Abstract
The growing field of nanotechnology and its many applications have led to the irregular release of nanoparticles (NPs), with unintended effects on the environment and continued contamination of water bodies. Metallic NPs are used more frequently in extreme environmental conditions due to their higher efficiency, which attracts more attention in various applications. Due to improper pre-treatment of biosolids, inefficient wastewater treatment practices, and other unregulated agricultural practices continue to contaminate the environment. In particular, the uncontrolled use of NPs in various industrial applications has led to damage to the microbial flora and caused irreplaceable damage to animals and plants. This study focuses on the effect of different doses, types, and compositions of NP on the ecosystem. The review also mentions the impact of various metallic NPs on microbial ecology, their interactions with microorganisms, ecotoxicity studies, and dosage evaluation of the NPs, mainly focused on the review article. However, further research is still needed to understand the complexity of interactions between NPs and microbes in soil and aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles (NPs) have gained significant attention in research due to their versatile applications in various fields, including biomedicine, agriculture, and manufacturing. Their unique properties, such as large surface-to-volume ratios, increased reactivity, and ability to penetrate cell membranes, make them useful in therapeutics, diagnostics, drug delivery, gene therapy, and bioimaging [1]. The widespread use of NPs in various areas makes them vulnerable to being released into the atmosphere, water sources, soil, and waste from landfills. This can lead to the accumulation of NPs in the environment, which can have negative impacts on the ecosystem. Additionally, metal nanoparticles (MNPs) can have antimicrobial properties, which can disrupt the balance of microorganisms in soil and water ecosystems. This can have cascading effects on the food chain and the organisms that depend on it. Metal nanoparticles (MNPs) can have antimicrobial properties, which can disrupt the balance of microorganisms in soil and water ecosystems. Overall, the unique properties of metal nanoparticles make them potentially harmful to the environment and the organisms that depend on it.
The nanomaterials (NM) toxicity study deals with the interaction of manufactured NPs with biological systems and the environment, focusing on the link between the physicochemical properties of NPs and the generation of toxic or hostile biological responses [2]. For toxicology studies, NPs of silver, gold, titanium dioxide, cerium dioxide, iron oxide, alumina, zinc oxide, carbon nanotubes, fullerenes, and nano-C60 have been widely studied in the literature [1].
Understanding the behavior of NMs under different environmental conditions, exposure pathways, and potential health consequences is crucial to mitigate the negative effects of NPs on the environment and human health [3]. When NPs are released into the environment, they can accumulate in various compartments, with exponential growth. Metallic NPs have strong resilience to harsh environments, and their slow dissolution allows them to persist in the environment for longer periods [4]. The terrestrial ecosystem is likely to be the primary repository for NMs discharged into the atmosphere. NPs can be exposed to organisms through various pathways once they are dispersed into the environment. As the presence and interaction of NMs in the environment increase, it is crucial to understand their behavior under different environmental conditions, exposure pathways, and potential health consequences for humans. Recent studies have shown that Ag NPs released into the environment can negatively impact various organisms including bacteria, nematodes, insects, plants, and mammals [5]. Inhalation of Ag NPs in humans can lead to multi-organ damage including liver, spleen, lung, and kidney [6].
Microbes play a crucial role in biogeochemical processes, including nitrogen fixation, sulfur metabolism, phosphorus cycle, carbon cycle, and waste and dead matter [7]. The unavailability of nutrients, organic carbon anthropogenic activity, and the introduction of pollutants such as Metal Oxide Engineered NPs (MO-ENPs) can cause changes in microbial community composition and activity [8]. A previous study revealed that manufactured NMs like QDs, nanowires, nanorods, nanosheets, and nano plastic could be released conditionally during their life cycles [9]. Although the detrimental effects of Ag NP on the soil bacterial population are well known, there is limited data on the effects of functionalization, concentration, exposure length, and soil texture on AgNP affect expression. NPs could break the bacterial membrane and generate ROS, which causes oxidative damage to their DNA [10].
Characterizing MO-ENP effects requires identifying and characterizing affected microbial groups and assessing their community productivity. Data on the effects of metal-engineered NMs (MENMs) exposure on bacteria and changes in their community composition is being studied, but data on other microorganism populations are scarce. MENMs released into the soil ecosystem impact the entire soil community, not just a particular species. Very little is documented about their interaction with the environment, the composition of their community, and the ecosystem functions that are affected. In the present review, we included recent studies on community interaction and soil ecosystem processes affected by exposure to MENMs, risk assessment, and dose assessment for releases of NPS in the soil. Furthermore, how the biogeochemical cycle is negatively affected, and carbon emissions increase due to the altered ratio of involved bacteria to fungi and how impacted trophic transfer and biomagnification within the food web are covered.
Origin of Nanoparticles in the Environment
NPs can be derived from natural and anthropogenic sources. Photochemical reactions, volcanic eruptions, forest fires, weathering, soil erosion, and plant and animal shedding skin and hair are natural processes that produce NPs. Naturally, ambient NPs vary in size, travel thousands of kilometers, and stay in the environment (air) for days. Several million tons of natural NPs are expected to be contained in air dust alone within a year. In addition, various artificial engineering activities contribute to the formation of NM in the environment, such as combustion, fly ash, cooking, chemical manufacturing, vehicle and aircraft exhaust, combustion of pollutants, etc., all contributing to adding NMs to the environment [11].
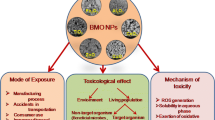
Artificial or anthropogenic NPs are released into the environment unintentionally or intentionally during various industrial, mechanical, and groundwater remediation activities and automobile exhaust. The characteristics, sources of origin, and the fate of metal NPs and their negative impacts on the environment are briefly presented in Fig. 1. New advances and industrial products, processes, and unethical dumping of waste also contribute to the leakage of NMs into the air, water, and soil [7]. The presence of particulate matter in the air, which falls out by gravity and aggregates to reach a specific size, is a significant environmental concern. Water serves as a medium for transportation and temporary reservoir for NPs, with sediments and soil serving as the ultimate recipients of nonvolatile compounds and dissolved particles in the environment [12].
Metal Nanoparticles
Metallic NPs have become the most studied area of research due to their exceptional physicochemical properties and high surface-to-volume ratio compared to their bulk material [13]. Metal monovalent and metal oxide NPs are synthesized by adding reducing/oxidizing precursors [14]. Various factors were responsible for the biochemical activity of NPs, such as size, shape, surface, purity, chemical stability, and the synthetic method adopted [15]. Cu NPs have a biphasic size distribution with broad peaks at 80 and 450 nm. The toxicity of Cu NPs was studied on zebrafish (Danio rerio), and the toxic response of soluble Cu ions (CuSO4) in dechlorinated tap water with a pH of 8.2 and hardness of 142 mg CaCO3/L was also checked and compared [16]. Noble metal NPs (NMNP) have played an important role in biomedicine over the past decades due to their significance in healthcare and personalized diagnostics [13].
Ag NPs and their composites with graphene oxide or carbon nanotubes have shown potential in photothermal therapy when used as Ag core–shell systems [17]. The biocompatibility of noble metal NPs biological systems, such as with cells and tissues, has given them broader applicability in analytics and diagnostic applications [18]. Because of their propensity to adsorb biomolecules, as well as their superior conductivity and stability, Au (gold) and Pt (platinum) NPs are commonly used in the development of novel biosensors and probes [19]. Resulted, NMNPs have been used as immunosensors [20] detection of biomolecules [21] and for nanoprobes to study the pathogenesis and disease progression, in vivo cell imaging, and clinical applications [22]. Despite all these advantages, there are still numerous doubts and arguments about the safety profile of NMNPs and noble metal nano compounds (MNCs) in the human body and microbial ecology.
Toxic Effects of Metal Nanoparticles on the Environment
Ecologists have warned that releasing potent antimicrobials in water streams and soil could negatively affect beneficial microorganisms in natural systems [23]. MNPs can accumulate in organisms over time, leading to long-term toxicity. They can also have an effect on the food chain by reducing the number of organisms at the base of the food chain, which can have cascading effects on the entire ecosystem. MNPs can be toxic to ecosystems because they can be easily taken up by organisms, and their small size allows them to cross cellular membranes and enter cells, where they can cause damage to DNA, proteins, and other cellular structures. The toxicity of MNPs can depend on the type of metal, the size and shape of the particles, and the specific organisms and environment they are found in. Some beneficial microbes affected by NPs and the mechanism of action are briefed in Table 1. Several factors determine a metal’s toxicity, including solubility, binding selectivity to a biological site, and so on [24]. Any functional or morphological alteration of the body caused by a pharmacological, chemical, or biological agent ingested, injected, inhaled, or absorbed is classified as a toxic effect or heavy metal poisoning. Ag NPs are very dangerous for mammalian cells in addition to bacteria [25]. Brain cells [26], liver cells [27], and mammalian stem cells have all been damaged by Ag NPs. Skin diseases such as argyria are caused by continued exposure to colloidal Ag or Ag salt deposited on the skin [28]. The Ag metal harms fish, algae, certain plants, crustaceans, fungi, and bacteria. For example, nitrogen-fixing heterotrophic bacteria and soil-forming chemolithotroph bacteria are affected by Ag metal [23]. NP ecotoxicity studies are rare and difficult to compute. Experimental results from simplified conditions indicate that certain NPs are toxic to many organisms, even in small quantities [29]. Examples include fullerenes NPs, QDs, metals, and metal oxide nanoparticles such as Ag, Cu, ZnO, SiO2, TiO2, and to a lesser extent, carbon nanotube NPs. To determine whether NPs threaten any organism and environment, important information on the dynamics, transfer, and intake influenced by environmental measurements is yet to be ascertained [12].
Effects of Metal Nanoparticles in Bacteria
NPs are commonly used as powerful antibacterial agents because they can be effective at low concentrations [14]. The mechanism of action of NPs on microbes is represented in Fig. 2. The slow dissolution of Ag-NPs affects microbial ecology due to their higher electrostatic attraction and affinity for biomolecules such as sulfur proteins, which allows Ag ions to percolate microbial cell walls and cytoplasmic membranes [30]. Gram-negative bacteria have a thinner cell wall than Gram-positive bacteria; this is why Ag-NPs are more threatening to gram-negative bacteria [31]. Therefore, for antibacterial activity, the uptake of Ag-NPs via adsorption is a prerequisite to quantifying the antibacterial activity of Gram-positive vs. gram-negative. Ag-NPs smaller than 10 nm have long been known to modify cell permeability, percolate bacterial cells, and cause cell harm. In the case of gram-positive bacteria, due to the thick cell wall, NPs may be prevented from penetrating cells and their accumulation in the cytoplasm [31]. Although these NPs have a high potential for destroying pathogenic microorganisms, they can also harm beneficial microflora if mistakenly spilled into the environment.
Nanotoxicity in Soil Ecosystem
In the soil ecosystem, soil bacteria are an integral part of the ecosystem and support various biogeochemical cycles such as nutrient cycling, nitrification, denitrification, carbon and mineral fixation, growth and proliferation of plant and soil species, etc. [49]. Plants, fungi, bacteria, insects, and earthworms make up soil communities, which control soil’s structural and chemical composition [49]. Moreover, they absorb and transform toxic compounds and destabilize them into non-toxic compounds by immobilization. Therefore, it is crucial to study the impact of various NPs on soil ecology carefully. Juliane Filser worked in “realistic environments” where various species are selected (sampled) or long-term studies are undertaken under field conditions [50]. In 2016, a comprehensive analysis of Ag and Cu NPs in the soil ecosystem was conducted. It was concluded that NPs, directly and indirectly, affect the overall soil ecology and soil organisms [51].
According to Filser, the dose that makes NPs an effective antibacterial agent is also lethal to other microbes such as fungi, soil insects, and other soil microflora. For example, Ag NPs with potential antibacterial activity suppress soil microbes’ proliferation at a lower concentration than other heavy metals [50]. Ag NPs also kill more bacteria than fungi, which changes the soil ecosystem [52]. Some fungi/mushrooms that bloom because of this are extremely harmful to other soil creatures. Those who have developed the ability to swallow metals harmlessly and reinject them into the environment have become highly immune to their toxic effects [29].
Earthworms play a vital role in soil health by proliferating, aerating the soil, and transporting organic waste to deeper areas. However, the presence of NPs in the soil can have a detrimental effect on earthworm populations. Typically, a higher concentration of NPs leads to greater damage. However, in recent studies, it has been observed that earthworms exhibit higher activity at low concentrations of NPs. This phenomenon suggests that some organisms can recognize and avoid high concentrations of metallic NP toxicity, but may still be harmed by persistent exposure at low levels. For example, Ag NPs have been found to have no effect at 100 mg/kg, but a negative effect was observed at concentrations of 3–5 mg/kg. This highlights the importance of understanding the impact of NPs at different concentrations in order to effectively manage and mitigate their effects on soil organisms [53]. Many nanoparticles tend to clump together, or “aggregate,” and exhibit different behavior at high concentrations. They do not disperse evenly in soil solutions, instead forming complex structures known as “hetero aggregates” that include bonds with the soil, which can mitigate negative effects. However, metallic NPs such as Cu, Zn, Ag, Ce, MgO, and others have been found to negatively impact microbial health. The mechanisms of toxicity include disruption of cell walls, dissolution through hydrophobic interactions, and release of metal ions within these microorganisms [54][54]. Ag NPs have shown toxic effects on beneficial agricultural microorganisms, such as nitrogen-fixing and ammonifying bacteria and chemolithotroph bacteria in soil communities [55]. These symbiotic bacteria maintain close relationships with leguminous plants, which provides them with a major source of fixed nitrogen supply. However, Ag NPs disturb the nitrification process and thus affect the balance of the ecosystem [53].
Nanotoxicity in Aquatic Ecosystem
In a study conducted in the USA and Europe, Ag-NPs, TiO2-NPs, and ZnO-NPs from sewage treatment have been hazardous to aquatic creatures [49]. In 2015, the negative effect of Ag NPs on exposed zebrafish embryos was observed to alter the expression profiles of neural development-related genes (gfap, huC, and ngn1), metal-responsive metallothioneins, and ABCC genes [37]. The harmful effects of iron oxide NPs on the freshwater alga Mougeotia sp. were mostly due to increased ROS, which depleted the antioxidant defense system, including catalase glutathione reductase and superoxide dismutase enzymes [56].
NPs have been perceived in various organs in marine vertebrates, including muscles, viscera [28], and the brain of Carassius carassius. After being in contact with constituents of the environmental matrix, such as ions, natural colloids, and other charged surfaces, NPs are likely to modify their mobility, aggregation, other properties, and toxicity [12]. In a recent investigation of Ag NPs absorption in zebrafish embryos, the no observed effect concentration (NOEC) was as low as 0.19 nM. Ag NPs impair osmoregulation in fish by disrupting the Na+, K+-ATPase, which aids active Na+ and Cl− uptake [37]. On mammalian germline stem cells, it showed a potent cytotoxic effect. Ag NPs at concentrations of 10 g/mL and higher were found to cause cell necrosis and apoptosis [25].
Nanotoxicity in Terrestrial Ecosystem
Earthworms comprise a considerable portion of the soil biomass (60–80%), and their biology is commonly accepted as an indicator of soil health. They are necessary for the integration and fragmentation of organic detritus and the mineralization and recycling of mineral nutrients [57]. Their burrowing behavior is also important for water purification and erosion stability. Furthermore, in vivo investigations in mice revealed that orally administered PS NPs (50 nm) may pass the GI barrier and enter the circulatory system [58]. PS-NPs have been shown to move through intestinal barrier models in vitro studies [53, 58]. This translocation depends on the characteristics of NPs, such as size, charge, and surface chemistry. In another work, 20, 50, 100, 200, and 500 nm fluorescent PS NPs were coated with d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and evaluated against Caco-2 and MDCK cell lines in the in-vitro model for GI barrier and blood–brain barrier (BBB), respectively. According to the findings, NPs with a diameter of 200 nm or less were able to pass both the GI and BBB barriers [22]. Some extremely hydrophilic NPs, such as poly (butyl cyanoacrylate), poly (lactic-co-glycolic acid), and poly (lactic acid), have been demonstrated to penetrate the BBB [59].
Threats to Humans
Metal nanoparticles can be toxic to humans if inhaled or ingested. They can cause damage to lung tissue and other organs, as well as potentially leading to cancer or other diseases. Long-term exposure to high levels of metal nanoparticles can also have negative effects on the nervous and immune systems. It is important to handle metal nanoparticles with proper safety precautions and to use personal protective equipment when working with them. There are no sets of regulations that address the existence of MPs in seafood, according to information published by the European Food Safety Authority in the year 2016. Besides, the average daily consumption of fish muscle is around 7 g per person per day. Humans could consume roughly five pieces of MPs per day [60]. As such, no research on the toxicity of MPs in humans was found in vivo. However, a recent report verified the presence of MPs in human feces.
Additionally, other research using simulated gut conditions has shown that ingested MP can interact with molecules in the gut, which can cause negative effects [61]. Di (2-ethylhexyl) phthalate (DEHP), a contaminant that is adsorbed at the surface of particles and is slowly released into seawater, has been shown to disappear relatively quickly under simulated gut conditions [28, 62, 63]. MPs eventually had harmful impacts on sex hormone metabolism in human adenocarcinoma cells. A recent cytotoxicity experiment on Au-NPs ranging in size from 0.8 to 15 nm revealed that several human cell types were sensitive to the small gold particles (1.4 nm), with EC50 values for apoptosis within 12 h ranging from 30 to 56 M [64].
Dose Assessment
The toxicity and degradation of the various NPs in the environment are not yet clearly identified. The concentrations of many NPs cannot be estimated with precision due to their small size and composition, their physiochemical characteristics, and the medium in which they are distributed. Therefore, it is crucial to concentrate on identifying and measuring the emissions of NPs in the environment, understanding their life cycle, assessing their toxicity and impact, both short-term and long-term. This is of paramount importance as NPs can persist for extended periods, with half-lives ranging from months to years, due to their high production rates. Additionally, these materials have the potential to accumulate and undergo changes depending on local environmental conditions over time [65].
Although the identification of NPs in natural ecosystems is difficult due to their highly dilute concentration and expensive instrumentation, some studies have been performed under laboratory conditions to determine possible ecotoxicological concentrations under varying environmental conditions. For example, AgNPs widely used in engineering applications at high exposure doses are more toxic to marine life in aquatic environments. The concentration of these NPs has a huge impact on their level of cytotoxicity. Chlorella vulgaris (marine microalgae) is highly toxic to AgNPs at 5 ug/mL. Similarly, Daphnia manga (water flea) showed acute toxicity at 1.8 ug/mL of AgNPs and Danio rerio (zebrafish) at 10.09 µg/mL [66]. Another widely used iron oxide NPs has major side effects on human health. For example, at 400 mg/L Fe3O4 NPs, there was a significant reduction in male sex cell (spermatozoon) motility. Additionally, at concentrations above 400–800 mg/L Fe3O4 NPs, there was a significant increase in oxidative stress molecules (malonaldehyde and total glutathione) [67].
The assessment of risk associated with NPs begins by identifying potential risks and pathways of human exposure in a manufacturing setting, which is a critical area of concern for safety. The initial step is to identify and describe the sources of NPs in the environment. The hazards are then evaluated in terms of their potential effects on different endpoints, and the likelihood of exposure is predicted. To evaluate the relative risk, both the dose–response relationship and the level of exposure are quantified. In the risk assessment process, hazard identification is followed by dose–response assessments, which can be conducted using laboratory studies or mathematical models. However, determining the dose–response relationship for NPs may not be straightforward, as dose based on mass concentration may be less relevant than dose based on surface area, and variations in the fabrication of NPs can lead to differences in surface reactivity and toxicity [12].
The lack of effective regulation on NPs applications and synthesis creates various health-related risks and potential damage to the ecosystem. Government agencies such as the Environmental Policy Agency (EPA) have made substantial efforts to regulate the use of materials and enable safe standard operating procedures for the proper disposal of NP wastes and their use in various applications.
Conclusion
With increasing industrial applications and urbanization, the synthesis and utilization of NPs have increased significantly. The article summarizes the harmful effects of various NPs on soil, aquatic, and terrestrial systems. The unrestricted use may increase the continuous accumulation of NPs in the ecosystem and cause adverse effects through biomagnification and bioaccumulation. Before products enter the market, an effective risk assessment is needed to prevent NP toxicity. Thus, with the implementation of critical measures, proper toxicity assessment, and a more detailed investigation of the release of NPs into the environment, the negative effects of NPs on beneficial microbial communities and human health can be protected. However, there is considerable disagreement in the literature on the toxicological effects of engineered NPs entering the environment. Green synthesis is receiving considerable attention, and developing eco-friendly NPs is one of the central goals of nanotechnology. The future of nanotechnology depends on the development of eco-friendly NP synthesis. It also focuses on the development of technologies to improve the quantification of NPs. It is important to pay more attention to the long-term toxicity of any NP before it reaches the environment.
Data Availability
The data will be available based on a request.
References
Erkekoglu, P., & Kocer-Gumusel, B. (2018). Toxicity assessment of nanopharmaceuticals. In Inorganic Frameworks as Smart Nanomedicines, pp. 565–603.
Sharifi, S., Behzadi, S., Laurent, S., Forrest, M. L., Stroeve, P., & Mahmoudi, M. (2012). Toxicity of nanomaterials. Chemical Society Reviews, 41(6), 2323–2343.
Brunelli, A., Calgaro, L., Semenzin, E., Cazzagon, V., Giubilato, E., Marcomini, A., & Badetti, E. (2021). Leaching of nanoparticles from nano-enabled products for the protection of cultural heritage surfaces: A review. Environmental Sciences Europe, 33(1), 1–19.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Hayyan, A., & Ibrahim, S. (2016). Environmental application of nanotechnology: Air, soil, and water. Environmental Science and Pollution Research, 23(14), 13754–13788.
Tortella, G. R., Rubilar, O., Durán, N., Diez, M. C., Martínez, M., Parada, J., & Seabra, A. B. (2020). Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. Journal of Hazardous Materials, 390(121974), 6.
Durán, N., Silveira, C. P., Durán, M., & Martinez, D. S. T. (2015). Silver nanoparticle protein corona and toxicity: A mini-review. J Nanobiotechnology, 13(1), 55.
Smita, S., Gupta, S. K., Bartonova, A., Dusinska, M., Gutleb, A. C., & Rahman, Q. (2012). Nanoparticles in the environment: Assessment using the causal diagram approach. Environmental Health, 11(1), 1–11.
Becker, J. M., Ganatra, A. A., Kandie, F., Mühlbauer, L., Ahlheim, J., Brack, W., Torto, B., Agola, E. L., McOdimba, F., Hollert, H., Fillinger, U., & Liess, M. (2020). Pesticide pollution in freshwater paves the way for schistosomiasis transmission. Scientific reports, 10(1), 1–13.
Arvidsson, R., Baun, A., Furberg, A., Hansen, S. F., & Molander, S. (2018). Proxy measures for simplified environmental assessment of manufactured nanomaterials. Environmental Science & Technology, 52(23), 13670–13680.
Abdal Dayem, A., Hossain, M. K., Lee, S. B., Kim, K., Saha, S. K., Yang, G. M., Choi, H. Y., & Cho, S. G. (2017). The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. International journal of molecular sciences, 18(1), 120.
Kabra, A. N., Ji, M. K., Choi, J., Kim, J. R., Govindwar, S. P., & Jeon, B. H. (2014). Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonas mexicana. Environmental Science and Pollution Research, 21(21), 12270–12278.
Lapied, E., Moudilou, E., Exbrayat, J. M., Oughton, D. H., & Joner, E. J. (2010). Silver nanoparticle exposure causes apoptotic response in the earthworm Lumbricus terrestris (Oligochaeta). Nanomedicine, 5(6), 975–984.
Habibullah, G., Viktorova, J., & Ruml, T. (2021). C urrent strategies for noble metal nanoparticle synthesis. Nanoscale Research Letters, 16(1), 1–12.
Sanchez-Dominguez, M., Boutonnet, M., & Solans, C. (2009). A novel approach to metal and metal oxide nanoparticle synthesis: The oil-in-water microemulsion reaction method. Journal of Nanoparticle Research, 11(7), 1823–1829.
Teske, S. S., & Detweiler, C. S. (2015). The biomechanisms of metal and metal-oxide nanoparticles’ interactions with cells. International Journal of Environmental Research and Public Health, 12(2), 1112–1134.
Cioffi, N., Ditaranto, N., Torsi, L., Picca, R. A., Sabbatini, L., Valentini, A., Novello, L., Tantillo,G., Bleve-Zacheo, T., & Zambonin, P. G. (2005). Analytical characterization of bioactive fluoropolymer ultra-thin coatings modified by copper nanoparticles. Analytical and bioanalytical chemistry, 381(3), 607–616.
Behnam, M. A., Emami, F., Sobhani, Z., Koohi-Hosseinabadi, O., Dehghanian, A. R., Zebarjad, S. M., Moghim, M. H., & Oryan, A. (2018). Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Advanced pharmaceutical bulletin, 8(1), 49.
Darabdhara, G., Das, M. R., Singh, S. P., Rengan, A. K., Szunerits, S., & Boukherroub, R. (2019). Ag and Au nanoparticles/reduced graphene oxide composite materials: Synthesis and application in diagnostics and therapeutics. Advances in Colloid and Interface Science, 271, 101991.
Naresh, V., & Lee, N. (2021). A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors, 21(4), 1109.
Doria, G., Conde, J., Veigas, B., Giestas, L., Almeida, C., Assunção, M., Rosa J., & Baptista, P. V. (2012). Noble metal nanoparticles for biosensing applications. Sensors, 12(2), 1657–1687.
Zhao, X., Zhao, H., Yan, L., Li, N., Shi, J., & Jiang, C. (2020). Recent developments in detection using noble metal nanoparticles. Critical Reviews in Analytical Chemistry, 50(2), 97–110.
Azharuddin, M., Zhu, G. H., Das, D., Ozgur, E., Uzun, L., Turner, A. P., & Patra, H. K. (2019). A repertoire of biomedical applications of noble metal nanoparticles. Chemical Communications, 55(49), 6964–6996.
Rana, S., & Kalaichelvan, P. (2013). 2013. International Scholarly Research Notices: Ecotoxicity of nanoparticles.
Rousk, J., Ackermann, K., Curling, S. F., & Jones, D. L. (2012). Comparative toxicity of nanoparticulate CuO and ZnO to soil bacterial communities. PLoS One, 7(3), e34197.
Braydich-Stolle, L. K., Lucas, B., Schrand, A., Murdock, R. C., Lee, T., Schlager, J. J., Hussain, S. M., & Hofmann, M. C. (2010). Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicological sciences, 116(2), 577–589.
Maqbool, Z., Hussain, S., Imran, M., Mahmood, F., Shahzad, T., Ahmed, Z., Azeem, F., & Muzammil, S. (2016). Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: A critical review. Environmental Science and Pollution Research, 23(17), 16904–16925.
Hussain, S. M., Hess, K. L., Gearhart, J. M., Geiss, K. T., & Schlager, J. J. (2005). In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in Vitro, 19(7), 975–983.
Feng, Y., Cui, X., He, S., Dong, G., Chen, M., Wang, J., & Lin, X. (2013). The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. EnviRonmental Science & Technology, 47(16), 9496–9504.
Hyde, K. D., Xu, J., Rapior, S., Jeewon, R., Lumyong, S., Niego, A. G. T., Abeywickrama, P. D., Aluthmuhandiram, J. V. S., Brahamanage, R. S., Brooks, S., Chaiyasen, A., Chethana, K. W. T., Chomnunti, P., Chepkirui, C., Chuankid, B., de Silva, N. I., Doilom, M., Faulds, C., Gentekaki, E., Gopalan, V.,Kakumyan, P., Harishchandra, D., Hemachandran, H., Hongsanan, S., Karunarathna, A.,Karunarathna, S. C., Khan, S., Kumla, J., Jayawardena, R. S., Liu, J. K., Liu, N., Luangharn, T., Macabeo, A P G., Marasinghe, D. S., Meeks, D., Mortimer, P E., Mueller, P., Nadir, S., Nataraja, K N., Nontachaiyapoom, S., Brien, M. O., Penkhrue, W., Phukhamsakda, C., Ramanan, U. S., Rathnayaka, A. R., Sadaba, R B., Sandargo, B., Samarakoon, B C., Tennakoon, D S., Siva, R., Sriprom, W., Suryanarayanan, T. S., Sujarit, K., Suwannarach, N., Suwunwong, T., Thongbai, B., Thongklang, N., Wei, D., Wijesinghe, S. N., Winiski, J., Yan, J., Yasanthika, E., & Stadler, M. (2019). The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diversity, 97(1), 1–136.
Wang, L., Hu, C., & Shao, L. (2017). The antimicrobial activity of nanoparticles: Present situation and prospects for the future. International Journal of Nanomedicine, 12, 1227.
Slavin, Y. N., Asnis, J., Häfeli, U. O., & Bach, H. (2017). Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology, 15(1), 1–20.
Haan, N. L., Zhang, Y., & Landis, D. A. (2020). Predicting landscape configuration effects on agricultural pest suppression. Trends in Ecology & Evolution, 35(2), 175–186.
Huang, J., Chong, C. A. O., Runqing, L. I., & Wenzhu, G. U. A. N. (2018). Effects of silver nanoparticles on soil ammonia-oxidizing microorganisms under temperatures of 25 and 5 C. Pedosphere, 28(4), 607–616.
VandeVoort, A. R., & Arai, Y. (2018). Macroscopic observation of soil nitrification kinetics impacted by copper nanoparticles: Implications for micronutrient nanofertilizer. Nanomaterials, 8(11), 927.
Pérez-Lucas, G., El Aatik, A., Vela, N., Fenoll, J., & Navarro, S. (2021). Exogenous organic matter as strategy to reduce pesticide leaching through the soil. Archives of Agronomy and Soil Science, 67(7), 934–945.
Chen, Z., Gao, S. H., Jin, M., Sun, S., Lu, J., Yang, P., Philip L Bond P. L.., Yuan Z., & Guo, J. (2019). Physiological and transcriptomic analyses reveal CuO nanoparticle inhibition of anabolic and catabolic activities of sulfate-reducing bacterium. Environment international, 125, 65–74.
Ma, Q., Zhang, Q., Yang, S., Yilihamu, A., Shi, M., Ouyang, B., Guan X., & Yang, S. T. (2020). Toxicity of nanodiamonds to white rot fungi Phanerochaete chrysosporium through oxidative stress. Colloids and Surfaces B: Biointerfaces, 187, 110658.
Du, J., Zhang, Y., Yin, Y., Zhang, J., Ma, H., Li, K., & Wan, N. (2020). Do environmental concentrations of zinc oxide nanoparticle pose ecotoxicological risk to aquatic fungi associated with leaf litter decomposition? Water Research, 178, 115840.
Arciniegas-Grijalba, P. A., Patiño-Portela, M. C., Mosquera-Sánchez, L. P., Sierra, B. G., Muñoz-Florez, J. E., Erazo-Castillo, L. A., & Rodríguez-Páez, J. E. (2019). ZnO-based nanofungicides: Synthesis, characterization and their effect on the coffee fungi Mycena citricolor and Colletotrichum sp. Materials Science and Engineering: C, 98, 808–825.
Chaves-Lopez, C., Nguyen, H. N., Oliveira, R. C., Nadres, E. T., Paparella, A., & Rodrigues, D. F. (2018). A morphological, enzymatic and metabolic approach to elucidate apoptotic-like cell death in fungi exposed to h-and α-molybdenum trioxide nanoparticles. Nanoscale, 10(44), 20702–20716.
Noori, A., White, J. C., & Newman, L. A. (2017). Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. Journal of Nanoparticle Research, 19(2), 1–13.
Priyanka, K. P., Harikumar, V. S., Balakrishna, K. M., & Varghese, T. (2017). Inhibitory effect of TiO2 NPs on symbiotic arbuscular mycorrhizal fungi in plant roots. IET nanobiotechnology, 11(1), 66–70.
Li, S., Liu, X. Q., Wang, F. Y., & Miao, Y. F. (2015). Effects of, ZnO nanoparticles ZnSO and arbuscular mycorrhizal fungus on the growth of maize. Huan Jing ke Xue= Huanjing Kexue, 36(12), 4615–4622.
Wang, F., Liu, X., Shi, Z., Tong, R., Adams, C. A., & Shi, X. (2016). Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants–a soil microcosm experiment. Chemosphere, 147, 88–97.
Rhiem, S., Riding, M. J., Baumgartner, W., Martin, F. L., Semple, K. T., Jones, K. C., Schäffer A., & Maes, H. M. (2015). Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells. Environmental Pollution, 196, 431–439.
Pereira, J. L., Antunes, S. C., Castro, B. B., Marques, C. R., Gonçalves, A. M., Gonçalves, F., & Pereira, R. (2009). Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: Commercial formulation versus active ingredient. Ecotoxicology, 18(4), 455–463.
Abd-Alla, M. H., Nafady, N. A., & Khalaf, D. M. (2016). Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agriculture, Ecosystems & Environment, 218, 163–177.
Sarabia-Castillo, C. R., & Fernández-Luqueño, F. (2016). TiO2, ZnO, and Fe2O3 nanoparticles effect on Rhizobium leguminosarum-Pisum sativum L. symbiosis. 3rd Biotechnology Summit 2016, Ciudad Obregón, Sonora, Mexico, 24–28 October 2016, 144–149.
Rajmohan, K. S., Chandrasekaran, R., & Varjani, S. (2020). A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian Journal of Microbiology, 60(2), 125–138.
Wilson, N. (2018). Nanoparticles: Environmental problems or problem solvers? BioScience, 68(4), 241–246.
McKee, M. S., & Filser, J. (2016). Impacts of metal-based engineered nanomaterials on soil communities. Environmental Science: Nano, 3(3), 506–533.
Shah, G. M., Amin, M., Shahid, M., Ahmad, I., Khalid, S., Abbas, G., Imran, M., Naeem MA., & Shahid, N. (2022). Toxicity of ZnO and Fe2O3 nano-agro-chemicals to soil microbial activities, nitrogen utilization, and associated human health risks. Environmental Sciences Europe, 34(1), 1–12.
Ferdous, Z., & Nemmar, A. (2020). Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. International Journal of Molecular Sciences, 21(7), 2375.
Hwang, G., Ahn, I. S., Mhin, B. J., & Kim, J. Y. (2012). Adhesion of nano-sized particles to the surface of bacteria: Mechanistic study with the extended DLVO theory. Colloids and Surfaces B: Biointerfaces, 97, 138–144.
Masrahi, A., VandeVoort, A. R., & Arai, Y. (2014). Effects of silver nanoparticle on soil-nitrification processes. Archives of Environmental Contamination and Toxicology, 66(4), 504–513.
Jagadeesh, E., Khan, B., Chandran, P., & Khan, S. S. (2015). Toxic potential of iron oxide, CdS/Ag2S composite, CdS and Ag2S NPs on a fresh water alga Mougeotia sp. Colloids and Surfaces B: Biointerfaces, 125, 284–290.
Medina-Sauza, R. M., Álvarez-Jiménez, M., Delhal, A., Reverchon, F., Blouin, M., Guerrero-Analco, J. A., ... & Barois, I. (2019). Earthworms building up soil microbiota, a review. Frontiers in Environmental Science, 7, 81.
Walczak, A. P., Kramer, E., Hendriksen, P. J., Tromp, P., Helsper, J. P., van der Zande, M., ... & Bouwmeester, H. (2015). Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology, 9(4), 453–461.
Zhou, Y., Peng, Z., Seven, E. S., & Leblanc, R. M. (2018). Crossing the blood-brain barrier with nanoparticles. Journal of Controlled Release, 270, 290–303.
Abbasi, S., Soltani, N., Keshavarzi, B., Moore, F., Turner, A., & Hassanaghaei, M. (2018). Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere, 205, 80–87.
Vandermeersch, G., Van Cauwenberghe, L., Janssen, C. R., Marques, A., Granby, K., Fait, G., Kotterman, M. J. J., Diogène, J., Bekaert, K., Robbens J., & Devriese, L. (2015). A critical view on microplastic quantification in aquatic organisms. Environmental Research, 143, 46–55.
Bakir, A., Rowland, S. J., & Thompson, R. C. (2014). Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environmental Pollution, 185, 16–23.
Rowdhwal, S. S. S., & Chen, J. (2018). Toxic effects of di-2-ethylhexyl phthalate: An overview. BioMed research international, 2018.
Pan, Y., Neuss, S., Leifert, A., Fischler, M., Wen, F., Simon, U., Schmid, G., Brandau, W., & Jahnen‐Dechent, W. (2007). Size‐dependent cytotoxicity of gold nanoparticles. Small, 3(11), 1941–1949.
Hardman, R. (2006). A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environmental Health Perspectives, 114(2), 165–172.
Kumar, C. V., Karthick, V., Kumar, V. G., Inbakandan, D., Rene, E. R., Suganya, K. U., Embrandiri, A., Dhas T. S., Ravi M., & Sowmiya, P. (2022). The impact of engineered nanomaterials on the environment: Release mechanism, toxicity, transformation, and remediation. Environmental Research, 212, 113202.
Özgür, M. E., Ulu, A., Balcıoğlu, S., Özcan, I., Köytepe, S., & Ateş, B. (2018). The toxicity assessment of iron oxide (Fe3O4) nanoparticles on physical and biochemical quality of rainbow trout spermatozoon. Toxics, 6(4), 62.
Author information
Authors and Affiliations
Contributions
J.R.: conceptualize the article, write the original draft, review, and edit the whole manuscript. V.K.: write the original draft, review, and edit the whole manuscript. P.A.: review the manuscript, design the artwork, and edit the whole manuscript. R.T.: review the manuscript, design the artwork, and edit the whole manuscript. R.K. and L.K.T.: review the manuscript, design the artwork, and edit the whole manuscript. S.D.: review the manuscript and artwork, and edit the whole manuscript. PKG: supervise, plan, design, and execute the idea of writing of the whole manuscript. All authors have read and approved the draft of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate.
Not applicable.
Consent for Publication.
All authors have read the manuscript and agreed to publish it in the journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rawat, J., Kumar, V., Ahlawat, P. et al. Current Trends on the Effects of Metal-Based Nanoparticles on Microbial Ecology. Appl Biochem Biotechnol 195, 6168–6182 (2023). https://doi.org/10.1007/s12010-023-04386-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04386-0