Abstract
Cystinuria continues to be one of the most challenging stone diseases. During the latest decades our knowledge of the molecular basis of cystinuria has expanded. Today 160 different mutations in the SLC3A1 gene and 116 in the SLC7A9 gene are listed. The full implications of type A, B or AB status are not yet fully understood but may have implications for prognosis, management and treatment. Despite better understanding of the molecular basis of cystinuria the principles of recurrence prevention have remained essentially the same through decades. No curative treatment of cystinuria exists, and patients will have a life long risk of stone formation, repeated surgery, impaired renal function and quality of life. Therapy to reduce stone formation is directed towards lowering urine cystine concentration and increasing cystine solubility. Different molecules that could play a role in promoting nucleation and have a modulating effect on cystine solubility may represent new targets for cystinuria research. Investigation of newer thiol-containing drugs with fewer adverse effects is also warranted. Determining cystine capacity may be an effective tool to monitor the individual patient’s response. Compliance in cystinuric patients concerning both dietary and pharmacological intervention is poor. Frequent clinical follow-up visits in dedicated centres seem to improve compliance. Cystinuric patients should be managed in dedicated centres offering the complete range of minimal invasive treatment modalities, enabling a personalized treatment approach in order to reduce risk and morbidity of multiple procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
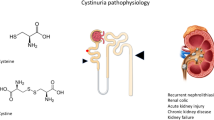
Cystinuria continues to be one of the most challenging stone diseases. Stone formation is due to mutations in the genes that encode the two subunits of the amino acid transport, resulting in failure of absorption of filtered dibasic amino acids including cystine in the proximal tubules [1]. Since cystine has a very limited solubility in the physiological range of urine pH, this leads to complicated and recurrent kidney stone formation. The stone disease starts early in life, often necessitating multiple interventions to prevent stone-related complications [2]. Cystinuria represents approximately 1 % of adult and 3–10 % of paediatric stone disease [3–8]. The worldwide prevalence of cystinuria is approximately 1 in 7000 neonates, ranging from approximately 1:2000 in the Mediterranean East Coast to 1:100,000 in Sweden [8–10].
Therapy to reduce stone formation is directed towards lowering urine cystine concentration and increasing cystine solubility, which is often demanding due to a lack of effective pharmacologic measures. A multimodal approach to diagnosis, surgical and medical treatment is mandatory for optimal management of cystinuric patients. During the latest decades our knowledge of the molecular basis of cystinuria has expanded, and new minimal invasive treatment modalities have evolved, enabling a personalized approach to stone management. This review will summarize current understanding of cystinuria, focusing on how patients with cystine stone disease should be evaluated and treated in the twenty-first century.
Genetic basis of cystinuria
The inheritance pattern of cystinuria is complex. Some patients show autosomal recessive inheritance. However, among heterozygotes the urinary excretion of cystine can vary considerably and stone formation is seen suggesting autosomal dominant inheritance with incomplete penetrance in some families [9]. Historically, different subtypes were classified according to the excretion pattern of cystine in obligate heterozygotes (parents of patients). Type I was characterised by a normal excretion pattern while non-type I were characterised by urinary hyper-excretion of cystine, sometimes resulting in stone formation [11]. The normal rate of cystine excretion is up to 30 mg/day. Homozygotes usually excrete more than 400 mg/day. Non-type I heterozygotes excrete 200–400 mg/dag while type I heterozygotes excrete <200 mg/day [12]. The traditional classification was imprecise, and a new classification based on the affected genes SLC3A1 and SLC7A9 was made by Della Strologo et al. [11].
Cystinuria type A (formerly type I) is caused by mutations in the SLC3A1 gene located on chromosome 2. The SLC3A1 gene encodes the heavy subunit of the renal amino acid transporter (rBAT), which is needed to localize the transporter to the plasma membrane [13]. The mode of inheritance appears to be truly autosomal recessive and the penetrance is high (increased urinary cystine excretion: 100 %; stone formation: 94 %) [14]. An affected person has two faulty copies of the gene and each of the parents carries one copy. Carriers usually have normal level of urine cystine and they have no increased risk of developing urolithiasis compared with the general population [13]. In an Italian study of 125 patients, the frequency of type A was found to be 45 % [11]. This is in agreement with a study concerning 164 probands from the International Cystinuria Consortium (ICC) [15]. In a UK cohort of 74 patients 55 % had cystinuria type A [16]. Predominance of SLC3A1 (64 %) was also found in a study of 93 south-eastern European patients [17].
Cystinuria type B (formerly non-type I) is caused by mutations in the SLC7A9 gene located on chromosome 19. The SLC7A9 gene encodes the light subunit of the renal amino acid transporter (b0, +AT), which compromises the catalytic, transporting component [13]. The mode of inheritance seems to be autosomal recessive or autosomal dominant with incomplete penetrance [18]. For homozygotes the penetrance is similar to type A [14]. Carriers such as parents, siblings and children often have raised urine cystine levels (86–90 % of cases) and stone formation is reported in 2–18 % of cases [11, 14]. In the previous mentioned Italian study and the study of ICC probands, the frequency of type B was found to be 53 and 56 %, respectively [11, 15]. The UK study reported a type B frequency of 31 % in their study population [16]. This is in line with the frequency found in the study of south-eastern European patients [17].
In more rare cases the patient has mutations in both genes (SLC3A1 + SLC7A9). This subgroup is classified as cystinuria type AB. The frequency is reported to be 1.2–4 % [11, 15, 16].
Cystinuria is also associated with two deletion syndromes (2p21 microdeletion syndrome and hypotonia cystinuria syndrome). These patients have large DNA deletions that remove not only the SLC3A1 gene but one or more neighbouring genes as well. Each syndrome has a distinct phenotype with severity reflecting the number of genes affected [13, 14].
Today 160 different mutations in the SLC3A1 gene and 116 in the SLC7A9 gene are listed in the Human Genome Mutation Database (http://www.hgmd.cf.ac.uk; accessed on April 2015). Most of the mutations are point mutations (nonsense/missense), but splicing mutations, deletions, duplications and complex rearrangements also have been described. The prevalence of some mutations varies significantly between ethnic groups [13, 17]. In most studies the detection rates for mutations in SCLC7A9 and SLC3A1 do not reach 100 % [2, 11, 16, 17]. Therefore, it has been suggested that mutations in other genes not yet discovered may be involved in cystinuria [2, 16, 17]. A part of the explanation may be that mutations in the two known genes are missed with the methods used.
Genotype–phenotype correlation
So far no clinical differences (age of presentation, number of stone episodes, interventions) have been found between type A and type B cystinuria [2, 11, 16]. In one study type AB patients were found to present with a later onset of disease compared with type A and type B patients, but this is based on very few patient reports [16]. Another study has showed that the presence of two mutations in the SLC7A9 gene results in a significantly earlier age of onset compared to one mutation [19]. In the majority of patients stone formation occurs before the age of 20, but a broad inter- and intra familial variation exists [11, 14, 16–18]. One study reported that males have more severe disease than females [11]. This is in contrast to a study from 2013, where no clinical differences between genders were found [17].
The biochemical phenotype has been correlated to the genotype in a few studies. One study showed that patients with at least one missense mutation in SLC3A1 had lower levels of urinary lysine, arginine and ornithine than patients with all other type of SLC3A1 mutations. There was, however, no difference in urinary cystine levels. The authors hypothesize that the lower levels of dibasic amino acids is due to a less severe effect of missense mutations compared to other types of mutations [16]. Another study found that homozygote patients for a specific mutation (T216M) in the SLC3A1 gene have significantly higher values of cystine and other dibasic amino compared to patients with other mutations [17].
The full implications of type A, B or AB status are not yet fully understood, although implications for prognosis, management and treatment have been suggested. The knowledge of the genotype may allow for predictive testing and early preventive care. Furthermore, the genotype is important for estimating recurrence risk in the family.
Diagnosis
An early and correct diagnosis is essential for successful treatment of cystinuric patients. Assessment in stone formers includes medical history, imaging, stone analysis and laboratory investigations. Cystinuria should be suspected in patients younger than 30 years with recurrent, large or bilateral stones, and in patients having siblings with stone disease. Cystinuria should also be suspected if a sulphur odour is noticed during laser lithotripsy. Diagnosis is established primarily by stone analysis and/or quantitative 24 h urine analysis. Genotyping is not performed routinely.
Stone analysis
Stone analysis should be performed whenever possible. Pure cystine stones represent the majority of cases (80 %). Mixtures of cystine and calcium oxalate, calcium phosphate or magnesium ammonium calcium phosphate have also been described [20]. Cystine stones are usually uniform, pale yellow stones having rough or smooth surfaces.
Laboratory investigations
Microscopic evaluation of morning urine might reveal the pathognomonic hexagonal cystine crystals in 25 % of cystinuric patients [8, 12, 21]. The cyanide–nitroprusside colorimetric quantitative test detects cystine at a threshold concentration of 75 mg/l, displaying a purple colour change when the sulfhydryl groups formed via interaction between cyanide and cystine react with nitroprusside [22–24]. The sensitivity and specificity are 72 and 95 %, respectively. The test is false positive in patients with homocystinuria, Fanconi’s syndrome and in patients taking ampicillin or sulpha-containing drugs [22, 24], and may also be positive in heterozygote carriers of cystinuria. A quantitative chromatographic analysis of a 24 h collected urine-sample confirms the diagnosis. The normal rate of cystine excretion is 0.13 mmol/day, whereas homozygotes usually excrete more than 1.7 mmol/day [12]. Heterozygotes may excrete normal or slightly elevated amounts of cystine [25].
The presence of other metabolic abnormalities such as hypercalciuria, hypocitraturia and hyperuricosuria will also be revealed using the chromatographic urine analysis.
Imaging
Cystine stones are weak radiopaque due to their sulphur content but less radiodense than calcium stones, and they may be difficult to detect on plain X-ray (kidney, ureter, and bladder radiograph: (KUB)). In one study 88 % of cystine stones were visualized by KUB [26]. Stone size and attenuation value expressed as Hounsfield units (HU) were not significantly different between visualized and not visualized stones, but the body mass index was significantly higher in the not visualized group [26]. The HU value of cystine stone varies. In the study by Patel et al. the majority (80 %) had a HU < 550 (424 ± 106 HU), while a distinct second group (20 %) had a HU > 850 (972 ± 134 HU) [26]. A low HU of 350–650 may support the suspicion of cystine stones, but there is significant overlapping between stones of different composition, especially for cystine and uric acid stones, making a definitive differentiation more challenging [27–29]. Dual-energy CT and automated image processing may improve the differentiation [30, 31].
CT scanning may also be clinically helpful in predicting fragility of the stone by evaluating morphology of the stone. The smooth subtypes are more resistant to fragmentation due to an irregular, interlacing crystal structure [32–34]. Renal ultrasonography is less accurate but represents the mainstay for follow-up of cystine stone formers to avoid frequent exposure to ionized radiation.
Surgical management
Despite aggressive medical therapy, cystinuric patients are likely to suffer frequent recurrent episodes of stones necessitating urologic intervention [35]. Surgical management does not differ significantly from other urinary tract stones [12]. However, cystinuric patients will often have experienced previous invasive procedures and high recurrence rates, demanding use of the least invasive methods to minimize the potential complications and morbidities of multiple procedures [2]. On the other hand, since residual fragments after stone treatment in cystinuric patients sooner or later will result in symptomatic stone disease, complete stone clearance is of upmost importance. Thus, the surgical challenge in cystinuria is to find the balance between the safety of the procedures and the need for complete stone clearance [36]. Contemporary treatment modalities include chemolysis, Shock Wave Lithotripsy (SWL), ureterorenoscopic stone removal (URS), percutaneous nephrolithotomy (PNL), and open or laparoscopic surgery.
Chemolysis
No systemic therapies can effectively dissolute cystine stones. Cystine is highly insoluble in the physiological range of urine pH. A significant increase in cystine solubility begins, when pH exceeds 7.0 [37]. Chemolytic dissolution of cystine stones may be done with the highly alkaline solution THAM (tris-hydroxymethyl-amminomethane) or the chelating solution acetylcysteine 2 %, or preferably the two solutions in combination creating a synergistic effect [35, 38]. During installation of chemolytic agents, prophylactic antibiotics are mandatory, and intra-pelvic pressure should be monitored to avoid pyelovenous and pyelolymphatic backflow that may result in septic or agent-specific toxic complications and alterations in serum chemistries. Instillation therapy for cystine stones are normally performed as secondary chemolysis for residual fragments after SWL, PCNL or URS, since chemolysis monotherapy of most cystine stones will require long periods of irrigation [35, 37, 39]. Due to advances in minimal invasive procedures chemolytic procedures are only rarely needed [2].
Shock wave lithotripsy (SWL)
Cystine calculi are generally considered SWL resistant. Pre-treatment imaging may, however, reliably predict a positive outcome of SWL. It has long been recognized that cystine stones present in two morphologically different types, and that stones with a “rough” morphology fragments more easily with shock waves than stones with “smooth” morphology [32]. The “rough” stones have large, blocky crystals at their surface, while the “smooth” stones have smaller crystals appearing paler in colour [32, 34]. Unfortunately, these qualities of the stones cannot be observed in the patients at diagnosis [33]. Cystine stones with “rough” morphology do, however, tend to contain internal void regions at NCCT, and it has been shown in an in vitro study that this is an indicator of fragility in SWL [34]. On the contrary, cystine stones appearing homogeneous by CT were resistant to shock waves [34]. Thus, patients suitable for SWL may be selected by the appearance of the stone on NCCT (Fig. 1).
It is the impression of the authors that cystine stones in the early course of a recurrence often are heterogeneous with void regions, highlighting the importance of regular follow-up, in order to be able to offer the patients the least invasive treatment options. Furthermore, it has been suggested that oral thiol therapy might produce more fragile calculi, which consequently may be more suitable for SWL [12, 40].
Ureterorenoscopic stone removal (URS)
Retrograde ureteroscopic stone management is a suitable treatment option for the majority of ureteral and pelvicalyceal stones [2, 36]. With development of smaller sized flexible endoscopes, this procedure also may be performed safely even in prepubertal children [41]. Holmium-YAG laser is the preferred method for stone disintegration, since it will allow usage of energy settings for both fragmentation and dusting, enabling an individualized approach to lithotripsy [42]. URS for management of recurrent cystine stones has been shown to be both efficient and safe. In 15 of 21 cystine stones [mean size 11 mm (range 5–30 mm)] complete stone clearance (71 %) was achieved in one setting with no major complications [43]. The limiting factor for stone clearance was size and not number of stones [43]. If patients accept that the retrograde procedure may become staged (more than one intervention), even stones larger than 2 cm may be safely approached retrogradely [44]. Since cystinuric patients usually will suffer from multiple recurrences, it is of upmost importance that all safety aspects of ureterorenoscopy are observed. If an access sheath is considered, the smallest size that allows passage of the ureteroscope should be used [45], in order to reduce risk of ureteral damage [46], since development of a ureteral stricture in these patients will harbour very high risk of losing a significant amount of nephrons. Intrarenal pressure should be kept as low as possible to reduce pyelovenous and pyelolymphatic backflow, thereby minimizing renal parenchymal damage and septic complications [47]. Also, if a JJ-catheter is considered postoperatively, indwelling time should be minimized to reduce the risk and morbidity of encrustation [2]. Usually JJ-stenting can be limited to 1–3 days [2].
Percutaneous nephrolithotomy (PNL)
For large and branched cystine calculi (staghorn stones) (Fig. 2), PNL is usually the preferred treatment option, offering the highest stone free rates [48]. For very complex calculi an endoscopic combined approach (ECIRS), combining PNL with flexible ureterorenoscopy, may be the best solution in order to avoid the morbidity of multiple accesses in this patient group that often needs repeated interventions [49]. Additionally, residual stones after PNL may have to be approached by SWL and/or chemolysis to achieve a complete stone-free rate, which is crucial to prevent early recurrence [20]. Newer miniaturized PNL approaches, in which the traditional tract size has been reduced to below 20 Fr., may prove to be of special value in cystinuric patients, since various studies have confirmed that reducing the tract size potentially also reduces the complications of percutaneous surgery [50].
As mentioned previously, imaging may help selecting cystine stones that are more suitable for endoscopic management than for SWL [34], and in this way a personalized stone approach may result in an overall higher success rate of both treatment modalities.
Stone prevention
Mechanisms of stone formation in general include overgrowth on interstitial apatite plaques, crystal formation within renal tubules and free solution crystallization [51]. Stone formation in cystinuria is caused by the over-excretion of cystine, leading to super-saturation and cystine free crystal forming within urine in the inner medullary collecting ducts, ducts of Bellini and collecting system [52].
No curative treatment of cystinuria exists, and patients will have a life long risk of stone formation and repeated surgery. Cystinuric patients are more prone to loss of renal function than calcium oxalate stone formers [53].
During the last decades there have been no major advances in the preventive management of cystinuria. The primary goal in prevention is to decrease the risk of stone formation, which may be achieved by decreasing the cystine concentration and increasing the solubility. A decrease in concentration can be achieved by hydration and dietary measures; an increase in solubility may be achieved by alkalinisation of the urine and cystine-binding drugs.
Homozygous cystinuria patients excrete 600–1400 mg cystine/day. A cystine concentration >1 mmol/l (250 mg/l) increases the risk of stone formation. The solubility of cystine is approximately 1 mmol/l (250 mg/l) at normal physiological urine pH, but increases to 2 mmol/l (500 mg/l) at a pH of 7.5 [54, 55].
Hydration
Hydration or hyperdiuresis is the oldest preventive measure in cystinuria, and has been known for more than 50 years [54, 55].
High fluid intake prevents stone formation by diluting the urine, thereby lowering urinary cystine concentration. The patient should drink enough to maintain a urine cystine concentration below 250 mg/l at all times, and therefore the fluid intake should be evenly distributed during the day, in order to force nightly urine production, opposing the physiological concentration of urine during night time.
In a study by Barbey et al. [56] the need for urological intervention was reduced in more than half of the patients treated with a combination of high fluid intake, alkalization and second line thiols, but patients poorly compliant with hyperdiuresis remained at risk for recurrence. Maintaining a daily urine volume of more than 3 l was essential for therapeutic success regardless of whether thiol derivatives were administered [56].
Recommendations of minimum fluid intake vary from 3 to 5 l/day in adults, and 3 l/day in children [8, 12, 23, 29, 57–64]. Some groups recommend 2 l/m2 body surface area [57, 59, 65, 66], and others a fluid intake resulting in urinary volumes exceeding 3–4 l/day [4, 12, 20, 29, 61, 67, 68]. As the excretion rate, as well as the solubility depending on urinary pH, varies in each patient, so will the necessary fluid intake. A patient with a cystine excretion of 500 mg/day at physiological urine pH, should drink enough to produce a minimum of 2 l/day of urine, and a patient with a cystine excretion of 1000 mg/day should have a 24 h urinary volume of more than 4 l. The fluid intake should be increased even more during periods of heavy perspiration due to exercise, fever or warm weather.
Alkalizing beverages such as mineral water rich in bicarbonate and low in sodium (1500 mg HCO3/L, maximum 500 mg sodium/L) are recommended.
Fluid intake in the form of fruit juices and herbal tea containing citric acid may also result in alkalization of urine by conversion of citrate into bicarbonate and thus raise the solubility of cystine [69–71].
In order to maintain a constant and sufficient state of hydration and a stable urinary volume, the patient may benefit from keeping a schedule of fluid intake and voiding, or monitor the gravity of urine, which should be less than 1.010 [72]. Long-term compliance to this regime is difficult to maintain, however.
Dietary measures
The dietary source of urinary cystine is the amino acid methionine. One way of reducing cystine excretion is by lowering methionine intake to a minimum, by consuming less animal protein such as egg, fish, chicken, beef and pork, and also wheat and some nuts and seeds. This will also decrease any acidotic effects resulting from the breakdown of animal protein, and thus increase urinary pH [58]. It has been shown that a short-term low animal protein diet significantly reduced urinary cystine excretion [73]. An effect on stone recurrence of a protein-restricted diet has not yet been shown in controlled trials, however.
It has been recommended to reduce the intake of animal protein to around 1 g/kg/day in adults [8, 12, 58, 61, 64, 68, 74]. Protein restriction should however not be applied in children and young adolescents, who are still growing. Long-term patient compliance may be low especially for those used to a high animal protein diet.
Similarly to drinking fruit juices, eating fruits and vegetables that contain organic anions such as malate and citrate will raise urine pH.
Reducing sodium intake to 1 mmol/kg per day has been shown to decrease cystine excretion significantly [75], and in spite of the defective proximal tubular reabsorption of cystine in cystinuria the reabsorption may be increased by restricting the intake of sodium [76]. The general recommendation of daily intake of sodium is no more than 2 g/day [8, 12, 59, 61, 62, 64, 68, 74, 77]. In order to achieve this, patients should avoid salting of the food, and fast food in general. There are however no randomized controlled trials to support these recommendations.
Pharmacologic treatment
Alkali supplementation
Since very few patients are able to stay free of recurrence solely on hyperhydration, alkali supplementation is part of first line therapy. The primary pharmacologic treatment is oral alkali supplementation. Sodium bicarbonate, sodium citrate, magnesium citrate and potassium citrate may be used, but potassium citrate is preferred since sodium loads increase the urinary cystine excretion [76]. Usual doses are 60–80 mEq per day. Slow release formulation drugs can be administered twice a day, whereas mixtures should be administered four times a day. Dose should be titrated to reach and maintain a urine pH of 7.0–7.5. At urinary pH > 7.5 there is a risk of calcium phosphate stone formation.
It is essential that patients monitor the urinary pH with dipsticks, although a clear differentiation in pH level in the range of 6.0–7.5 may be difficult using standard dipsticks.
Side effects to alkali supplementation are usually mild, and most frequently include abdominal discomfort (pain, diarrhoea and nausea). Gastric ulceration has been described, and potassium citrate should be used with caution in patients with impaired gastrointestinal motility. There is a potential risk of hyperkalaemia, and monitoring serum potassium is advisable [78]. Sodium bicarbonate may be used, when potassium citrate is contraindicated due to hyperkalaemia [29, 36].
Acetazolamide, a carbonic anhydrase inhibitor, increases the urinary bicarbonate excretion, but is often poorly tolerated [79], and not widely used.
Thiols
When first line treatment with hydration and urine alkalization are not sufficient to keep the patient free of recurrence, second line pharmacological therapy with a cystine-binding thiol agent is indicated. Thiols are organosulfur compounds that contain a sulfhydryl group that combine with cystine to form a more soluble drug-cysteine complex. Available agents include d-penicillamine (dimethyl-cysteine), α-mercaptopropionylglycine (alpha-MPG also know as tiopronin) that produce cysteine complexes, which are 50 times more soluble than cystine [12], and captopril.
Penicillamine was first used to treat patients with cystinuria in 1963 [80]. Dahlberg et al. showed that stone recurrence, stone passage, and stone growth were lowered when d-penicillamine was added to conservative treatment [81]. Treatment with d-penicillamine or alpha-MPG compared to conservative treatment has shown a reduction of stone events from 1.6 to 0.45 per years [71]. The effect is dose dependent. An increase in dose by 250 mg/day decreases the urinary cystine level by 75–100 mg/day [36]. The typical dose of d-penicillamine in adults ranges between 0.5 and 2 g/day, given in 3 or 4 divided doses. The dose should ideally be adjusted to reduce unbound urinary cystine concentration to below 250 mg/l. The usual dosage in children is between 20 and 40 mg/kg [8].
Alpha-MPG (tiopronin) is a second-generation chelating agent with a mechanism of action similar to d-penicillamine but with a 30 % higher dissolution capacity, and with less adverse effects. The US Food and Drug Administration approved alpha-MPG in 1988. One study showed that alpha-MPG reduced the rate of stone formation by 81 % in patients previously treated with D-penicillamine, and by 94 % in treatment naive patients [82]. Treatment with d-penicillamine or tiopronin compared with conservative management (with hydration and alkali) significantly decreased stone events by 32–65 % [56, 71].
The recommended initial dose in adults is 250 mg/day, gradually increasing the dosage to 400–1200 mg/day in three divided doses. Maximum dose is 2 g/day. Recommended dose of tiopronin in children is 10–15 mg/kg/day [82, 83].
The efficacy of thiol drugs is dependent on urinary pH and therefore should always be combined with urine alkalization [84]. Adverse effects are common and include rash, fever, arthralgia, leukopenia, gastrointestinal intolerance, proteinuria and nephritic syndrome. Long-term therapy may lead to vitamin B-6 (pyridoxine) deficiency, why vitamin B-6 supplementation (50 mg/day) is recommended.
Captopril, an angiotensin-converting enzyme (ACE) inhibitor, also contains a thiol and forms a captopril-cysteine disulphide, which have been shown to increase cystine solubility in vitro by about 200 times [85]. It has been used as an alternative to d-penicillamine and tiopronin due to fewer side effects. The literature is mainly case based and the results are contradictory. The first use of captopril in cystinuria was published in 1987 in two patients in which cystine excretion was reduced with 70 and 93 %, and additionally proteinuria was reduced [85]. A positive effect has also been described in two cases treated for 1 year without recurrence or side effects [86]. Cohen et al. treated nine patients with captopril in addition to standard fluid and alkalization therapy and found a non-significant decrease in new stone formation and stone growth [87]. Other authors have found captopril ineffective in reducing cystine excretion and not clinically useful [88–90]. Captopril is mainly used in patients intolerant for other thiols. The recommended dose is 50 mg three times daily.
Recommended first and second line preventive treatment in cystinuria is presented in Fig. 3.
Monitoring
Since the effect of thiols are dose dependent, ideally dose should be titrated. Routine analysis of cystine is not suitable for monitoring, because not only free but also bound cystine is measured. For therapy monitoring it is essential to differentiate between free cystine, cysteine and thiol-cysteine complexes. Only high-performance liquid chromatography (HPLC) analysis can differentiate between these. Solid phase assay of urinary cystine seems to be an accurate method for determining urine cystine super-saturation and cystine capacity in the presence of cystine-binding thiol drugs [25, 91–93].
Patients should also be monitored for side effects such as proteinuria, hematologic complications, copper, zinc and pyridoxine deficiency, and liver function abnormalities [4, 12].
Compliance
Compliance in urolithiasis patients concerning both dietary and pharmacological intervention in general is poor. This also applies for patients with cystinuria. The main reason for discontinuation of medical treatment is adverse reactions.
In a multicentre study 69 % of patients treated with d-penicillamine discontinued due to adverse reactions, compared with 31 % for alpha-MPG [82].
Pietrow et al. retrospectively studied the course of 26 patients at a comprehensive kidney stone centre with an average follow-up of 38.2 months, and found that 42 % achieved initial success, but failed to maintain this after an average of 16 months, and only 15 % were able to achieve and maintain a cystine concentration of less than 300 mg/l [94]. A vigilant approach and thorough patient education is warranted to improve compliance.
Compliance presumably improves in dedicated hands. Haritopoulos et al. found that the introduction of a dedicated cystinuria clinic halved the intervention rate [95]. The average number of surgical procedures per patient per year was reduced from 0.74 to 0.34, at the “expense” of more frequent clinical attendances in the latter group [95]. Of course a historical comparison like this may be questioned methodologically, but it is likely that compliance and clinical outcome will improve in dedicated and experienced centres. Frequent clinical follow-up visits might improve compliance; on the other hand you could speculate that it might also lead to more frequent interventions like retrograde flexible ureterorenoscopy of early small stone recurrences.
Importance of follow-up
Being a genetic disorder often causing severe morbidity, cystinuria demands close, life-long follow-up. The primary goal is to keep urinary cystine levels low thereby preventing stone formation.
Appropriate follow-up includes:
-
Measurement of treatment efficacy (laboratory investigations)
-
Imaging
-
Monitoring of adverse reactions to medication
-
Monitoring and preservation of kidney function
-
Counselling to increase patient compliance
Treatment efficacy
The effectiveness of therapy should be determined by measurement of urinary pH and 24 h urinary cystine excretion. Urinary pH should be >6.5 to increase solubility of cystine [20]. It is important to keep in mind that total urine cystine measurement is not efficient when using thiol-containing drugs, as most cystine-assays do not distinguish cystine from soluble thiol-cysteine drug complexes. Cystine capacity, which is a solid-phase assay of urinary cystine, is a more reliable and accurate method to measure the ability of the urine to solubilize vs. precipitate cystine when the urine cystine load is increased, thereby reflecting undersaturation = positive capacity or supersaturation = negative capacity [23]. Thus, cystine capacity may be an effective tool to monitor patient response and guide medication dosage.
Imaging
Image diagnostic monitoring of stone activity is mandatory. Patients with severe disease should be followed with imaging studies every 3 months, renal ultrasonography being an ideal initial choice of modality, since in these patients with frequent recurrences, ionized radiation should be avoided when possible. Patients with minimal or no stone activity should be followed every 6 months [62]. Ultrasonography is precise for detecting renal atrophy and hydronephrosis and fairly sensitive in detecting larger stones. However, low-dose NCCT may be required when ultrasonography is unequivocal, and when a symptomatic patient is considered for surgical intervention. Ultra Low dose CT protocols may be performed with radiation doses equivalent to or below an ordinary KUB [96, 97]. Thus, with regard to image follow-up the uroradiologists should be involved in planning individualized protocols.
Adverse effects
Treatment with sulfhydryl compounds such as d-penicillamine and tiopronin implies risk of severe adverse effects [62]. Therefore, patients receiving thiol medications should be followed-up regularly allowing the physician to detect neutropenia, thrombocytopenia, anaemia, proteinuria and decreased kidney function, rashes and copper/zinc deficiencies. Unfortunately, a significant amount of cystinuric patients has poor compliance due to medical adverse reactions and lack of insight into the pathophysiology of the disease. Moreover, the large amount of pills and fluid to be administered per day can be difficult to accept [94]. It has, however, been shown that active management and follow-up in compliant patients decreases the need of surgical intervention [98].
Preservation of kidney function
Renal insufficiency has been reported to occur in 5–17 % of cystinuric patients [53, 62], and homozygotes have a statistically higher serum creatinine compared to other urolithiasis patients [53]. End-stage renal failure occurs in <5 % [99]. Medical prevention of stone formation is proposed to preserve renal function by preventing damage of the renal parenchyma caused by cystine crystals deposited within the ducts of Bellini [52]. Careful follow-up including relevant laboratory analyses and renographic examinations may reveal unilateral changes in renal function that can be handled at an early stage by more aggressive therapy to avoid further renal deterioration.
Future directions
Despite better understanding of the molecular basis of cystinuria the principles of recurrence prevention have remained essentially the same through decades [20]. To date, the mechanisms of cystine precipitation in urine remain incompletely understood. Interestingly, severity of stone disease cannot be entirely explained by differences in urine cystine excretion levels [100], which may be due to the fact that other molecules facilitating the initial interaction between cystine molecules may have a prominent role in promoting nucleation [101]. It has been suggested that urinary excretion of nucleosides and vanillylmandelic acid (VMA) might have a modulating effect on cystine solubility [102]. It was found that nucleoside concentrations remained significantly associated with lower cystine solubility after correction for gender and for urine concentration [102]. These findings may represent new targets for cystinuria research. In vitro findings have shown that increasing urine pH greatly increases the effect of thiol drugs to solubilize cystine, highlighting the importance of combining thiol with alkali therapy [84]. Clinical trials should be performed to confirm these in vitro findings. Investigation of newer thiol-containing drugs [cysteamine and 2,3 dimercaptosuccinic acid (DMSA)] has not yet lead to clinical use, however, taking the severity of this particular stone disease into consideration, this should not reduce the desire to look for new drugs [71, 103]. Improved understanding of the molecular biology of cystine transport hopefully will lead to more targeted therapeutic options in the near future, reducing both recurrences and side effects [104].
Methods for diagnosing cystinuria have been established, however, a validated method of assessing and monitoring disease activity is still lacking [2].
Failure of pharmacological therapy may to a large extend be contributed compliance problems. It has been shown that the need for urological intervention for recurrent stones decreased with medical compliance [98], and that introduction of a dedicated cystinuria clinic halved intervention rate [95]. Centralizing the management of patients with cystinuria to dedicated centres, therefore, may be an equally important step towards better management of this complicated group of stone patients. Such centres should be able to offer the full range of minimal invasive treatment modalities, enabling a personalized approach to stone management. Such a multimodal approach including methods to increase compliance to therapy should be evaluated from an evidence-based perspective, including patient reported outcome and quality of life measures [105, 106].
Conclusion
Cystinuria represent a major therapeutic challenge, and pharmacotherapy of this serious stone disease has not improved in decades despite new knowledge on the molecular-genetic basis of the disease. Future studies on pathophysiology and new treatment options are highly warranted. Imaging may help defining patients suitable for SWL vs. endourological procedures. Cystinuric patients should be managed in dedicated centres offering the complete range of minimal invasive treatment modalities, enabling a personalized treatment approach in order to reduce risk and morbidity of multiple procedures. Furthermore, a multimodal approach has been shown to increase compliance of hydration and medical therapy, decreasing the need for invasive treatment. Early diagnosis and close follow-up are mandatory, minimizing renal damage from both stone disease itself and interventional stone therapy. Although much is to be desired in terms of treatment efficiency, by embracing all the tools of a personalized stone approach, loss of nephrons in cystinuric patients in the twenty-first century should be a rare event.
References
Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacin M (2010) Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6:424–434
Thomas K, Wong K, Withington J, Bultitude M, Doherty A (2014) Cystinuria—a urologist’s perspective. Nat Rev Urol 11:270–277
Faerber GJ (2001) Pediatric urolithiasis. Curr Opin Urol 11:385–389
Claes DJ, Jackson E (2012) Cystinuria: mechanisms and management. Pediatr Nephrol 27:2031–2038
Elmaci AM, Ece A, Akin F (2014) Pediatric urolithiasis: metabolic risk factors and follow-up results in a Turkish region with endemic stone disease. Urolithiasis 42:421–426
Leusmann DB, Blaschke R, Schmandt W (1990) Results of 5,035 stone analyses: a contribution to epidemiology of urinary stone disease. Scand J Urol Nephrol 24:205–210
Polinsky MS, Kaiser BA, Baluarte HJ (1987) Urolithiasis in childhood. Pediatr Clin North Am 34:683–710
Knoll T, Zöllner A, Wendt-Nordahl G, Michel MS, Alken P (2005) Cystinuria in childhood and adolescence: recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol 20:19–24
Online Mendelian Inheritance in Man, OMIM (TM). Johns Hopkins University, Baltimore, MD. MIM number: 104614 and 604144. http://omim.org/entry/220100. Accessed 30 April 2015
Krombach P, Knoll GW-N (2011) Cystinuria and Cystine Stones. In: Rao PN, Kavanagh JP, Preminger GM (eds) Urinary Tract Stone Disease. Springer-Verlag, London
Dello Strologo L, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, Ponzone A, Gallucci M, Bisceglia L, Zelante L, Jimenez-Vidal M, Font M, Zorzano A, Rousaud F, Nunes V, Gasparini P, Palacín M, Rizzoni G (2002) Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol 13:2547–2553
Saravakos P, Kokkinou V, Giannatos E (2014) Cystinuria: current diagnosis and management. Urology 83:693–699
Eggermann T, Venghaus A, Zerres K (2012) Cystinuria: an inborn cause of urolithiasis. Orphanet J Rare Dis 7:19
Eggermann T, Zerres K, Nunes V, Font-Llitjós M, Bisceglia L, Chatzikyriakidou A, dello Strologo L, Pras E, Creemers J, Palacin M (2012) Clinical utility gene card for: cystinuria. Eur J Hum Genet 20(2). doi:10.1038/ejhg.2011.163
Font-Llitjós M, Jiménez-Vidal M, Bisceglia L, Di Perna M, de Sanctis L, Rousaud F, Zelante L, Palacín M, Nunes V (2005) New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet 42:58–68
Wong KA, Mein R, Wass M, Flinter F, Pardy C, Bultitude M, Thomas K (2015) The genetic diversity of Cystinuria in a UK population of patients. BJU Int 116(1):109–116. doi:10.1111/bju.12894
Popovska-Jankovic K, Tasic V, Bogdanovic R, Miljkovic P, Golubovic E, Soylu A, Saraga M, Pavicevic S, Baskin E, Akil I, Gregoric A, Lilova M, Topaloglu R, Sukarova Stefanovska E, Plaseska-Karanfilska D (2013) Molecular characterization of cystinuria in south-eastern European countries. Urolithiasis 41:21–30.doi:10.1111/bju.12894
Barbosa M, Lopes A, Mota C, Martins E, Oliveira J, Alves S, De Bonis P, Mota M, Dias C, Rodrigues-Santos P, Fortuna AM, Quelhas D, Lacerda L, Bisceglia L, Cardoso ML (2012) Clinical, biochemical and molecular characterization of cystinuria in a cohort of 12 patients. Clin Genet 81:47–55
Halbritter J, Baum M, Hynes AM, Rice SJ, Thwaites DT, Gucev ZS, Fisher B, Spaneas L, Porath JD, Braun DA, Wassner AJ, Nelson CP, Tasic V, Sayer JA, Hildebrandt F (2015) Fourteen monogenic genes account for 15% of Nephrolithiasis/Nephrocalcinosis. J Am Soc Nephrol 26(3):543–551. doi:10.1681/ASN.2014040388
Tiselius HG (2010) New horizons in the management of patients with cystinuria. Curr Opin Urol 20:169–173
Thomas JC, DeMarco RT, Donohoe JM, Adams MC, Brock JW, Pope JC (2005) Pediatric ureteroscopic stone management. J Urol 174:1072–1074
Finocchiaro R, D’Eufemia P, Celli M, Zaccagnini M, Viozzi L, Troiani P, Mannarino O, Giardini O (1998) Usefulness of cyanide-nitroprusside test in detecting incomplete recessive heterozygotes for cystinuria: a standardized dilution procedure. Urol Res 26:401–405
Sumorok N, Goldfarb DS (2013) Update on cystinuria. Curr Opin Nephrol Hypertens 22:427–431
Nakagawa Y, Coe FL (1999) A modified cyanide-nitroprusside method for quantifying urinary cystine concentration that corrects for creatinine interference. Clin Chim Acta 289:57–68
Goldfarb DS, Coe FL, Asplin JR (2006) Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int 69:1041–1047
Patel SR, Wagner LE, Lubner MG, Nakada SY (2014) Radiopacity and hounsfield attenuation of cystine urolithiasis: case series and review of the literature. J Endourol 28:472–475
Torricelli FC, Marchini GS, De S, Yamaçake KG, Mazzucchi E, Monga M (2014) Predicting urinary stone composition based on single-energy noncontrast computed tomography: the challenge of cystine. Urology 83:1258–1263
Motley G, Dalrymple N, Keesling C, Fischer J, Harmon W (2001) Hounsfield unit density in the determination of urinary stone composition. Urology 58:170–173
Mattoo A, Goldfarb DS (2008) Cystinuria. Semin Nephrol 28:181–191
Ferrandino MN, Pierre SA, Simmons WN, Paulson EK, Albala DM, Preminger GM (2010) Dual-energy computed tomography with advanced postimage acquisition data processing: improved determination of urinary stone composition. J Endourol 24:347–354
Chevreau G, Troccaz J, Conort P, Renard-Penna R, Mallet A, Daudon M, Mozer P (2009) Estimation of urinary stone composition by automated processing of CT images. Urol Res 37:241–245
Bhatta KM, Prien EL, Dretler SP (1989) Cystine calculi–rough and smooth: a new clinical distinction. J Urol 142:937–940
Kim SC, Hatt EK, Lingeman JE, Nadler RB, McAteer JA, Williams JC (2005) Cystine: helical computerized tomography characterization of rough and smooth calculi in vitro. J Urol 174:1468–1470
Kim SC, Burns EK, Lingeman JE, Paterson RF, McAteer JA, Williams JC (2007) Cystine calculi: correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urol Res 35:319–324
Ng CS, Streem SB (1999) Contemporary management of cystinuria. J Endourol 13:647–651
Biyani C, Cartledge JJ (2006) Cystinuria—diagnosis and management. EAU-EBU Updates Series 4:175–183
Elkoushy MA, Violette PD, Andonian S (2012) Percutaneous instillation of chemolytytic, chemotherapeutic, and antifungal agents. In: Smith AD, Badlani GH, Preminger GM, Kavoussi LR (eds) Smith’s Textbbok of Endourology, 3rd edn. Blackwell Publishing Ltd
Hesse A, Tiselius HG, Siener R, Hoppe B (2009) Cystine stones. In: (eds) Urinary Stones. Diagnosis, treatment, and prevention of recurrence., 3rd edn. S. Karger AG, Basel
Ahlstrand C, Tiselius HG (1993) Treatment of cystine urolithiasis by a combination of extracorporeal shock wave lithotripsy and chemolysis. J Stone Dis 5:32–38
Kachel TA, Vijan SR, Dretler SP (1991) Endourological experience with cystine calculi and a treatment algorithm. J Urol 145:25–28
Azili MN, Ozcan F, Tiryaki T (2014) Retrograde intrarenal surgery for the treatment of renal stones in children: factors influencing stone clearance and complications. J Pediatr Surg 49:1161–1165
Kronenberg P, Traxer O (2014) In vitro fragmentation efficiency of holmium: yttrium-aluminum-garnet (YAG) laser lithotripsy—a comprehensive study encompassing different frequencies, pulse energies, total power levels and laser fibre diameters. BJU Int 114:261–267
Ruggera L, Zanin M, Beltrami P, Zattoni F (2011) Retrograde transureteral approach: a safe and efficient treatment for recurrent cystine renal stones. Urol Res 39:411–415
Cohen J, Cohen S, Grasso M (2013) Ureteropyeloscopic treatment of large, complex intrarenal and proximal ureteral calculi. BJU Int 111:E127–E131
Al-Qahtani SM, Letendre J, Thomas A, Natalin R, Saussez T, Traxer O (2014) Which ureteral access sheath is compatible with your flexible ureteroscope. J Endourol 28:286–290
Traxer O, Thomas A (2013) Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol 189:580–584
Jung HU, Frimodt-Møller PC, Osther PJ, Mortensen J (2006) Pharmacological effect on pyeloureteric dynamics with a clinical perspective: a review of the literature. Urol Res 34:341–350
Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS, AUA NGP (2005) Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol 173:1991–2000
Scoffone CM, Cracco CM, Cossu M, Grande S, Poggio M, Scarpa RM (2008) Endoscopic combined intrarenal surgery in Galdakao-modified supine Valdivia position: a new standard for percutaneous nephrolithotomy. Eur Urol 54:1393–1403
Ganpule AP, Bhattu AS, Desai M (2015) PCNL in the twenty-first century: role of microperc, miniperc, and ultraminiperc. World J Urol 33:235–240
Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38:147–160
Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM (2006) Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int 69:2227–2235
Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ (2002) The impact of cystinuria on renal function. J Urol 168:27–30
Dent CE, Senior B (1955) Studies on the treatment of cystinuria. Br J Urol 27:317–332
Dent CE, Friedman M, Green H, Watson LC (1965) Treatment of cystinuria. Br Med J 1:403–408
Barbey F, Joly D, Rieu P, Méjean A, Daudon M, Jungers P (2000) Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 163:1419–1423
Joly D, Rieu P, Méjean A, Gagnadoux MF, Daudon M, Jungers P (1999) Treatment of cystinuria. Pediatr Nephrol 13:945–950
Bihl G, Meyers A (2001) Recurrent renal stone disease-advances in pathogenesis and clinical management. Lancet 358:651–656
Al-Hermi B, Abbas B (2003) Cystinuria in arab countries. Saudi J Kidney Dis Transpl 14:358–366
Grases F, Costa-Bauza A, Prieto RM (2006) Renal lithiasis and nutrition. Nutr J 5:23
Porena M, Guiggi P, Micheli C (2007) Prevention of stone disease. Urol Int 79(Suppl 1):37–46
Rogers A, Kalakish S, Desai RA, Assimos DG (2007) Management of cystinuria. Urol Clin North Am 34:347–362
Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R (2010) An update and practical guide to renal stone management. Nephron Clin Pract 116:c159–c171
Fattah H, Hambaroush Y, Goldfarb DS (2014) Cystine nephrolithiasis. Transl Androl Urol 3:228–233
Goodyer P (2004) The molecular basis of cystinuria. Nephron Exp Nephrol 98:e45–e49
Copelovitch L (2012) Urolithiasis in children: medical approach. Pediatr Clin North Am 59:881–896
Tiselius HG (2004) Recurrence prevention in patients with urinary tract stone disease. Sci World J 4:35–41
Xu H, Zisman AL, Coe FL, Worcester EM (2013) Kidney stones: an update on current pharmacological management and future directions. Expert Opin Pharmacother 14:435–447
Sakhaee K (1994) Cystinuria: pathogenesis and treatment. Miner Electrolyte Metab 20:414–423
Sakhaee K (1996) Pathogenesis and medical management of cystinuria. Semin Nephrol 16:435–447
Chow GK, Streem SB (1996) Medical treatment of cystinuria: results of contemporary clinical practice. J Urol 156:1576–1578
Rutchik SD, Resnick MI (1997) Cystine calculi. Diagnosis and management. Urol Clin North Am 24:163–171
Rodman JS, Blackburn P, Williams JJ, Brown A, Pospischil MA, Peterson CM (1984) The effect of dietary protein on cystine excretion in patients with cystinuria. Clin Nephrol 22:273–278
Heilberg IP, Goldfarb DS (2013) Optimum nutrition for kidney stone disease. Adv Chronic Kidney Dis 20:165–174
Jaeger P, Portmann L, Saunders A, Rosenberg LE, Thier SO (1986) Anticystinuric effects of glutamine and of dietary sodium restriction. N Engl J Med 315:1120–1123
Lindell A, Denneberg T, Edholm E, Jeppsson JO (1995) The effect of sodium intake on cystinuria with and without tiopronin treatment. Nephron 71:407–415
Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR (2014) Medical management of kidney stones: AUA guideline. J Urol 192:316–324
Fjellstedt E, Denneberg T, Jeppsson JO, Tiselius HG (2001) A comparison of the effects of potassium citrate and sodium bicarbonate in the alkalinization of urine in homozygous cystinuria. Urol Res 29:295–302
Sterrett SP, Penniston KL, Wolf JS, Nakada SY (2008) Acetazolamide is an effective adjunct for urinary alkalization in patients with uric acid and cystine stone formation recalcitrant to potassium citrate. Urology 72:278–281
Crawhall JC, Scowen EF, Watts RW (1963) Effect of penicillamine on cystinuria. Br Med J 1:588–590
Dahlberg PJ, van DB, Kurtz SB, Wilson DM, Smith LH (1977) Clinical features and management of cystinuria. Mayo Clin Proc 52:533–542
Pak CY, Fuller C, Sakhaee K, Zerwekh JE, Adams BV (1986) Management of cystine nephrolithiasis with alpha-mercaptopropionylglycine. J Urol 136:1003–1008
Lindell A, Denneberg T, Hellgren E, Jeppsson JO, Tiselius HG (1995) Clinical course and cystine stone formation during tiopronin treatment. Urol Res 23:111–117
Asplin DM, Asplin JR (2013) The Interaction of thiol drugs and urine pH in the treatment of cystinuria. J Urol 189:2147–2151
Sloand JA, Izzo JL (1987) Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 147:1409–1412
Perazella MA, Buller GK (1993) Successful treatment of cystinuria with captopril. Am J Kidney Dis 21:504–507
Cohen TD, Streem SB, Hall P (1995) Clinical effect of captopril on the formation and growth of cystine calculi. J Urol 154:164–166
Dahlberg PJ, Jones JD (1989) Cystinuria: failure of captopril to reduce cystine excretion. Arch Intern Med 149(713):717
Coulthard M, Richardson J, Fleetwood A (1991) Captopril is not clinically useful in reducing the cystine load in cystinuria or cystinosis. Pediatr Nephrol 5:98
Michelakakis H, Delis D, Anastasiadou V, Bartsocas C (1993) Ineffectiveness of captopril in reducing cystine excretion in cystinuric children. J Inherit Metab Dis 16:1042–1043
Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL (2000) Clinical use of cystine supersaturation measurements. J Urol 164:1481–1485
Coe FL, Clark C, Parks JH, Asplin JR (2001) Solid phase assay of urine cystine supersaturation in the presence of cystine binding drugs. J Urol 166:688–693
Dolin DJ, Asplin JR, Flagel L, Grasso M, Goldfarb DS (2005) Effect of cystine-binding thiol drugs on urinary cystine capacity in patients with cystinuria. J Endourol 19:429–432
Pietrow P, Auge BK, Weizer AZ, Delvecchio FC, Silverstein AD, Mathias B, Albala DM, Preminger GM (2003) Durability of the medical management of cystinuria. J Urol 169:68–70
Haritopoulos K, Fojtik P, Cross W, Cartledge J (2010) Impact of a metabolic stone clinic on management of patients with cystinuria: 5 years follow-up. Clin Ter 161:341–344
Kluner C, Hein PA, Gralla O, Hein E, Hamm B, Romano V, Rogalla P (2006) Does ultra-low-dose CT with a radiation dose equivalent to that of KUB suffice to detect renal and ureteral calculi. J Comput Assist Tomogr 30:44–50
Kulkarni NM, Uppot RN, Eisner BH, Sahani DV (2012) Radiation dose reduction at multidetector CT with adaptive statistical iterative reconstruction for evaluation of urolithiasis: how low can we go. Radiology 265:158–166
Pareek G, Steele TH, Nakada SY (2005) Urological intervention in patients with cystinuria is decreased with medical compliance. J Urol 174:2250–2252 discussion 2252
Lindell A, Denneberg T, Granerus G (1997) Studies on renal function in patients with cystinuria. Nephron 77:76–85
Coe FL, Evan A, Worcester E (2005) Kidney stone disease. J Clin Invest 115:2598–2608
Rimer JD, An Z, Zhu Z, Lee MH, Goldfarb DS, Wesson JA, Ward MD (2010) Crystal growth inhibitors for the prevention of l-cystine kidney stones through molecular design. Science 330:337–341
Masotti A, Laurenzi C, Boenzi S, Pastore A, Taranta A, Bellomo F, Muraca M, Dionisi-Vici C, Bertucci P, Dello Strologo L, Emma F (2014) Gender-related effects on urine l-cystine metastability. Amino Acids 46:415–427
Belldina EB, Huang MY, Schneider JA, Brundage RC, Tracy TS (2003) Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br J Clin Pharmacol 56:520–525
Wendt-Nordahl G, Sagi S, Bolenz C, Alken P, Michel MS, Knoll T (2008) Evaluation of cystine transport in cultured human kidney cells and establishment of cystinuria type I phenotype by antisense technology. Urol Res 36:25–29
Kartha G, Calle JC, Marchini GS, Monga M (2013) Impact of stone disease: chronic kidney disease and quality of life. Urol Clin North Am 40:135–147
Penniston KL, Nakada SY (2013) Development of an instrument to assess the health related quality of life of kidney stone formers. J Urol 189:921–930
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Andreassen, K.H., Pedersen, K.V., Osther, S.S. et al. How should patients with cystine stone disease be evaluated and treated in the twenty-first century?. Urolithiasis 44, 65–76 (2016). https://doi.org/10.1007/s00240-015-0841-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0841-x