Abstract
We searched to review experimental and clinical trials concerning the capabilities of impacting on the ureteric and pelvic activity by means of pharmacological stimulation. Ureteropyeloscopy may cause high renal pelvic pressure. The normal pressure is in the range of 5–15 mmHg whereas pressure of 410 mmHg has been measured during endoscopy. The threshold pressure for intrarenal reflux is about 35 mmHg. Studies in animals have revealed that high renal pelvic pressures may cause permanent damage to the renal parenchyma. Furthermore, it has been demonstrated that elevated pressures may entail an increased risk of several complications related to endourological procedures including bleeding, perforation and infection. In other words, means by which intrarenal pressure could be lowered during endourological procedures might be beneficial with respect to clinical outcomes. In vitro experiments support the existence of different receptors in the ureter and renal pelvis. The ureteric and pelvic responses to the corresponding neurotransmitters have been determined. It seems that α-adrenergic and cholinergic agents are stimulating whereas β-adrenergic agents inhibit ureteric activity. The effect may depend on the mode of administration. Drugs exerting advantageous effects in the pyeloureter may cause undesirable systemic side effects when administered intravenously. In animal studies, renal pelvic pressure can be significantly lowered by topical administration of β-adrenergic agonists without systemic side effects. In vivo human studies are necessary to clarify the exact dose–response relationship and the degree of urothelial absorption of a drug before clinical use may be adopted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ureteropyeloscopy is regarded by most urologists as a minimal invasive procedure followed by few complications. The primary peroperative worries are risk of perforation or even rupture of the ureter. There have been, however, some reports of other risks. Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy has been presented, suggesting that cancer cells may be spread because of elevated pelvic pressure [1]. Furthermore, high pelvic pressures could result in pyelorenal backflow with potential risk of bacteraemia and sepsis [2, 3]. Accordingly, the challenge of lowering the intrarenal pressure during endourological procedures is interesting from a clinical point of view.
Today NSAIDs and α-adrenergic blockers are used for medical modulation of the motility in the upper urinary tract in the case of renal colic secondary to ureterolithiasis [4–6]. Prostaglandin synthetase inhibitors and cyclooxygenase inhibitors have been shown to mediate spasmolytic effects in the ureter [7, 8]. NSAIDs are currently considered a first-line treatment of renal colic because of the analgesic effect. Indomethacin was found to reduce smooth muscle activity in human renal pelvis [4] and in pigs indomethacin reduced intrapelvic pressure when administered intravenously [9]. The α-blocker tamsulosin has in several studies been proven to mediate an increased expulsion rate of ureteric stones [6, 10, 11]. These findings suggest that patients with small stones in the distal ureter may benefit from this kind of medical therapy because it may induce spontaneous stone passage. Influence on renal activity and pelvic pressure during endourological procedures resulting in more beneficial outcomes, i.e. fewer complications and higher success rates, would extend the clinical perspectives of pharmacological modulation in the upper urinary tract.
In animals, high renal pelvic pressures have been shown to cause irreversible damage to the renal parenchyma [3, 12, 13] (Tables 1, 2). Intraluminal renal pelvic pressure depends on several factors: the tension in the pelvic wall, the compliance in the ureteropelvic junction, the actual urine flow, external pressures exerted by surrounding structures and the actual system capacity [14].
The normal pelvic pressure is in the range 5–15 mmHg [3, 13–15]. During ureterorenoscopy, renal pelvic pressure as high as 410 mmHg has been measured [16]. The pressures vary according to the surgical devices used for instrumentation, the mode of irrigation (e.g. the height of the irrigation bag, use of manual syringe or mechanical roller-pump device) and the filling of the bladder. In the case of pre-existing ureteric obstruction, the pressure may be lower according to the distended urinary tract [3, 16]. The mere introduction of the flexible ureteroscope into the ureter raises the renal pelvic pressure in the range 20–25 mmHg, “the scope effect” [3] and a further rise of 20 mmHg may be found in the case of a full bladder. Individual variations in anatomy and compliance of the upper urinary tract will influence the pressure as well [14].
During ureteroscopy in pigs, renal pelvic pressures were measured in the range 28–122 mmHg depending on the height of the irrigation bag (25–90 cm) and bladder filling [3]. Previous studies in man [16] disclosed pressures of 345–410 mmHg when a syringe was used for irrigation. Although the pelvic pressures remained lower, in the range 70–110 mmHg, when a roller-pump mechanical irrigation device was used, the magnitude of the pressures gives rise to concern about the conceivable injury to the kidney parenchyma but especially to pelvic rupture and septic complications.
In a study on cadaveric human kidneys, intrarenal reflux occurred at 30 mmHg [17]. Pyelovenous reflux, which is often associated with infection, was observed at intrarenal pressures of 37–52 mmHg. Pyelotubular reflux was evident at 22–30 mmHg [17]. Intrarenal reflux rarely occurred at pressure levels below 35 mmHg, but was evident at pressures above 45 mmHg [18]. In a similar study, the threshold pressure for intrarenal backflow was about 35 mmHg, although abnormal conditions (low urine flow, ischemic damage and vesico-ureteric reflux) lower the critical pressure [13].
The fornix is susceptible to ruptures during raised intra renal pressures [19]. When this happens the primary site of extravasation is the sinus, pyelosinous backflow. From the sinus, backflow may extend towards the hilum and hence into the retroperitoneal space as well as into the veins and lymphatics [13, 19]. In animal studies, pyelosinous backflow is recorded at intrarenal pressures of 60–70 mmHg in rabbits [19] and at about 200 mmHg in piglets [20]. Several complications have been attributed to pyelosinous backflow although calyceal ruptures following raised intrarenal pressures most often seem to heal spontaneously [13]. However, recurrent episodes of increased pressure may lead to irreversible changes such as fibrolipomathosis [21], perirenal pseudocysts [22], retroperitoneal oedema [23], perinephritic abscess [24] and adhesions with nodular masses of tissue reaction and haemorrhage simulating a pelvic tumour [25].
Urine is a well-known causative agent of fibrotic reaction whenever it escapes from its normal pathway [26]. It seems to be possible that calyceal rupture seen in cases with prolonged intrarenal pressure and backflow may allow leakage of urine containing Tamm–Horsfall protein and bacteria into the interstitial tissue which gives rise to an inflammatory process and subsequent fibrosis. This series of events may be responsible for papillary damage [13].
In animal studies, high-pressure (>150 mmHg) ureterorenoscopic irrigation was found to produce denudation of the calyceal urothelium with submucosal oedema and congestion [3, 16]. Acutely, renal tubules subjected to high irrigant pressure demonstrated marked vacuolization and degeneration, whereas tubules subjected to low pressure appeared normal [3]. Seventy per cent of porcine kidneys subjected to high-pressure irrigation showed histological signs of metaplasia, pericalyceal vasculitis and tubular degeneration after 4–6 weeks. These changes were less pronounced in kidneys subjected to lower pressures (<75 mmHg) [16]. Rupture of the intrarenal collecting system occurred at 330 mmHg.
A peroperative complication rate of 5.6% has been reported after ureteroscopy in 191 patients [27]. Postoperative complications including pain, fever and sepsis occurred in 8.9%. The intraoperative complication rate was 10.2% in a study including 182 ureteroscopic procedures [28]. The most frequent complications were bleeding and perforation of the ureter. Renography is not routinely performed after ureteroscopic procedures and to our knowledge there have not been any investigations in man evaluating the potential harm on the functional kidney parenchyma these procedures may involve. In particular, the maximum pressure safely tolerated by the kidney without following permanent parenchymal scarring has not been determined. Only from animal experiments do we know that kidneys subjected to high pressures may be irreversibly injured [3, 16].
Evidently, it may be of great importance to keep renal pelvic pressure as low as possible during endourological procedures although threshold values are not known. Therefore, we have searched the literature with particular interest in how renal pelvic and ureteric pressures may be controlled pharmacologically.
Physiology of the upper urinary tract
Previously, the ureter was considered a simple tube connecting the kidney and bladder and with a continuous flow of urine depending exclusively on gravity. Today we know that urine transport from the kidney to the bladder is a function of pelvic and ureteric peristalsis [14, 29–31] and that different receptor types are represented in the renal pelvic and ureteric smooth muscle cells [32–34].
In both man and animals, peristaltic activity begins with the origin of electrical activity in the proximal portion of the urinary collecting system [14, 34, 35]. A main theory is that spontaneous depolarizations and succeeding action potentials in the calyceal and renal pelvic smooth muscle cell membranes generate the pelvic peristalsis which propagates through the ureter [14, 30, 34]. The theory is supported by the finding of “pacemaker-cells” and recording of electrical activity in the human and pig calyces, renal pelvis and ureter [14, 35]. Canine experiments revealed that the calyces and renal pelvis exercise control over the function of the ureter, both in determining the distributive properties of ureteric peristalsis and in defining its range of effectiveness [30]. A concept for the generation of ureteric peristalsis in multicalyceal kidneys is that several primary oscillators exist in the calyces; in the pyeloureteric junction, a secondary pacemaker functions with an intrinsic frequency slightly lower than that of the calyceal pacemakers [35]. Both processes cooperate in the generation of ureteric peristalsis. The frequency gradient between calyceal and pelvic pacemaker activity is essential in initialization and controlling orthograde peristalsis [36], and chronic ureteric obstruction disrupts this coordinating mechanism [31].
Two histochemically distinct types of smooth muscle cells have been revealed in the musculature in the proximal portion of the human urinary tract [37]. It seems that one type of the cells is “atypical” in that they are devoid of cholinesterase in comparison to the other type. These cells are present in the walls of the major calices and pelvis but ceases in the pelveoureteric region. It is proposed that the atypical cells are capable of spontaneous contractility and perform a pacemaker function responsible for the initiation of ureteric peristalsis [37].
Another theory is that pelvic activity is stimulated by stretch of the pelvic musculature which agrees with the observation that peristalsis varies with baseline pelvic pressure [14]. This has led to the hypothesis that at high urine flows (high diuresis) the pelvic pressure rises and the smooth musculature is subjected to stress which subsequently increases the frequency of pelvic activity [38, 39]. Therefore, it may be that the combination of urinary distension of the pelvis and ureter and the propagation of an action potential from the calyces is necessary for the induction of ureteric peristaltic activity.
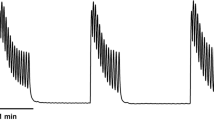
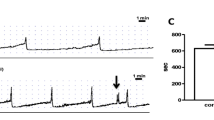
A study of pressure and flow in the pig renal pelvis showed a four-phase relationship in the flow range 0–20 ml/min [29]. In the first phase, covering normal urine output, urine transport takes place in isolated boli with no increase in pelvic filling pressure. Only in this phase does the ureteropelvic junction function adequately and protects the renal pelvis. Phase 2, at flow rates between 4 and 6 ml/min, is characterized by a significant pressure increase because passive filling is inhibited by the preceding peristaltic contraction ring. In phase 3 (6 ml/min) the increment in pelvic pressure decreases, probably as a function of leakage between boli (Tables 3, 4). When the flow rate exceeds 6 ml/min, a linear relation between pressure and flow is found because the ureter functions as an open tube with continuous flow, phase 4.
Existence of excitatory α-adrenergic and inhibitory β-adrenergic as well as muscarinic cholinergic receptors and the finding of high levels of catecholamines in the ureter have been demonstrated [40]. Sakamoto et al. [41] evidenced the existence of five different muscarinic receptor subtypes in the human ureter using an immunostain antibody method. α1 adrenergic receptor subtypes have been recognized along the human ureter utilizing immunohistochemical techniques [42]. By means of a receptor-binding assay, Park et al. [33] demonstrated the distribution of β-adrenoceptors in the human ureter. Specimens of human ureters were placed in a tissue bath and the adrenergic agents epinephrine, norepinephrine and isoprenaline were added. A relaxant effect on the ureter was found since the amplitude and frequency of ureteric contractions declined indicating that especially β2 and β3 receptors induce relaxation of the ureteric smooth muscles. The rank order of relaxing potency for the three catecholamines was isoproterenol > epinephrine > norepinephrine. Although epinephrine and norepinephrine are mainly considered α-adrenergic, they may predominantly exert β-adrenergic effects when administered directly into the ureter [43]. Conclusively the authors stated that β1, β2 and β3 adrenergic receptors are expressed in human ureteral smooth muscle and that primarily β2 and β3 adrenoceptors mediate the ureteric relaxation induced by adrenergic stimulation [33]. Most investigators agree that α-adrenergic agents tend to stimulate ureteric activity [40, 44–46] whereas several experiments have found β-adrenergic agonists like isoproterenol to inhibit ureteric peristalsis [40, 44, 47, 48]. In contrast, cholinergic (parasympathetic) agonists potentate ureteric contractility by directly stimulating cholinergic receptors [40, 47, 49].
Pharmacological modulation of ureteric activity and renal pelvic pressure
In vitro experiments
Morita et al. [50] showed in excised canine ureters that norepinephrine elevates baseline renal pelvic pressure accelerating the ureteric peristaltic rate. Adding acetylcholine pacemaker discharges were initially quickened but after 10 s the baseline and contraction pressures declined, whereas peristalsis was stimulated by acetylcholine.
Deane [47] examined the effects of different catecholamines on freshly harvested ureters from domestic pigs placed in Krebs’ solution in the so-called “Trendelenburg apparatus”. The length of the ureteric segment was measured in response to addition of a variety of drugs. Isoproterenol depressed ureteric contractility. When propranolol, a β-receptor blocking agent, was added the effect of isoproterenol was blocked. Propranolol administered with norepinephrine intensified the stimulatory α-adrenergic effects on ureteric activity induced by norepinephrine. This suggests the presence of both α-adrenergic stimulatory and β-adrenergic inhibitory receptors in the ureter. Furthermore, the presence of α-adrenergic stimulatory and β-adrenergic inhibitory receptors in the rabbit ureter has been shown by adding norepinephrine, isoproterenol and phenylepinephrine and their respective antagonists to a solution containing isolated rabbit ureter [51]. Both norepinephrine and phenylepinephrine were shown to elicit a stimulatory effect on ureteric contraction force whereas isoproterenol inhibited contractility. The α-adrenergic antagonist regitine decreased the stimulatory effect of norepinephrine and phenylepinephrine. Propranolol tended to potentiate the stimulatory effect of norepinephrine.
The effects of the prostaglandin inhibitors indomethacin and diclofenac on peristalsis were studied in isolated strips of human ureters [4]. Indomethacin reduced smooth muscle activity in the renal pelvis thereby lowering intrarenal pressure. Normally, a rise in renal pelvic pressure stimulates the synthesis of prostaglandins in the kidney which causes a temporary rise in renal blood flow and pelvic pressure [52]. Prostaglandin inhibitors suppressed this effect [4]. These observations were substantiated by results from in vivo experiments in pigs during artificially induced ureteric obstruction [9]. Maximum pelvic pressure and the rate of pressure increase were significantly reduced by intravenous indomethacin. The unselective COX-inhibitor diclofenac has been shown to inhibit ureteral contractions and possess spasmolytic properties in several studies [7, 8, 53, 54]. The inhibition of ureteric activity was reversed by prostaglandin F2 alpha [7].
The effects of the calcium-channel blocker verapamil and the methylxanthin derivate theophylline have been examined by suspending strips of human ureter in a tissue bath [55]. Subsequently, after incubation with drugs, changes in contractility were recorded. Verapamil caused ureteric relaxation probably through calcium channel blockade. Surprisingly, theophylline, a well-known smooth muscle relaxant in other tissues, primarily in the bronchioles, had minimal effect. In vivo, peristalsis was inhibited by theophylline when administered intraluminally in the ureter, suggesting a secondary phosphodiesterase inhibition.
In an in vitro study on canine ureter lidocaine caused increased contractility and a marked reduction of flow in the ureters when examined in a tissue bath [56]. The authors concluded that the cessation of flow through the ureter was a result of continuous contraction of the ureter. The generation of “aperistalsis” and flow abolishment caused by lidocaine makes this drug inappropriate for clinical use. The mode of action in the ureteric smooth musculature is not well known as no receptors are present for lidocaine. It may diffuse through the mucosa and muscularis and elicit a direct effect on the cell membrane causing a release of calcium into the cell [57].
Intravenous administration of pharmacological agents
Danuser et al. [58] studied the effects of the β-agonists isoproterenol and fenoterol and of phenylepinephrine administered intravenously in pigs. Phenylepinephrine induced increasing contraction frequency and amplitude in a dose-dependent manner. Distinct cardiovascular side effects were, however, observed. Phenylepinephrine effects were reversed by an α-adrenergic antagonist. Isoproterenol decreased contraction frequency to 13% compared to controls and this effect was blocked by propranolol. Similar relaxing effects on the ureteric activity were seen with administration of fenoterol. In reducing pelvic pressure the β-agonists elicited high efficacy, but the authors pointed out that the perfect drug exerting maximal inhibiting effect on ureteric peristalsis without causing systemic side effects still had to be identified.
These results agree with experimental studies on dog ureters [40, 44, 59, 60] in which α-adrenergic agents such as epinephrine and norepinephrine stimulated ureteric activity, an effect that was suppressed by α-adrenergic blockers. Similarly urecholine, a cholinergic agent, increased the frequency of ureteric contractions and renal pelvic pressure. Isoproterenol decreased ureteric wall tension, ureteric peristalsis and renal pelvic pressure. These effects were inhibited by a β-adrenergic blocking agent.
Anticholinergic agents elicited a marked effect on the ureteric activity and renal pelvic pressure in a study in rabbits [61]. A fall in baseline ureteric pressure was recorded and ureteric peristalsis was temporarily abolished. The authors pointed out that the reproducibility of this study in man may be uncertain because the corresponding doses of atropine probably would be toxic.
Serotonin (5-hydroxytryptamine) has been shown to have a stimulatory effect on pelvic pressure and contraction rate and force of ureteric peristalsis in anaesthetized dogs [62]. The contractions induced were so intense that they completely prevented the passage of fluid through the ureteric lumen during the first minutes after administration of serotonin. Intravenous administration of morphine did not influence the pelvic pressure or peristalsis in humans [57, 63].
In a canine study, the anaesthetic agent pentobarbital was demonstrated to cause total blockade of ureteric peristalsis. Pacemaker activity was completely abolished resulting in accumulation of urine in the renal pelvis and subsequently passive transport through the ureter [64]. Thus, the authors concluded that clinical use might be controversial.
Per orally administrated drugs in patients with ureteric stones
As described above, the influence on pyeloureteric dynamics is well known for adrenergic agents and their inhibitors from animal studies. To facilitate spontaneous stone passage researchers have examined drugs with potential impact on ureteric smooth muscle cells. Tamsulosin, a selective α-blocker with α1-A and α1-D antagonism has been tested in several studies. The addition of tamsulosin to conventional treatment was found to be beneficial in terms of stone clearance of lower ureteric stones [65]. In a randomized trial comparing corticosteroid plus tamsulosin with corticosteroid plus an antispasmotic agent, an increased expulsion rate and a decreased expulsion time was significant in the group treated with tamsulosin [10]. Additionally, the tamsulosin group required less analgesia and decreased hospitalization time. In a subsequent randomized study, tamsulosin was shown to be superior to nifedipine (a calcium blocker) and phloglucinol in expulsion rate and time and the need for endoscopic intervention [66]. Autorino at al. confirmed the efficacy of tamsulosin in the treatment of distal ureteric stones in a randomized study including two groups of each 32 patients receiving tamsulosin and diclofenac versus diclofenac. After 2 weeks, stone expulsion rate was 88% in the group treated with tamsulosin and diclofenac versus 60% in the group treated with diclofenac (P = 0.01) [67]. The reported incidence of side effects due to tamsulosin was relatively low in all studies compared to those reported in the treatment of LUTS probably as a result of the short duration of treatment. The efficacy of α-adrenergic blockers has made several authors conclude that a conservative approach should be considered in the management of uncomplicated distal ureteric stones.
Two randomized clinical studies have shown that the calcium antagonist nifedipine also improves stone expulsion rate and time [68, 69]. The mode of action is suggested to be inhibition of the active calcium channel pump in the ureteric smooth muscles. Side effects in these series were significant and implied hypotension, asthenia and headache.
Topical administration of pharmacological agents in the ureter
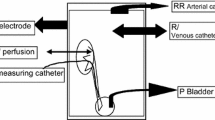
Holst et al. [43, 70, 71] have proved that norepinephrine significantly reduces the pressure increase in pigs during a pressure flow study of the upper urinary tract. Moreover, it was demonstrated that the effect is reproducible and occurs in a dose-dependent manner. Maximal effect of relaxation of the pyeloureter was obtained without side effects from cardiovascular or renal functions even though significant increases in plasma levels of norepinephrine were measured. Addition of a non-selective β-blocking agent diminished the effect of norepinephrine. These observations are in contrast to the majority of studies concerning intravenous administration of adrenergic agents, which suggest a stimulatory effect of norepinephrine on ureteric activity. The authors propose that norepinephrine stimulates β-receptors when administered endoluminally, whereas intravenous administration stimulates α-receptors. The studies show that a desired local effect on pyeloureteric dynamics is obtainable by endoluminal administration of a drug.
Danuser et al. [58] evaluated the effects on the pig ureter of a diversity of agents administered topically. Isoproterenol and fenoterol, non-selective β-adrenergic agonists, inhibited frequency and amplitude of ureteric contractions but caused systemic side effects in a dose-dependent manner suggesting an absorption by the urothelium. These effects were blocked by propranolol. Epinephrine stimulated ureteric activity and no increase in heart rate was observed.
The influence of verapamil, prostaglandin F2α, norepinephrine and phenylepinephrine on the pig ureter when administered topically in the ureter was first studied by Selmy et al. [72]. It was shown that verapamil significantly reduced the peristaltic rate and no systemic side effects were recorded, indicating minimal or no urothelial absorption. Phenylepinephrine and norepinephrine perfusion increased renal pelvic pressure and augmented the frequency of ureteric peristalsis. Prostaglandin F2α tended to increase ureteric peristalsis and pelvic pressures in high doses but no significant findings were obtained. None of the drugs affected heart rate or blood pressure.
A 5-HT agonist induced increased ureteric contractions when administered topically in pigs. Systemic side effects were only seen following intravenous administration [62, 73, 74]. The 5-HT antagonist ketanserin had opposite effects. Responses were dose-dependent in both cases.
Weiss et al. [75] inhibited pig ureteric motility in vivo by adding the potassium channel opener PKF 217–744b and the calcium antagonist nicorandil intraluminally. The drugs decreased spontaneous contraction frequency in pigs and man and no systemic side effects were seen when administered topically. The authors stated that potassium channel openers may be promising drugs for clinical application in patients with renal colics.
The effects of topical lidocaine on motility of the human ureter in vivo were evaluated by Andersson and Ulmsten [57]. Topical administration of lidocaine into the ureter elicited a brief stimulatory effect followed by total abolishment of ureteric contractions. The peristaltic activity gradually resumed its prelidocaine level within 15–30 min. The depressing effect of lidocaine on the ureteric pacemakers simulating lidocaine’s effect on the cardiac pacemakers was, however, questioned. It is suggested that lidocaine might exert a direct effect on the cell membrane causing a release of calcium into the cell.
Clinical aspects and perspectives
Modulation of ureteric peristalsis and thereby ureteric pressure is an everyday urological practice. Promotion of stone passage using calcium antagonists and especially α-blockers has achieved good results [6, 10, 65]. On the other hand, modulation of renal pelvic and ureteric pressure during ureteropyeloscopy is not used routinely. The reviewed animal experiments, however, reveal that many pharmacological agents might be beneficial.
As stated in the introduction, ureteropyeloscopy is regarded as a minimal invasive procedure followed by few complications but to our knowledge nobody has looked for long-term damage to the kidneys. Animal studies have shown that high renal pelvic pressure may cause harm. Prolonged exposure of the kidney to high renal pelvic pressures (>100–150 mmHg) may result in irreversible renal damage not seen during endoscopic procedures with lower pelvic pressures [3, 13, 16, 19]. The risk of pyelorenal backflow with the potential for producing bacteraemia and sepsis is increased as well [3]. The relationship between exposure pressure, exposure time, infectious complications and renal damage, however, still needs to be clarified. Besides, it is obvious that variations in the patient’s anatomy, compliance of the upper urinary tract and degree of obstruction may play a role as well. To uncover the safety limits with regard to maximum intrarenal pressure tolerated by the kidney parenchyma, experimental studies including renographic examination before and after ureteropyeloscopy are necessary. Additionally, the pressure increase corresponding to variable flow rates through the ureteroscope should be determined.
Besides possessing the potential of reducing complications and permanent damage to the renal parenchyma, lowering the pressure may also facilitate both diagnostic and therapeutic endourological procedures. With these purposes, the use of ureteric access sheaths has been tried out. Despite the presence of an access sheath during flexible ureteroscopy, renal pelvic pressures above 40 mmHg have been measured [76]. Moreover, the necessity of further instrumentation in order to decrease the pressure may involve other disadvantages. In other words, the use of drugs capable of inhibiting pyeloureteral dynamics when administered topically into the urinary tract without causing any side effects might be advantageous with regard to clinical practice.
Many different drugs have been studied and no single drug can be assigned as the most effective to modulate ureteric and renal pelvic pressures. But the studies in this review show that endoluminal or topical administration is superior in consideration to local gain and systemic risks. Several drugs with potential usefulness have undesirable systemic side effects when given intravenously [58]. In contrast, topical administration appears to be safe. Especially, β-adrenergic agonists are effective and safe to lower renal pelvic pressure—at least in the pig [43, 58]. Elucidation of pressure reducing capacity, dose-responsiveness and side effects when using combinations of two or several drugs could be interesting for future experimental investigations. For example, the combination of a β-adrenergic agonist and an α-adrenergic blocker which have both shown capability in reducing ureteric and pelvic pressure may be advantageous. Combination with a calcium antagonist or NSAID may have complementary effects as well. The exact dose–response relationship, the most advantageous administration form, the mode of action inside and outside the pelveoureteral system and the capability of consistent and continuous effect need to be clarified before clinical use of a drug may be profitable and secure.
Conclusion
Evidence is put forward that it is advantageous to keep pelvic pressure as low as possible during endourological procedures. The existence of different receptors in the ureter and renal pelvis has been uncovered. Experimental studies have paid attention to the potential of pyeloureteric endoluminal administration of drugs in order to modulate upper urinary tract dynamics. The use of diagnostic and therapeutic endourological procedures is currently increasing. It is highly relevant to study possible advantages of drug addition to the irrigation fluid to lower pelvic pressure for the outcome of endourological procedures. If we intend to perfect the endoscopic operation, it is time to go from animal studies to human studies.
References
Lim DJ, Shattuck MC, Cook WA (1993) Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy. J Urol 149:109
Sonda LP, Fischer CP, Gross MD, Skinner RW (1985) Pyelovenous backflow: Implications for coagulum pyelolithotomy and nephroscopy. J Urol 133:894
Schwalb DM, Eshghi M, Davidian M, Franco I (1993) Morphological and physiological changes in the urinary tract associated with ureteral dilation and ureteropyeloscopy: an experimental study. J Urol 149:1576
Zwergel UE, Zwergel TB, Neisius DA, Ziegler M (1990) Effects of prostaglandin synthetase inhibitors on the upper urinary tract. Experimental studies on isolated preparations and urodynamic measurements in men. Urol Res 18:429
Ergene U, Pekdemir M, Canda E, Kirkali Z, Fowler J, Coskun F (2001) Ondansetron versus diclofenac sodium in the treatment of acute ureteral colic: a double blind controlled trial. Int Urol Nephrol 33:315
Sowter SJ, Tolley DA (2006) The management of ureteric colic. Curr Opin Urol 16:71
Lennon GM, Bourke J, Ryan PC, Fitzpatrick JM (1993) Pharmacological options for the treatment of acute ureteric colic. An in vitro experimental study. Br J Urol 71:401
Sivrikaya A, Celik OF, Sivrikaya N, Ozgur GK (2003) The effect of diclofenac sodium and papaverine on isolated human ureteric smooth muscle. Int Urol Nephrol 35:479
Frokiaer J, Nielsen AS, Knudsen L, Djurhuus JC, Pedersen EB (1993) The effect of indomethacin infusion on renal hemodynamics and on the renin-angiotensin system during unilateral ureteral obstruction of the pig. J Urol 150:1557
Dellabella M, Milanese G, Muzzonigro G (2003) Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. J Urol 170:2202
De SM, Autorino R, Di LG, Damiano R, Giordano D, Cosentino L, Pane U, Di GF, Mordente S, D’Armiento M (2006) Medical expulsive treatment of distal-ureteral stones using tamsulosin: a single-center experience. J Endourol 20:12
Saltzman B, Khasidy LR, Smith AD (1987) Measurement of renal pelvis pressures during endourologic procedures. Urology 30:472
Thomsen HS (1984) Pyelorenal backflow. Clinical and experimental investigations. Radiologic, nuclear, medical and pathoanatomic studies. Dan Med Bull 31:438
Djurhuus JC (1980) Aspects of renal pelvic function, Thesis, Borns Bogtryk, Københavns Universitet
Mortensen J, Bisballe S, Jorgensen TM, Tagehoj-Jensen F, Djurhuus JC (1982) The normal pressure-flow relationship of pyeloureter in the pig. Urol Int 37:68
Wilson WT, Preminger GM (1990) Intrarenal pressures generated during flexible deflectable ureterorenoscopy. J Endourol 4:2
Boccafoschi C, Lugnani F (1985) Intra-renal reflux. Urol Res 13:253
Hodson CJ (1969) The effects of disturbance of flow on the kidney. J Infect Dis 120:154
Thomsen HS, Dorph S, Olsen S (1982) Pyelorenal backflow in rabbits following clamping of the renal vein and artery: radiologic and microscopic investigation. Acta Radiol Diagn 23:143
Thomsen HS, Larsen S, Talner LB (1982) Pyelorenal backflow during retrograde pyelography in normal and ischemic porcine kidneys. Eur Urol 8:291
Olsson O (1972) Frequency of backflow in acute renal colic. Acta Radiol Diagn 12:469
Schwartz A, Caine M, Hermann G, Bittermann W (1966) Spontaneous renal extravasation during intravenous urography. AJR 98:27
Main J (1967) Spontaneous rupture of the renal pelvis in the only functioning kidney. Br J Surg 54:1033
Harrow BR (1966) Spontaneous urinary extravasation associated with renal colic causing a perinephritic abscess. AJR 98:47
Fajers CM, Idborn H (1957) Peripelvic reflux simulating a tumour of the renal pelvis. Urol Int 5:197
Hodson CJ (1981) Reflux nephropathy. A personal historical review. AJR 137:451
Hollenbeck BK, Schuster TG, Faerber GJ, Wolf JS, Jr. (2001) Comparison of outcomes of ureteroscopy for ureteral calculi located above and below the pelvic brim. Urology 58:351
Chow GK, Patterson DE, Blute ML, Segura JW (2003) Ureteroscopy: effect of technology and technique on clinical practice. J Urol 170:99
Mortensen J (1986) Hydrodynamics of the pyeloureter of the pig. Thesis. Neurourol Urodyn 5:87
Constantinou CE (1974) Renal pelvic pacemaker control of ureteral peristaltic rate. Am J Physiol 226:1413
Djurhuus JC, Constantinou CE (1982) Chronic ureteric obstruction and its impact on the coordinating mechanisms of peristalsis (pyeloureteric pacemaker system). Urol Res 10:267
Boyarsky S, Kirshner N, Labay P (1966) Catecholamine content of the normal dog ureter. Invest Urol 4:97
Park YC, Tomiyama Y, Hayakawa K, Akahane M, Ajisawa Y, Miyatake R, Kiwamoto H, Sugiyama T, Kurita T (2000) Existence of a beta3-adrenoceptro and its functional role in the human ureter. J Urol 164:1364
Weiss RM (1992) Physiology and pharmacology of the renal pelvis and ureter. Campbells urology, vol 6. WB Saunders, Philadelphia, p 111
Hannappel J, Golenhofen K, Hohnsbein J, Lutzeyer W (1982) Pacemaker process of ureteral peristalsis in multicalyceal kidneys. Urol Int 37:240
Hannappel J (1985) Myogenic function of normal and chronically dilated pyeloureters. In: Urodynamics II, vol 13. Springer, Berlin Heidelberg New York
Dixon JS, Gosling JA (1982) The musculature of the human renal calices, pelvis and upper ureter. J Anat 135:129
Bülbring E (1955) Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol 128:200
Bozler E (1942) The activity of the pacemaker previous to the discharge of a muscular impulse. Am J Physiol 136:543
Rose JG, Gillenwater JY (1974) The effect of adrenergic and cholinergic agents and their blockers upon ureteral activity. Invest Urol 11:439
Sakamoto K, Rajasekaran M (2005) Characterization of muscarinic receptor subtypes in human ureter. In: Conference proceeding. 23rd World Congress of Endourology, Amsterdam, August 2005
Madeb R, Nicholson C, Golijanin D, Bourne P (2005) Immunohistochemical and molecular analysis of alpha-1-adrenergic receptors in the human ureter. In: Conference proceeding. 23rd World Congress of Endourology, Amsterdam, August 2005
Holst U, Dissing T, Rawashdeh YF., Frokiaer J, Djurhuus JC, Mortensen J (2003) Norepinephrine inhibits the pelvic pressure increase in response to flow perfusion. J Urol 170:268
McLeod DG, Reynolds DG, Swan KG (1973) Adrenergic mechanisms in the canine ureter. Am J Physiol 224:1054
Tindall AR (1972) Preliminary observations on the mechanical and electrical activity of the rat ureter. J Physiol 223:633
Weiss RM, Zabinski MP, Biancani P (1974) Adrenergic control of ureteral tonus. Invest Urol 12:30
Deane RF (1967) Functional studies of the ureter: its behaviour in the domestic pig as recorded by the technique of Trendelenburg. Br J Urol 39:31
Kiil F, Kjekshus J (1967) The physiology of the ureter and renal pelvis. In: Proceedings of the 3rd international congress on nephrology, vol 2. Washington, p 321
Morita T, Wada I, Saeki H, Tsuchida S (1987) Ureteral urine transport: Changes in bolus volume, peristaltiq frequency, intraluminal pressure and volume of flow resulting from autonomic drugs. J Urol 137:132
Morita T, Suzuki T, Kondo S, Tsuchida S (1983) Effects of noradrenaline and acetylcholine on ureteral peristalsis. Tohoku J Exp Med 141:489
Weiss RM (1975) Autonomic mediators of ureteral function. Fed Proc 34:362
Holmlund D, Svanvik J (1982) The treatment of ureteral colic and biliary pain. Scand J Urol Nephrol Suppl 75
Mastrangelo D, Wisard M, Rohner S, Leisinger H, Iselin CE (2000) Diclofenac and NS-398, a selective cyclooxygenase-2 inhibitor, decrease agonist-induced contractions of the pig isolated ureter. Urol Res 28:376
Brough RJ, Lancashire MJ, Prince JR, Rose MR, Prescott MC, Payne SR, Testa HJ (1998) The effect of diclofenac (voltarol) and pethidine on ureteric peristalsis and the isotope renogram. Eur J Nucl Med 25:1520
Figenshau RS, Ames CD, Jerde TJ, Minor SD (2005) Acute effects of verapamil and theophylline on ureteral smooth muscle activity: In vitro and in vivo study. In: Conference proceeding 23rd World Congress of Endourology, Amsterdam August 2005
Katz G (1993) Effect of lidocaine on ureteral motility and fluid transport in vitro. Urology 41:489
Andersson KE, Ulmsten U (1975) Effects of spinal anaesthesia, lidocaine, and morphine, on the motility of the human ureter in vivo. Scand J Urol Nephrol 9:236
Danuser H, Weiss R, Abel D, Walter B, Scholtysik G, Mettler D, Studer UE (2001) Systemic and topical drug administration in the pig ureter: effect of phosphodiesterase inhibitors alpha1, beta and beta2-adrenergic receptor agonists and antagonists on the frequency and amplitude of ureteral contractions. J Urology 166:714
Kondo S, Morita T., Saeki H, Tsuchida S (1985) Effects of autonomic drugs on in vivo recording of electromyograms of canine renal pelvis and ureter. Urol Int 40:260
Morita T, Suzuki T, Kondo S, Saeki H, Nishimoto T, Tsuchida S (1985) Relationship between pelviureteral peristaltic frequency and urine flow change evoked by autonomic drug administration. Tohoku J Exp Med 146:265
Sjögren C, Ulmsten U (1977) The effects of some anticholinergic compounds on the rabbit ureter. Scand J Urol Nephrol 11:149
Catacutan-Labay P, Boyarsky S, Gerber C (1966) The effect of serotonin (5-hydroxytryptamine) on ureteral peristalsis. Invest Urol 4:224
Boyarsky S, Labay P (1972) Ureteral dynamics: pathophysiology, drugs and surgical implication. Williams and Wilkins, Baltimore
Yamaguchi O, Tsuchida S (1979) Dynamics of upper urinary tract during pacemaker blockade induced by pentobarbital. Tohoku J Exp Med 127:9
Kupeli B, Irkilata L, Gurocak S, Tunc L, Kirac M, Karaoglan U, Bozkirli I (2004) Does tamsulosin enhance lower ureteral stone clearance with or without shock wave lithotripsy? Urology 64:1111
Dellabella M, Milanese G, Muzzonigro G (2005) Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 174:167
Autorino R, De SM, Damiano R, Di LG, Perdona S, Russo A, Quarto G, Cosentino L, D’Armiento M (2005) The use of tamsulosin in the medical treatment of ureteral calculi: where do we stand? Urol Res 33:460
Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, Mineo F (1994) Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol 152:1095
Porpiglia F, Destefanis P, Fiori C, Fontana D (2000) Effectiveness of nifedipine and deflazacort in the management of distal ureter stones. Urology 56:579
Holst U, Rawashdeh YF., Andreasen F., Djurhuus JC, Mortensen J. (2005) Endoluminal pelvic perfusion with norepinephrine causes only minor systemic effects and diminishes the increase in pelvic pressure caused by perfusion. Scand J Urol Nephrol 39:443
Holst U, Jakobsen JS, Djurhuus JC, Andreasen F, Mortensen J (2005) Belysning af virkningsmekanismen bag noradrenalins hæmning på aktiviteten i øvre urinveje. En eksperimentel undersøgelse på grise (personal communication)
Selmy GI, Hassouna MM, KhalafI M, Elhilali MM (1994) Effects of verapamil, prostaglandin F2 alpha, phenylephrine, and noradrenaline on upper urinary tract dynamics. Urology 43:31
Abrahams VC, Pickford M (1956) The effect of 5-hydroxytryptamine on the ureter and on the blood pressure of dogs; and of adrenaline, noradrenaline, and posterior pituitary extracts on the ureter. Br J Pharmacol Chemother 11:44
Hauser DS, Mevissen M, Weiss R, Portier CJ, Scholtysik G, Studer UE, Danuser H (2002) Effects of ketanserin and DOI on spontaneous and 5-HT-evoked peristalsis of the pig ureter in vivo. Br J Pharmacol 135:1026
Weiss R, Mevissen M, Hauser DS, Scholtysik G, Portier CJ, Walter B, Studer UE, Danuser H (2002) Inhibition of human and pig ureter motility in vitro and in vivo by the K(+) channel openers PKF 217–744b and nicorandil. J Pharmacol Exp Ther 302:651
Auge BK, Pietrow PK, Lallas CD, Raj GV, Santa-Cruz RW, Preminger GM (2004) Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 18:33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, H.U., Frimodt-Møller, P.C., Osther, P.J. et al. Pharmacological effect on pyeloureteric dynamics with a clinical perspective: a review of the literature. Urol Res 34, 341–350 (2006). https://doi.org/10.1007/s00240-006-0069-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-006-0069-x