Abstract
Metal oxide nanoparticles (MO-NPs) with multifunctional properties are used extensively in various industries and released into the environment as industrial effluents and waste nano-products. These non-degradable, toxic MO-NPs are accumulating in the environment, debilitating the ecosystem and their biological communities. In this review article, a real-time scenario of MO-NP toxicity towards the soil and aquatic ecosystem and their mode of toxicity have been addressed in detail. The up-to-date information presented here suggests serious consideration of the consequences before random utilization of MO-NPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
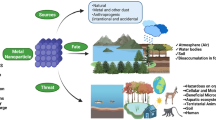
The advent and the worldwide growth of nanoparticle (NP)-based industries have been referred as the next industrial revolution by Lux Research 2008. In past 2 decades, successful synthesis of engineered nanoparticles (ENPs), especially metal oxide nanoparticles (MO-NPs) with fascinating physico-chemical properties, such as transparency to visible light, semi-conductivity, intrinsic UV-absorbing capacity, etc., has facilitated the golden age of these industries (Bondarenko et al. 2013). NP-rich industrial effluents are continuously disposed to the environment, resulting in alteration of soil and aquatic equilibrium, thus challenging to their living population (Ciacci et al. 2012). These risks are growing in tandem with increasing demand of nano-products in the global market (Morales-Diaz et al. 2017). A schematic presentation of eco-toxicological aspects of MO-NPs is shown in Fig. 1.
In recent years, many of studies have reported MO-NP induced toxicity in mammalian cells, specifically humans (Yamamoto et al. 2004; Bondarenko et al. 2013), and plants (Morales-Diaz et al. 2017; Siddiqi and Husen 2017). Although a few scientific reports are available focusing the eco-toxicity of individual MO-NP on a specific biological species, complete information comprising the MO-NP toxicity as a whole towards the entire soil and aquatic habitats is yet to be explored extensively.
This review article is a compact attempt to understand the real-time MO-NP induced eco-toxicity and its threshold level towards the entire soil and aquatic habitats. The most abundantly used MO-NPs, such as TiO2, ZnO, CuO, etc., have been studied with special consideration. This review strongly demonstrates the necessity of monitoring the random use of toxic MO-NPs to avoid the irreversible environmental impairment in the near future.
Global Production, Production Strategy and Application of MO-NPs
Global Production
In the year 2000, the very first national nanotechnology programme was launched in the United States, and in 2010 itself, the worldwide funding for NT was 1.78 billion dollars (Sargent 2012). Effectively, mammoth quantity of SiO2, TiO2, and other MO-NPs were produced (Piccinno et al. 2012). Projections indicate this exponentially increasing market value of NT to reach as high as $1 trillion by 2020 (Chakraborty et al. 2016).
Production Strategy
NPs are traditionally synthesized by a number of physical, chemical, and biological routes. The physical methods in practice include gas condensation technique, spray pyrolysis, laser pyrolysis, vapour deposition (Dhand et al. 2015), etc. Highly pure NPs with desired shape and size can be achieved this way, but complicated instrumentation and high-power consumption make these processes inconvenient and expensive. Chemical methods include sol–gel, microemulsion, hydrothermal, chemical vapour synthesis, etc. (Dhand et al. 2015), but employment of highly toxic and non-biodegradable chemicals causes environmental hazard and limits its biomedical applications (Boxi et al. 2016). Recently, biological synthesis of NPs has come to the fore where biomolecules secreted by plants or microorganisms being used as reducing agents for the material salts (Dhand et al. 2015).
Applications
The MO-NPs are critically used in manufacturing industries to reinforce the physical properties of bulk materials or to achieve enhanced surface features like scratch resistance, water repellence, reflectivity, photo-activity, etc. (Bondarenko et al. 2013). These are most commonly used in sensor and sensing devices, catalyst designing, sunscreens, cosmetics, electronic devices, textiles, agriculture, diagnostic imaging, potential cancer treatment, antimicrobial applications, etc. (Exbrayat et al. 2015; Dhand et al. 2015). Conclusively, MO-NPs have become an integral part of our daily life.
Eco-toxicology
NPs as Contaminant
NPs existed in the environment from the beginning of the earth’s history as volcanic dust, soil-particles, rock erosion, etc. With time, living systems had learnt to withstand the ill effects and interference of these natural NPs (Exbrayat et al. 2015). In the past 2 decades, synthesis of ENPs has introduced multipurpose industries but imposed new challenges to the living population. Even though some researchers deny the size-dependent mechanical toxicity of MO-NPs (Yamamoto et al. 2004; Warheit et al. 2006), manipulating the surface area, surface chemistry, and ionic characters of these ENPs for achieving desired properties has increased their toxicity and bioavailability to much higher level than their bulk form (http://europa.eu.int/comm/health/ph_risk/committees/04_scenihr/04_scenihr_en.htm). These toxic NPs are continuously disposed and accumulated in the environment resulting consequent ecological imbalance (Hu et al. 2010). The major sources of the NP contaminants in environment are summarized in Fig. 2.
MO-NPs in Aquatic Eco-system
Aquatic ecosystem consists of many organisms ranging from producers to decomposers (Bondarenko et al. 2013). The MO-NP toxicity on growth and survival of the producers causes depletion of dissolved oxygen in the water bodies. Again, lethal effects on decomposers limit biodegradation process resulting accumulation of waste materials that contribute to the pollution in aquatic environment (Navarro et al. 2008; Siddiqi and Husen 2017). Consumers at different levels, such as Zebrafish (Chakraborty et al. 2016), Daphnids and other crustaceans (Heinlaan et al. 2008), sea urchin (Fairbairn et al. 2011), rainbow trout (Federici et al. 2007), some amphibians, and molluscs (Exbrayat et al. 2015), are the most reported aquatic habitats to suffer from MO-NP toxicity (Table 1).
Toxicity of the MO-NPs are relative to their solubility in aqueous medium. TiO2 and CeO2-NPs aggregate and sediment fast (~ 30–60 min) and are completely insoluble in seawater (Keller et al. 2010) that limits their toxicity and bioavailability, too. In contrast, ZnO and CuO-NPs are readily soluble and release metal ions (Zn++ and Cu++) in aqueous solution, making them more toxic and bioavailable (Chang et al. 2012). In some cases, bulk ZnSO4 releases more metal ions in aqueous medium exerting higher toxicity than nano-ZnO, which supports the size independent toxicity of MO-NPs (Yamamoto et al. 2004; Warheit et al. 2006; Heinlaan et al. 2008). Similarly, influence of Cu++ ions in CuO-NP toxicity has been reported by Bondarenko et al. (2013).
Hence, without even entering the cell, MO-NP may exert toxicity by altering the ionic character of the micro-environment in close vicinity of cell-particle contact area. However, each metal ion is unique in its mode of toxicity and the toxicity is highly species-specific (Bondarenko et al. 2013; Exbrayat et al. 2015).
MO-NPs in Soil Environment and Its Impact on Soil Habitats
Nanoparticles are introduced into the soil environment during transportation, consumer’s use, and improper disposal (Navarro et al. 2008). The NP toxicity becomes more complex when it combines with the organic and inorganic substances present in the soil matrix. Dissolved or particulate organic matters present in the soil environment may get adsorbed onto the NP surface and influence its ionic character in a number of ways. In humus-rich soil, negatively charged substances are adsorbed onto the MO-NP surface and forms a negative charge bearing complex. The resulting complex increases MO-NP stability by reducing the chances of agglomeration (Ben-Moshe et al. 2010; Fang et al. 2009). Nature of humus, such as hydrophobicity and the capacity to change ionic character of MO-NPs, improves the stability of NPs in soil (Ghosh et al. 2008). Stability of the MO-NPs also influences the NP transportation through the soil matrix (Fig. 3). Aggregation of the MO-NPs make them deposit heavily on the soil surface (Dunphy et al. 2006). Likewise, oppositely charged soil surface and NPs form a conjugate and immobilize the NPs onto the soil surface (Cornelis et al. 2011). As an example, positively charged Al2O3-NPs are relatively less mobile but in phosphate rich soil (negatively charged) mobility increases due to phosphate sorption onto the surface (repulsive force) (Darlington et al. 2009). On the contrary, the electrostatic interaction between the soil surface and the MO-NPs determines the NP mobility through the soil gradient. The identical charge on the NP and soil surfaces create repulsive force that favours NP mobility towards deeper level causing ground water contamination.
Hence, accumulation of MO-NP and their consequent toxicity in soil environment is highly associated with the soil chemistry, composition, and its biological community. Again, the toxicological implications of MO-NPs affect the soil habitats significantly, and thus it has been specially considered (Table 2).
Toxicity of MO-NPs on Living Population
Toxicity of MO-NPs is highly species-specific and depends on its environmental chemistry. Among all industrially used MO-NPs, ZnO and CuO are reported to be most toxic in recent studies. Toxicity of the abundantly used MO-NPs is discussed individually with special focus to the microbial community and plant kingdom.
ZnO-NP
ZnO is one of the most studied and most toxic MO-NPs in the present age. According to Jiang et al. (2009), among ZnO, Al2O3, TiO2, and SiO2-NPs, ZnO is the most toxic causing 100% mortality to E. coli, B. subtilis, and P. flurescens. Effect of ZnO-NP on soil bacterial community also has been studied by Ge et al. (2014). Another study stated that nano-ZnO affects the bacterial taxa associated with nitrogen fixation (order Rhizobiales), methane oxidation (family Methylobacteriaceae), and recalcitrant organic compound decomposition (family Sphingomonadaceae and Streptomycetaceae) (Ge et al. 2012). Arakha et al. (2015) reported the higher inhibition of tested group of bacteria by negatively charged ZnO-NP compared with the positively charged one, which reveals the influence of surface charge on the antimicrobial propensity of the MO-NP.
ZnO induces seed germination inhibition in Zea mays plant and collapses the epidermis and cortex cells in the plant root of Lolium perenne was reported by Lin and Xing (2007, 2008). In aquatic environment, ZnO-NP delays embryo and larvae development in Zebrafish and decreases their hatching rate and survival significantly (Zhu et al. 2008).
CuO-NP
Toxicity-based categorising of the MO-NPs is a difficult task because of their species-specific reactivity. However, according to literature, CuO-NP can be ranked alongside ZnO-NP. In some instances, CuO-NP was reported to be more toxic to beneficial rhizosphere soil isolate P. chlororaphis 06, than ZnO-NP (Dimkpa et al. 2011). Similarly, Baek and An (2011) observed toxicity of CuO-NP to be higher on E. coli, B. subtilis, and S. aureus compared with NiO, ZnO, and Sb2O3-NPs. Kim et al. (2013) studied the soil enzyme activities in CuO-NP treated and untreated soil. A considerable reduction in dehydrogenase, phosphatase and β-glucosidase (62, 80, and ~ 60%, respectively) activity were observed in NP treated soil. However, the toxicity of NPs is highly influenced by plant species and soil characteristics. Frenk et al. (2013) studied the toxicity of CuO-NP on soil bacterial community, which is highly influenced by soil composition. The NP was found to be less toxic in soil samples containing larger clay and organic matter. A similar study has been reported by Ben-Moshe et al. (2013), which states the negative impact of CuO-NP on soil bacterial community. CuO-NP-induced morphological and genetic alterations in leaf litter decomposing fungus have been studied by Pradhan et al. (2011).
TiO2-NP
TiO2 is the most abundant, industrially used NP, reported to be less toxic to a living population compared with ZnO and CuO-NPs. In the presence of ultraviolet irradiation, it releases reactive oxygen species (ROS), which scavenges biomolecules, resulting in cell damage. Generation of ROS is restricted or limited in absence of UV light. Adams et al. (2006) studied the higher toxicity of TiO2-NP on Gram-positive Bacillus subtilis compared with the Gram-negative E. coli. The growth inhibitory effect of TiO2-NP under darkness reveals the involvement of alternative mode of toxicity other than ROS generation. The toxic effect of TiO2-NP on Zea mays plants has been reported by Asli and Neumann (2009). The NP accumulation around the root cell wall resulted in hindrance of hydraulic conductivity of the primary root and induction of water stress in shoot of the young seedlings. However, no intracellular accumulation of NP has been observed.
Other MO-NPs
Other MO-NPs, such as Fe3O4, CeO2, Al2O3, etc., are much less toxic (Fairbairn et al. 2011; Zhu et al. 2008) compared with those already discussed. He et al. (2011) reported the Fe3O4 induced alteration in anthrosol soil bacterial community that consequently influences the soil chemistry and property. Non-toxicity of CeO2-NP has been studied on the sea urchin (Lytechinus pictus) at concentrations up to 10 mg L−1 (Fairbairn et al. 2011). Similarly, non-toxicity of alumina-NP (Al2O3) against Zebrafish embryo has been reported by Zhu et al. (2008).
Mechanism of Toxicity
Three main mechanisms have been implicated for MO-NP-induced toxicity: generation of reactive oxygen species (ROS), dissolution property in aqueous phase, and exertion of oxidative stress.
Generation of ROS is the most common mode of MO-NP toxicity (Boxi et al. 2016; Chakraborty et al. 2016), which reacts with biomolecules, and inhibits the biological system’s ability to detoxify the reactive intermediates or to repair cellular damage. The MO-NP induced extracellular ROS causes oxidative damage to the cell membrane, resulting in severe cellular impairment while the intracellular ROS breaks DNA strands or alters gene expression (Chang et al. 2012).
Dissolution property of MO-NPs play significant role in their individual toxicity, which has already been discussed in section “MO-NPs in aquatic eco-system”. Released metal ions in aqueous medium enter the cell by ion/voltage-gated channels (Colvin et al. 2003) and exert toxicity in different ways, such as: (1) inducing intracellular ROS generation by various chemical reactions, (2) chelating with essential biomolecules, (3) dislodging metal ions in specific metallo-proteins resulting in functional protein inactivation, and (4) increasing metal ion concentration, thus disrupting the cellular metal cation homeostasis (Chang et al. 2012).
Exertion of oxidative stress is another common mechanism of toxicity, especially for the MO-NPs insoluble in aqueous phase (Gurr et al. 2005; Xiong et al. 2011). In the absence of light, when ROS generation is arrested, NP-induced intracellular oxidative stress modifies cellular proteins, lipids, and nucleic acids, stimulating the antioxidant defence system and leading to cell death (Adams et al. 2006; Handy et al. 2008). The mechanistic details of MO-NP toxicity have been schematically presented in Fig. 4.
Conclusions
The attractive physico-chemical properties of the MO-NPs are extensively exploited in multipurpose industries and released to the environment as industrial effluents and consumed nano-products. Discovery and use of some MO-NPs induced massive eco-toxicological and bio-cellular damages have alarmed the scientific community to consciousness. Although some studies have been performed on MO-NP toxicity in recent times, a broad research focusing on eco-toxicological threats remains untouched to date. Much extensive research and critical analysis is needed at a molecular level for better understanding the mechanistic details and species-specific toxicity of MO-NPs. An interdisciplinary approach for developing standard techniques and methodologies for NP toxicity assessment has become mandatory and have to be followed strictly before the utilization of any NP at an industrial scale. The random consumption of these NPs in different industries has to be restricted to protect the environment for our own well-being and future generations.
References
Adams LK, Lyon DY, McIntosh A, Alvarez PJJ (2006) Comparative eco-toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Res 40:3527–3532
Arakha M, Saleem M, Mallick BC, Jha S (2015) The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep 5:9578–9588
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407:1461–1468
Asli S, Neumann M (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Tot Environ 409:1603–1608
Ben-Moshe T, Dror I, Berkowitz B (2010) Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 81:387–393
Ben-Moshe T, Frenk S, Dror I, Minz D, Berkowitz B (2013) Effects of metal oxide nanoparticles on soil properties. Chemosphere 90:640–646
Blinova I, Ivask A, Heinlaan M, Kahru A (2010) Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ Pollut 158:41–47
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Boxi SS, Mukherjee K, Paria S (2016) Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 27:085103
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Canas JE, Qi B, Li S, Maul JD, Cox SB, Das S, Green MJ (2011) Acute and reproductive toxicity of nano-sized metal oxides (ZnO and TiO2) to earthworms (Eisenia fetida). J Environ Monit 13:3351–3357
Chakraborty C, Sharma AR, Sharma G, Lee SS (2016) Zebrafish: a complete animal model to enumerate the nanoparticle toxicity. J Nanobiotechnol 14:65–78
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Choi SJ, Kim RO, Yoon S, Kim WK (2016) Developmental toxicity of Zinc Oxide nanoparticles on Zebrafish (Danio rario): a transcriptomic analysis. PLoS ONE 11:e0160763
Ciacci C, Canonico B, Bilanicova D, Fabbri R, Cortese K, Gallo G, Marcomini A, Pojana G, Canesi L (2012) Immunomodulation by different types of N-oxides in the hemocytes of the marine bivalve Mytilus Galloprovincialis. PLoS ONE 7:e36937
Coleman JG, Johnson DR, Stanley JK, Bednar AJ, Weiss JRCA, Boyd RE, Steevens JA (2010) Assessing the fate and effect of nano aluminium oxide in the terrestrial earthworm, Eisenia fetida. Environ Toxicol Chem 29:1575–1580
Collins D, Luxton T, Kumar N, Shah S, Walker VK (2012) Assessing the impact of copper and zinc oxide nanoparticles on soil: a field study. PLoS ONE 7:e42663
Colvin RA, Fontaine CP, Laskowski M, Thomas D (2003) Zn2+ transporters and Zn2+ homeostasis in neurons. Eur J Pharmacol 479:171–185
Cornelis G, Ryan B, McLaughlin MJ, Kirby JK, Beak D, Chittleborough D (2011) Solubility and batch retention of CeO2 nanoparticles in soils. Environ Sci Technol 45:2777–2782
Darlington TK, Neigh AM, Spencer MT, Guyen OTN, Oldenburg SJ (2009) Nanoparticle characteristics affecting environmental fate and transport through soil. Environ Toxicol Chem 28:1191–1199
Dhand C, Dwivedi N, Loh XJ, Ying ANJ, Verma NK, Beuerman RW, Lakshminarayanan R, Ramakrishna S (2015) Methods and strategies for the synthesis of diverse nanoparticles and their application: a comprehensive overview. RSC Adv 5:105003–105037
Dimkpa CO, Calder A, Britt DW, McLean JE, Anderson AJ (2011) Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions. Environ Pollut 159:1749–1756
Doyle JJ, Ward JE, Mason R (2016) Exposure of bivalve shellfish to titania nanoparticles under an environmental-spill scenario: encounter, ingestion and egestion. J Mar Biol Assoc UK 96:137–149
Drobne D, Jemec A, Pipan Tkalec Z (2009) In vivo screening to determine hazards of nanoparticles: nanosized TiO2. Environ Pollut 157:1157–1164
Dunphy GKA, Finnegan MP, Banfield JF (2006) Influence of surface potential on aggregation and transport of titania nanoparticles. Environ Sci Technol 40:7688–7693
Exbrayat JM, Moudilou EN, Lapied E (2015) Harmful effects of nanoparticles on animals. Nanotechnology. https://doi.org/10.1155/2015/861092
Fairbairn EA, Keller AA, Madler L, Zhou D, Pokhrel S, Cherr GN (2011) Metal oxide nanomaterials in seawater: linking physicochemical characteristics with biological response in sea urchin development. J Hazard Mater 192:1565–1571
Fang J, Shan XQ, Wen B, Lin JM, Owens G (2009) Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environ Pollut 157:1101–1109
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Frenk S, Ben-Moshe T, Dror I, Berkowitz B, Minz D (2013) Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS ONE 8:e84441
Ge Y, Schimel JP, Patricia A, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45:1659–1664
Ge Y, Schimel JP, Holden PA (2012) Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl Environ Microbiol 78:6749–6758
Ge Y, Priester JH, Werfhorst LCVD, Walker SL, Nisbet RM, An YJ, Schimel JP, Torresdey JLG, Holden PA (2014) Soybean plant modify metal oxide nanoparticle effects on soil bacterial community. Environ Sci Technol 48:13489–13496
Ghosh S, Mashayekhi H, Pan B, Bhowmik P, Xing B (2008) Colloidal behaviour of aluminium oxide nanoparticles as affected by pH and natural organic matter. Langmuir 24:12385–12391
Gurr JR, Wang AS, Chen CH, Jan KY (2005) Ultra-fine titanium dioxide particles in the absence of photo-activation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73
Hai-zhou Z, Guang-hua LU, Jun X, Shao-ge J (2012) Toxicity of nanoscale CuO and ZnO to Daphnia magna. Chem Res Chin Univ 28:209–213
Hammond SA, Carew AC, Helbing CC (2013) Evaluation of the effects of titanium dioxide nanoparticles on cultured Rana catesbeiana tailfin tissue. Front Genet 4:1–7
Handy RD, Von der Kammer F, Lead JR, Hassellov M, Owen R, Crane M (2008) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hanna SK, Miller RJ, Lenihan HS (2013) Impact of engineered zinc oxide nanoparticles on the individual performance of Mytilus galloprovincialis. PLoS ONE 8:e61800
He S, Feng Y, Ren H, Zhang Y, Gu N, Lin X (2011) The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J Soils Sediments 11:1408–1417
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316
Hooper HL, Jurkschat K, Morgan AJ, Bailey J, Lawlor AJ, Spurgeon DJ, Svendsen C (2011) Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix. Environ Int 37:1111–1117
Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY (2010) Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 42:586–591
Hu W, Culloty S, Darmody G, Lynch S, Davenport J, Ramirez-Garcia S, Dawson KA, Lynch I, Blasco J, Sheehan D (2014) Toxicity of copper oxide nanoparticles in the Blue Mussel, Mytilus edulis: a redox proteomic investigation. Chemosphere 108:289–299
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2), on algae and daphnids. Environ Sci Pollut Res 13:225–232
Jemec A, Drobne D, Remskar M, Sepcic K, Tisler T (2008) Effects of ingested nano-sized titanium dioxide on terrestrial isopods (Porcellio scaber). Environ Toxicol Chem 27:1904–1914
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano and micro-scaled oxide particles. Environl Pollut 157:1619–1625
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967
Kim S, Sin H, Lee S, Lee I (2013) Influence of metal oxide particles on soil enzyme activity and bioaccumulation of two plants. J Microbiol Biotechnol 23:1279–1286
Kool PL, Diez Ortiz M, Van Gestel CAM (2011) Chronic toxicity of ZnO nanoparticles, non-nano ZnO and ZnCl2 to Folsomia candida (Collembola) in relation to bioavailability in soil. Environ Pollut 159:2713–2719
Lapied E, Nahmani JY, Moudilou E, Chaurand P, Labille J, Rose J, Exbrayat JM, Oughton DH, Joner EJ (2011) Ecotoxicological effects of an aged TiO2 nanocomposite measured as apoptosis in the anecic earthworm Lumbricus terrestris after exposure through water food and soil. Environ Int 37:1105–1110
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Lovern SB, Klaper R (2006) Daphnia magna mortility when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem 25:1132–1137
Lux Research (2008) Nanomaterials state of the market Q3 2008: stealth success, broad impact. Report. https://portal.luxresearchinc.com/research/document_excerpt/3735. Accessed 04 May 2017
Ma H, Bertsch PM, Glenn TC, Kabengi NJ, Williams PL (2009) Toxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegans. Environ Toxicol Chem 28:1324–1330
McShane H, Sarrazin M, Whalen JK, Hendershot WH, Sunahara GI (2012) Reproductive and behavioural responses of earthworms exposed to nano-sized titanium dioxide in soil. Environ Toxicol Chem 31:184–193
Morales-Diaz AB, Ortega-Ortiz H, Juarez-Maldonado A, Cadenas-Pliego G, Gonzalez-Morales S, Benavides-Mendoza A (2017) Application of nanoelements in plant nutrition and its impact in ecosystem. Adv Nat Sci Nanosci Nanotechnol 8:013001
Mortimer M, Kasemets K, Kahru A (2010) Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology 269:182–189
Navarro E, Baun A, Behra R, Hartmann N, Filser J, Miao AJ, Quigg A, Santschi P, Sigg L (2008) Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanoparticle Res 14:1109
Pipan-Tkalec Z, Drobne D, Jemec A, Romih T, Zidar P, Bele M (2010) Zinc bioaccumulation in a terrestrial invertebrate fed a diet treated with particulate ZnO or ZnCl2 solution. Toxicology 269:198–203
Pradhan A, Seena S, Pascoal C, Cássio F (2011) Can metal nanoparticles be a threat to microbial decomposers of plant litter in streams. Microb Ecol 62:58–68
Priester JH, Ge Y, Mielkea RE, Horst AM, Moritz SC, Espinos K, Gelb J, Walker SL, Nisbet RM, An YJ, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL, Holden PA (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. PNAS 109:14734–14735
Roh JY, Park YK, Park K, Choi J (2010) Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol 29:167–172
Sargent JF (2012) Nanotechnology: a policy primer. http://www.fas.org/sgp/crs/misc/RL34511.pdf. Accessed 04 May 2017
Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) (2005) The appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologie. http://europa.eu.int/comm/health/ph_risk/committees/04_scenihr/04_scenihr_en.htm. Accessed 04 May 2017
Siddiqi KS, Husen A (2017) Plant response to engineered metal oxide nanoparticles. Nanoscale Res Lett 12:92–110
Subhaskumar S, Selvanayagam M (2014) First report on: acute toxicity and gill histopathology of fresh water fish Cyprinus carpio exposed to zinc oxide (ZnO) nanoparticle. Int J Sci Res Publ 4:1–4
Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6156
Thit A, Selck H, Bjerregaard HF (2013) Toxicity of CuO nanoparticles and Cu ions to tight epithelial cells from Xenopus laevis (A6): effects on proliferation, cell cycle progression and cell death. Toxicol In Vitro 27:1596–1601
Wang H, Wick RL, Xing B (2009) Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut 157:1171–1177
Warheit D, Webb T, Sayes C, Colvin V, Reed K (2006) Pulmonery instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci 91:227–236
Wehmas LC, Anders C, Chess J, Punnoose A, Pereira CB, Greenwood JA, Tanguaya RL (2015) Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol Rep 2:702–715
Xiong D, Fang T, Yu L, Sima X, Zhu W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Yamamoto A, Honma R, Sumita M, Hanawa T (2004) Cytotixicity evaluation of ceramic particles of different sizes and shapes. J Biomed Mater Res 68A:244–256
Zhao X, Wang S, Wu Y, You H, Lv L (2013) Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aqua Toxicol 136–137:49–59
Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y (2008) Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Env Sci Health Part A 43:278–284
Acknowledgements
KM gratefully acknowledges the support from the UGC, India [Awardee No. F.15-1/2015-17/PDFWM-2015-17-WES-31430(SA-II)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mukherjee, K., Acharya, K. Toxicological Effect of Metal Oxide Nanoparticles on Soil and Aquatic Habitats. Arch Environ Contam Toxicol 75, 175–186 (2018). https://doi.org/10.1007/s00244-018-0519-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0519-9