Abstract
The association between serum gonadal steroids and urolithiasis in males received only limited attention. Calcium oxalate urolithiasis is induced by administration of ethylene glycol in drinking water. It appears that the administration of natural antioxidants has been used to protect against nephrolithiasis in human and experimental animals. The purpose is to study the potential role of antioxidants as inhibitors of high plasma androgenic markers or hyperandrogenicity in the pathogenesis of ethylene glycol-induced nephrolithiasis in Wistar rats. Male Wistar rats were studied in 4-week period. Group 1 (control) was fed a standard commercial diet. Group 2 received the same diet with 0.5 % of ethylene glycol. Group 3 received EG plus the diet and water added with antioxidant nutrients and lime juice as the dietary source of citrate. Group 4 and Group 5 were treated similar to Group 2 and Group 3 with 0.75 % of ethylene glycol. For antioxidant supplementation, the standard diet enriched with 4,000.0 μg vitamin E and 1,500.0 IU vitamin A for each rat per day added to the diet once a week, and provided daily with 5.0 mg vitamin C, 400.0 μg vitamin B6, 20.0 μg selenium, 12.0 mg zinc, and 2.0 mg boron for each rat per day in their drinking water. After treatment period, collection of blood was performed and kidneys were removed and used for histopathological examination. The results based on various assays, measuring size of crystal deposition, and histological examinations showed that high concentration of androgens acts as promoter for the formation of renal calculi due to ethylene glycol consumption and the inhibitory role of antioxidant complex in the formation of renal calculi disease. Data revealed that the size and the mean number of crystal deposits determined in EG 0.75 % treated groups (G4) were significantly higher than the EG-treated groups, added with antioxidant nutrients and lime juice (G5). The mean concentration of androgens in Group 4 increased after EG 0.75 % administration, and decreased after antioxidants supplementation in Group 5. Elevated concentration of androgens (as promoters of the formation of renal calculi) as a result of EG consumption and their decreasing following antioxidant supplementations along with the slight decrease in malondialdehyde level provides a scientific rational for preventive and treatment roles of antioxidant nutrient complex in kidney stone disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a multifactorial disorder influenced by both intrinsic and environmental factors.
It is a complex process that is a consequence of an imbalance between promoters and inhibitors in the kidney. Among the studied factors, male gender with a three times higher incidence of urolithiasis than female gender is considered as a risk factor, but the influence of sex hormones on urinary stone formation remains undetermined [1] and the pathogenesis of this male predisposition still remains to be elucidated. The two possibilities for this male predominance are either that male hormone may promote or that female hormones may inhibit kidney stone formation. Although clinical proof for this hypothesis is lacking, there is some initial experimental evidence to implicate sex hormones in the pathogenesis of stone disease [2].
Elevated free radical production as indicated by increase in malondialdehyde (MDA) concentration due to EG consumption in the formation of nephrolithiasis confirms that renal tissue is under oxidative stress. This hypothesis is strengthened by the report that patients with kidney stones have less activity of antioxidant enzymes with increased lipid peroxidation [3].
Data suggest that testosterone can promote and estrogen may inhibit calcium oxalate stone formation in EG-treated rats [4], likely because of their effects on oxalate synthesis and oxidative stress [5]. Testosterone appears to promote stone formation by suppressing osteopontin expression in the kidneys and increasing urinary oxalate excretion. Estrogen appears to inhibit stone formation by increasing osteopontin expression in the kidneys and decreasing urinary oxalate excretion [6].
Therefore, the aim of the current study was to test the effects of a combination of natural antioxidants such as vitamins A, C, E and B6; zinc and selenium plus boron with a proposed antioxidant capacity against ethylene glycol (EG)-induced nephrolithiasis caused by hyperandrogenicity.
Materials and methods
Male Wistar rats weighing 150–200 g were obtained from the Animal House of Physiology Group, Baqiyattallah University of Medical Sciences. Seven rats in each group (control vs. 4 treatment groups) were weighed and randomly kept in plastic cages in a controlled environment with a 12-h light/dark cycle and a constant temperature (22–25 °C) and humidity (55–65 %), with free access to food and water. They had access to normal rat chow diet and water for 7 days before the beginning of experimental protocols. Animals were provided clean cages weekly, for 4 weeks. All experiments were performed in accordance with the “Public Health Service Policy on Humane Care and Use of Laboratory Animals (NIH Office of Laboratory Animal Welfare, revised 2002) and the guidelines of the care of animals and approved by the Baqiyattallah University of Medical Sciences’ Research and Ethics Committee.
Diets and animal treatments
Rats in all groups were fed with standard rat chow from Pars Animal Food Co. (Tehran, Iran) and water ad libitum throughout study. According to the manufacturer, it contained 9.0 IU vitamin A, 18.0 μg vitamin E, 3.0 μg vitamin B6, 0.20 μg selenium and 85.0 μg zinc per g of dry food, with no added vitamin C and boron. The daily supplementation of the nutrients in the diet or the water consumed by the animals was calculated given that a 150–200 g animal food intake is approximately 20.0 g/day and drinks water at the rate of 10–12 ml/100 g body weight/day. Therefore, the supplementation rate was considered to provide the amounts of antioxidant nutrients approximately five to seven times of the natural daily intake. Accordingly, Group 1 (control) was fed a standard commercial diet (rat chow). Group 2 received the same diet with 0.5 % of ethylene glycol (EG) (Sigma-Aldrich Co., St. Louis, MO, USA) in drinking water for 28 days ad libitum to induce the kidney stone formation. Group 3 received the standard diet enriched with 4,000.0 μg vitamin E and 1,500.0 IU vitamin A for each rat per day added to the diet once a week, and provided daily with 5.0 mg vitamin C, 400.0 μg vitamin B6, 20.0 μg selenium, 12.0 mg zinc, and 2.0 mg boron for each rat per day in their drinking water plus 0.5 % of EG. Group 4 and Group 5 treated similarly, except receiving 0.75 % of EG in water. Commercial lime juice as the dietary source of citrate was added to the water at the level of 1 ml/rat/day for groups 3 and 5.
To provide the antioxidant nutrients, 300.0 IU (200.0 mg) of a softgel capsule of vitamin E as dl-alpha tocopheryl acetate (Vitane Pharmaceutical Inc., Costa Mesa, CA, USA) and 75,000.0 IU of a softgel capsule of vitamin A as Palmitate (Daana Pharma. Co., Tabriz, Iran) were dissolved in 3.0 ml of corn oil and added to 85.0 g of standard diet (~12.0 g/rat) and given once a week, to provide vitamin E and vitamin A at doses as mentioned above. The non-antioxidant groups were given 3.0 ml of corn oil alone added to 85.0 g of standard diet once a week.
Pharmaceutical vitamin C (ascorbic acid) 250.0 mg tablets (Osvah Pharmaceutical Co., Tehran, Iran), pyridoxine HCl (vitamin B6) 40.0 mg tablets (Ramopharmin Pharmaceutical Lab., Tehran, Iran), selenium (selenium amino acid chelate) 200.0 μg dietary supplement capsules (Alfa Vitamins Lab., Inc., Doral, FL, USA), zinc (zinc sulfate) 50.0 mg capsules (Alhavi Pharmaceutical Co., Tehran, Iran), and Boric acid (Merck-Germany) as the source of boron were used and added to their drinking water to provide the above-mentioned doses/rat/day, respectively. Lime juice (Mahram Co. Group, Shiraz, Iran) was purchased from local market. Fresh food and water containing the above chemicals were provided three times per week and the consumption was monitored and recorded.
The experimental procedure adopted for the study is detailed as follows.
-
Group 1 or control (CONT): the standard diet and drinking water;
-
Group 2 (EG 0.5 %): same as Group 1 + 0.5 % of ethylene glycol (EG) in drinking water;
-
Group 3 (EG 0.5 % + AX): same as Group 2 + antioxidant nutrients, boron and lime juice;
-
Group 4 (EG 0.75 %): same as Group 1 + 0.75 % of ethylene glycol (EG) in drinking water;
-
Group 5 (EG 0.75 % + AX): same as Group 4 + antioxidant nutrients, boron and lime juice;
Four weeks after the experimental periods, rats were restrained from food for 12 h but with access to drinking water were anesthetized for the collection of blood by cardiac puncture with a syringe and needle for further biochemical studies. Since, in rats, some parameters such as steroid hormones are subject to circadian rhythm; therefore, blood samples were collected at the peak time in the afternoon between 14.00 and 16.00 hours and plasma samples stored frozen until analysis. Kidney samples from Groups 1, 4 and 5 who received higher dose of EG were collected for histology examination.
Left kidney from each animal was placed and fixed in 10 % formalin and dehydrated in a gradient of ethanol, embedded in paraffin, and then cut into 5-μm serial sections. Serial sections were cut and stained by hematoxylin and eosin and von Kossa (for phosphate detection). Nine slides from lateral, middle, and medial sections were selected randomly. The histopathological changes and calcium oxalate crystal deposition at 40× magnifications under light microscope were studied in six fields of each section, composed of three fields in medulla and three fields in cortex. Aggregations of crystal deposits in the renal tubules were counted in 54 microscopic fields and an average was calculated. The size of crystal deposition was measured using Motic system Image analyzer [Motic Image 2000 1.2 micro-optic industrial group Co. LTD) and categorized as zero (no crystal), small (1–10 μm), medium (>10–20 μm), and large (>20 μm)].
Malondialdehyde (MDA) content, representing lipid peroxidation, determined by the thiobarbituric acid reactive method was measured by Photometric, Bioassay Systems, CA, USA, with the intra-assay coefficients of variation (CVs) % and assay sensitivity of 5.5 and 0.1 mmol/l.
Commercially available assay kits were used to determine the blood hormone levels.
Total testosterone (T), free testosterone (FT), dihydrotestosterone (DHT), and estradiol (E2) were analyzed by ELISA, Diagnostics Biochem, Canada Inc., Ontario, Canada. The intra-assay coefficients of variation (CVs) % and assay sensitivity were 5.1 and 0.022 ng/ml for T; 3.4 and 0.17 pg/ml for FT; 2.1 and 6.0 pg/ml for DHT; and 4.4 and 1.0 pg/ml for E2, respectively. Sex hormone binding globulin (SHBG) was analyzed by ELISA kit, CUSABIO BIOTECH CO. Ltd. Wuhan, Hubei, China, with the intra-assay coefficients of variation (CVs) % and assay sensitivity of 6.4 and 1.0 ng/ml.
Statistical analysis
Data are expressed as mean ± SD and a Statistical Package for the Social Sciences [(SPSS 18.0), New York: McGraw-Hill] was used to perform all comparisons. One-way ANOVA (LSD post hoc test) was used for comparison of the mean of the hormones. A P value of ≤0.05 was considered statistically significant for the differences.
Results
The rats in all groups adjusted to the treatments well, and consumed food and water normally.
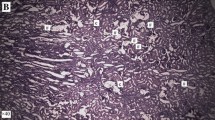
The effect of different treatments on the mean number of crystal deposits in the microscopic fields in the kidney specimens and on the plasma hormones in different groups is shown in Tables 1 and 2. The results of histopathological examinations are presented in Fig. 1.
Microscopic images of kidney sections from a vehicle control animals (G1), b lithiatic group (G4), c group treated with supplements (G5) and d crystal deposit on the surface of the papillary tip in group treated with supplements (G5). a Normal tubules and collecting ducts are shown in a rat’s kidney in negative control group (G1) [C.H&E (hematoxylin–eosin), C.vK (von Kossa (for phosphate detection))]. b Multiple tubular calculi (red arrows) in renal tubules (S.H&E, S.vK) with marked histological changes including tubular dilation (black arrows), with epithelial damage secondary to multiple calculi accompanied with inflammatory infiltration (red arrows) in peritubular space from ethylene glycol treated rat (G4). c Significant reduction and less number of crystal deposits (red arrows) in rats treated with ethylene glycol and supplements (G5) (T.H&E, T.vK). d Fewer crystal deposit on the surface of the papillary tips (red arrows) with renal tubular dilation (black arrows) in a kidney of rat treated with supplements (G5) (PT.H&E, PT.vK). Crystals observed by light microscope (H&E, Von Kossa staining, ×40–×400)
In the 4 weeks study, the mean number of deposits in EG 0.75 % group (G4) was higher than the EG 0.75 % + AX group (G5). Crystal deposition was completely absent in kidneys in the control group (G1) (Table 1).
The results of MDA as a bio-marker for the production of reactive oxygen species and the plasma hormone analysis in all groups are presented in Table 2.
A slight difference in the plasma concentration of MDA was noted between EG 0.75 % group (G4) and EG 0.75 % + AX group (G5), observing a lower concentration in G5.
There was a higher difference in the plasma concentration of T in EG 0.75 % group (G4) compared with Group 1 (G1), (P = 0.047), followed by a significant reduction with G5 (P = 0.011) and G2 (P = 0.004).
A higher plasma concentration of FT in EG 0.5 % group (G2) observed compared with Group 1 (G1), (P = 0.007), and decreased in Group 3 (G3), (P = 0.000), Group 4 (G4), (P = 0.03), and it was significantly lower in G5 (P = 0.000). The P value for FT between G4 and G5 was 0.08.
The plasma concentration of DHT in EG 0.75 % group (G4) was significantly higher than Group 1 (G1), (P = 0.040); and Group 2 (G2), (P = 0.004); and showed a significant reduction after antioxidant treatment in G5 (P = 0.011).
No significant different value was observed in the alteration of plasma concentration of E2 in the groups.
A significant lower plasma concentration of SHBG was noted between G4 compared with G1 (P = 0.048), G2 (P = 0.004) and G5 (P = 0.009).
With EG 0.75 % intoxication, lower plasma concentration of active steroid hormones (T, free T and DHT) in Group 5 (G5) treated with antioxidant nutrients correlated with less number of crystal deposits compared to Group 4 (G4).
Conclusion
The size and the mean number of crystal deposits determined in EG 0.75 % treated groups (G4) were significantly higher than the EG-treated groups, added with antioxidant nutrients and lime juice (G5). The kidney is a highly vulnerable organ to damage caused by reactive oxygen substances, due to the abundance of long-chain-polyunsaturated fatty acids. MDA is an end product of lipid peroxidation induced by free radical, and its level decreased after antioxidant consumption, reflecting lower level of lipid peroxidation and oxidative stress in Group 5 (G5). A significant higher plasma concentration of FT in EG 0.5 % group (G2) decreased in Group 3 (G3), (P = 0.000), and was significantly lower in G5 (P = 0.000) after antioxidant supplementation. The P value for FT between G2 and G4 was also significant (P = 0.03). The mean concentration of androgens in Group 4 increased after EG 0.75 % administration, and decreased after antioxidant supplementation in Group 5.
With EG 0.75 % intoxication, higher plasma concentration of active steroid hormones (T, free T and DHT) in Group 4 (G4) is well correlated with more number of crystal deposits compared to Group 5 (G5), treated with antioxidant nutrients.
Renal epithelial cell injury by reactive oxygen species is pre-requisite step in the pathogenesis of urolithiasis and there is increasing evidence that reactive oxygen species (ROS) and development of oxidative stress (OS) are produced during idiopathic calcium oxalate (CaOx) nephrolithiasis. The kidney is a highly vulnerable organ to damage caused by ROS, due to the abundance of long-chain-polyunsaturated fatty acids. It appears that the administration of natural antioxidants and lime juice as a source of citrate has been able to protect against nephrolithiasis in these experimental animals.
Oxidative stress has a critical role in the pathophysiology of several kidney diseases, and several systemic diseases induce oxidative stress in kidney [7].
Elevated free radical production as shown by increase in MDA concentration due to EG consumption in the formation of nephrolithiasis confirms that renal tissue is under oxidative stress. This hypothesis is strengthened by the report that patients with kidney stones have less activity of antioxidant enzymes with increased lipid peroxidation [3]. Antioxidant and reactive oxygen scavengers have been shown to be effective in animals for protecting kidney [7].
Moreover, the administration of antioxidants has been used to protect against nephrotoxicity in human and experimental animals. In the kidney, these treatments are reported to diminish the increase in MDA and the decrease in protective enzyme activity that are induced by chemical and pharmacological agents [8].
To evaluate the association between serum antioxidant levels and the prevalence of kidney stones, the likely role of oxidative tissue damage in the pathophysiology of stone disease is demonstrated with vitamin E to protect renal tubes from peroxidative injury [9], the coadministration of vitamin E and selenium [10], vitamin A [11], ascorbic acid [12], pyridoxine [13], zinc [14], boron [15], and lime juice as a source of citrate with a protective activity against urolithiasis [16], in which the results clearly demonstrated the ability of the lime juice to prevent the development of papillary and renal parenchymatous calcifications on the kidney, consequently preventing the development of papillary and parenchymatous calculi.
The incidence of urolithiasis has been increasing throughout the past three decades, and in men it has been repeatedly reported to be three times higher than that in women [1], mainly occurring in the third and fourth decades of life when the level of serum testosterone is also the highest [17].
Elevated concentration of androgens (as promoters of the formation of renal calculi) as a result of EG consumption decreased following antioxidant supplementations.
To elucidate the role of high steroid levels as a risk factor in kidney stone formation, further investigation on the relation between male steroids and urolithiasis is of importance and should be considered in evaluation of the etiology of the disease.
Although clinical proof for male predisposition hypothesis is lacking, there is some initial experimental evidence to implicate sex hormones in the pathogenesis of stone disease [2]. In some animal studies, it has been shown that the administration of testosterone increases urinary oxalate excretion and enhances the formation of calcium oxalate stones [4]. Testosterone appears to promote stone formation by suppressing renal osteopontin expression and increasing urinary oxalate excretion, while estrogen appears to inhibit stone formation by increasing osteopontin expression in the kidneys and decreasing urinary oxalate excretion [6]. Active dihydrotestosterone (DHT) is produced from testosterone by cytosolic enzyme, 5α-reductase and has been believed to be partially responsible for exaggerated hyperoxaluria observed in the rat ethylene glycol model of urolithiasis [2]. Recently, the potential role for the gonadal steroids in the pathogenesis of urolithiasis in male sex was proposed [5] and the relationship of kidney calculi with high plasma total and free testosterone is reported [18].
Our findings along with other studies provide evidence for the influence of sex hormones on the pathogenesis of calcium oxalate stone. Finlayson [19] postulated that lower serum testosterone levels may contribute to the protection of women and children against oxalate stone disease. In contrast, men have been found to have higher mean of oxalate levels than women [20], while it is reported that plasma oxalate concentration and kidney calcium oxalate deposition are increased by androgens, but decreased by estrogens [21].
Data suggest that testosterone can promote and estrogen may inhibit calcium oxalate stone formation in EG-treated rats [4], likely because of their effects on oxalate synthesis and oxidative stress [5].
In the present study, after EG exposure, a positive trend was observed between high plasma androgen concentrations and incidence of kidney stones, indicating a potential role for the gonadal steroids in the pathogenesis of urolithiasis in male rats. It is reported that testosterone is known to increase the hepatic levels of glycolic acid oxidase (GAO), an important enzyme in the metabolic pathway for urinary oxalate synthesis resulting in hyperoxaluria, urinary oxalate excretion increased 12.8-fold after 4 weeks of EG treatment and it was concluded that dihydrotestosterone (DHT) was partially responsible for the observed exaggerated hyperoxaluria [2].
In patients with nephrolithiasis, serum total and free testosterone revealed to be twice as healthy men [18], confirming a positive relationship and a potential role for the gonadal steroids in the pathogenesis of urolithiasis in male sex [22–24]. Administration of exogenous testosterone influenced the lipid peroxidation and carbonyl stress and decreased the antioxidant defense in the testes [25].
The rationale for various assays performed was to cover and consider the role of responsible steroid hormones involved in production of stones. Elevated concentration of androgens (as promoters of the formation of renal calculi) as a result of EG consumption and their decreasing following antioxidant supplementations provides a scientific rational for preventive and treatment roles of antioxidant nutrient complex in kidney stone disease. Although previous studies have shown that just the administration of vitamin E or other antioxidant to the rats on EG is sufficient to totally eliminate crystal deposition in the kidneys [10–14], but the result is not always 100 % or at a full rate of elimination. Thus, according to the data from studies, we aimed to study a group of these antioxidants as a complex along with a citrate source, with this in mind to have a dietary recommendation for human nutrition in urolithiasis. We intentionally used a mixture of antioxidants to increase the power of these dietary agents against total elimination of crystal deposition in the kidneys. It is clearly shown that a combination of excess dietary antioxidants may have a protective effect against free radical injury in stone formation.
We would suggest that this complex might be considered in the treatment of kidney stone formation and may benefit individuals with current kidney stone disease, which requires further elucidation by human studies.
References
Curhan GC (2007) Epidemiology of stone disease. Urol Clin North Am 34:287–293
Fan J, Glass MA, Chandhoke PS (1989) Effect of castration and finasteride on urinary oxalate excretion in male rats. Urol Res 26:71–75
Patel P, Patel M, Saralai M, Gandhi T (2012) Antiurolithiatic effects of Solanum xanthocarpum fruit extract on ethylene-glycol-induced nephrolithiasis in rats. J Young Pharm 4:164–170
Lee YH, Huang WC, Huang JK, Chang LS (1996) Testosterone enhances whereas estrogen inhibits calcium oxalate stone formation in ethylene glycol treated rats. J Urol 156:502–505
Yoshioka I, Tsujihata M, Momohara C, Akanae W, Nonomura N, Okuyama A (2010) Effect of sex hormones on crystal formation in a stone-forming rat model. Urology 75:907–913
Yagisawa T, Ito F, Osaka Y, Amano H, Kobayashi C, Toma H (2001) The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J Urol 166:1078–1082
Ozbek E (2012) Induction of oxidative stress in kidney. Int J Nephrol. doi:10.1155/2012/465897
Naziroglu M, Karaoğlu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Huang HS, Ma MC, Chen J (2009) Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am J Physiol Renal Physiol 296:F34–F45
Santhosh Kumar M, Selvam R (2003) Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem 14:306–313
Bardaoui M, Sakly R, Neffat F, Najjar MF, El Hani A (2010) Effect of vitamin A supplemented diet on calcium oxalate renal stone formation in rats. Exp Toxicol Pathol 62:573–576
Selvam R (2002) Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res 30:35–47
Ortiz-Alvarado O, Miyaoka R, Kriedberg C, Moeding A, Stessman M, Monga M (2011) Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone-forming patients. Urology 77:1054–1058
Atakan IH, Kaplan M, Seren G, Aktoz T, Gül H, Inci O (2007) Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol 39:351–356
Naghii MR, Einollahi B, Rostami Z (2012) Preliminary evidence hints at a protective role for boron in urolithiasis. J Altern Complement Med 18:207–209
Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Eljahiri Y et al (2007) Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 7:18
Travison TG, Araujo AB, Hall SA, McKinlay JB (2009) Temporal trends in testosterone levels and treatment in older men. Curr Opin Endocrinol Diabetes Obes 16:211–217
Li JY, Zhou T, Gao X, Xu C, Sun Y, Peng Y et al (2010) Testosterone and androgen receptor in human nephrolithiasis. J Urol 184:2360–2363
Finlayson B (1974) Symposium on renal lithiasis. Renal lithiasis in review. Urol Clin North Am 1:181–212
Curhan GC (1999) Epidemiologic evidence for the role of oxalate in idiopathic nephrolithiasis. J Endourol 13:629–631
Fan J, Chandhoke PS, Grampsas SA (1999) Role of sex hormones in experimental calcium oxalate nephrolithiasis. J Am Soc Nephrol suppl 14:S376–S380
Naghii MR, Hedayati M (2010) Determinant role of gonadal sex hormones in the pathogenesis of urolithiasis in a male subject—a document for male predominancy (case study). Endocr Regul 44:143–146
Watson JM, Shrewsberry AB, Taghechian S, Goodman M, Pattaras JG, Ritenour CW et al (2010) Serum testosterone may be associated with calcium oxalate urolithogenesis. J Endourol 24:1183–1187
Shakhssalim N, Gilani KR, Parvin M, Torbati PM, Kashi AH, Azadvari M et al (2010) An assessment of parathyroid hormone, calcitonin, 1,25 (OH)2 vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol Res 39:1–7
Tothova L, Celec P, Ostatnikov D, Okuliarova M, Zeman M, Hodosy J (2012) Effect of exogenous testosterone on oxidative status of the testes in adult male rats. Andrologia. doi: 10.1111/and.12032
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naghii, M.R., Mofid, M., Hedayati, M. et al. Antioxidants inhibition of high plasma androgenic markers in the pathogenesis of ethylene glycol (EG)-induced nephrolithiasis in Wistar rats. Urolithiasis 42, 97–103 (2014). https://doi.org/10.1007/s00240-013-0620-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-013-0620-5