trans Nonferrous Met Soc 23:484–493. doi:10.1016/S1003-6326(13)62489-9
Dhakate R, Singh SV (2008) Heavy metal contamination in groundwater due to mining activities in Sukinda valley, Orrisa. A case study. J Geogr Reg Plan 1(4):58–67
District Census Report of Kohistan (DCR) (1998a) Population Census Organization. Statistic Division. Ministry of Economic Affairs and Statistic Government of Pakistan, pp 1–17
District Census Report of Shangla (DCR) (1998b) Population Census Organization. Statistic Division. Ministry of Economic Affairs and Statistic. Government of Pakistan, pp 1–17
Emmanuel E, Pierre MG, Perrodin Y (2009) Groundwater contamination by microbiological and chemical substances released from hospital wastewater and health risk assessment for drinking water consumers. Environ Int 35:718–726. doi:10.1016/j.envint.2009.01.011
Ettler V, Krıbek B, Majer V, Knesl I, Mihaljevic M (2012) Differences in the bioaccessibility of metals/metalloids in soils from mining and smelting areas (Copperbelt, Zambia). J Geochem Explor 113:68–75. doi:10.1016/j.gexplo.2011.08.001
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. doi:10.1186/1745-6673-1-22
Gul N, Shah MT, Khan S, Khattak NU, Muhammad S (2015) Arsenic and heavy metals contamination, risk assessment and their source in drinking water of the Mardan District, Khyber Pakhtunkhwa, Pakistan. J Water Health 13(4):1073–1084
IARC (2012) A Review of human carcinogens: metals, arsenic, fibres and dusts. International Agency for Research on Cancer: Monographs on the Evaluation of Carcinogenic Risks to Humans, 100C
Jordao CP, Pereira MG, Bellato CR, Pereira JL, Matos AT (2002) Assessment of water systems for contaminants from domestic and industrial sewages. Environ Monitor Assess 79(1):75–100
Kavcar P, Sofuoglu A, Sofuoglu SC (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2):216–227. doi:10.1016/j.ijheh.2008.05.002
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing China. Environ Pollut 152(3):686–692. doi:10.1016/j.envpol.2007.06.056
Khan K, Lu Y, Khan H, Zakir S, Ullah I, Khan S, Khan AA, Luo W, Tieyu W (2013a) Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J Environ Sci 25:2003–2013. doi:10.1016/S1001-0742(12)60275-7
Khan S, Shahnaz M, Jehan N, Rehman S, Shah MT, Din I (2013b) Drinking water quality and human health risk in Charsadda district, Pakistan. J Clean Prod 60:93–101. doi:10.1016/j.jclepro.2010.02.016
Khan S, Shah IA, Muhammad S, Malik RN, Shah MT (2015) Arsenic and Heavy Metal Concentrations in Drinking Water in Pakistan and Risk Assessment: A Case Study. Hum Ecol Risk Assess Int J 21(4):1020–1031
Lehloesa LJ, Muyima NYO (2000) Evaluation of the impact of household treatment procedures on the quality of groundwater supplies in the rural community of Victoria district, Eastern Cape. Tech Note Water 26(2):285–290
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 30(168):1–2
Muhammad N, Banoori N, Akbar A, Azizullah A, Khan M, Qasim M, Rahman H (2016) Microbial and toxic metal contamination in well drinking water: potential health risk in selected areas of Kohat, Pakistan. Urban Water J 28:1–7
Nawab J, Ali S, Rehman UU, Khan A, Khan S, Khan W (2015a) Health risk associated with heavy metals via surface and ground water consumption in Shangla district of northern Pakistan. J Himal Earth Sci 2:14–48
Nawab J, Khan S, Shah MT, Gul N, Ali A, Khan K, Qing H (2015b) Heavy metal bioaccumulation in native plants in chromite impacted sites: a search for effective remediating plant species. Clean—Soil Air Water 44:37–46. doi:10.1002/clen.201400605
Nawab J, Khan S, Shah MT, Khan K, Huang Q, Ali R (2015c) Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. Int J Phytoremediation 17(9):801–813. doi:10.1080/15226514.2014.981246
Nawab J, Khan S, Ali S, Sher H, Rehman Z, Khan K, Tang J, Ahmad A (2016) Risk Assessment of Heavy metals and Biological Contamination in Drinking Water of Malakand Agency, Northern Pakistan. Environ Monitor Assess 188:286. doi:10.1007/s10661-016-5296-1
Nordberg G, Jin T, Bernard A (2002) Low bone density and renal dysfunction following environmental cadmium exposure in China. J Hum Environ 31:478–481
Pak-EPA (2008) Pakistan environmental protection agency. National Environmental Quality Standards. Ministry of Environment. Pakistan.http://www.pakepa.org/neqs.html. Accessed 10.05.10
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM (2005) Lead toxicity update: a brief review. Med Sci Monit 11(10):329–336
Pekey H, Karaka D, Bakoglu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analysis. Mar Pollut Bull 49(9–10):809–818. doi:10.1016/j.marpolbul.2004.06.029
SDPI (2012) Climate change adaptation: introducing & promoting energy efficient technologies in the flood affected remote underdeveloped rural areas. Energy Efficient Cooking Stoves (EECS)—a case study
Shah MT, Ara J, Muhammad S, Khan S, Tariq S (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67. doi:10.1016/j.gexplo.2012.04.008
Ullah Z, Khan H, Waseem A, Mahmood Q, Farooq U (2013) Water quality assessment of the River Kabul at Peshawar, Pakistan: Industrial and urban wastewater impacts. J Water Chem Technol 35(4):170–176
US Environmental Protection Agency (US EPA) (2005) Guidelines for carcinogen risk assessment. Risk Assessment Forum. Washington, DC. EPA/630/P-03/001F
US Environmental Protection Agency (US-EPA) (1999) Guidance for performing aggregate exposure and risk assessments. Office of Pesticide Programs. Washington, DC
WHO (World Health Organization) (2006) Guidelines for drinking water quality, First Addendum to third ed. Recommendations, vol 1, Geneva, Switzerland
Zabihullah Q, Rashid A, Akhtar N (2006) Ethnobotanical survey in kot Manzaray baba valley Malakand agency, Pakistan. Pak J Plant Sci 12(2):115–121
Acknowledgement
The financial support for this research work was provided by Higher Education commission of Pakistan (21-395/SRGP/R&D/HEC/2014) to the first author. The authors also acknowledge the Chairperson and Madam Shaheen, Department of Soil and Environmental Sciences Khber Puktunkwa Agricultural University Peshawar Pakistan for heavy metal analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nawab, J., Khan, S., Khan, M.A. et al. Potentially Toxic Metals and Biological Contamination in Drinking Water Sources in Chromite Mining-Impacted Areas of Pakistan: A Comparative Study. Expo Health 9, 275–287 (2017). https://doi.org/10.1007/s12403-016-0240-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0240-8