Abstract
Objective
To investigate the effect of α-lipoic acid (α-LA) in the prevention and treatment of ethylene glycol-induced calcium oxalate deposition in a rat model and preliminary exploration of the mechanism.
Methods
Sixty male Wistar rats were divided randomly and equally into six groups including two α-LA prevention groups, two α-LA therapeutic groups, one controlled group and one intervention group. Besides controlled group, other group received 1% glycol solution and 2 ml 2% ammonium chloride for 4 weeks as a stone inducer. The prevention groups received 0.1 and 0.2 mg/kg body weight/day/rat α-LA as food supplement during inducing stone and therapeutic groups received 0.1 and 0.2 mg/kg body weight/day/rat α-LA for 4 weeks after 4 weeks stone inducing.
Results
The volume of urine, the pH and magnesium levels in the preventive and therapeutic groups were higher in a dose-independent manner (p < 0.05), and urinary calcium was lower (p < 0.05). Antioxidant stress enzyme activity (glutathione, superoxide dismutase, catalase) in serum and kidney homogenates in the preventive and therapeutic groups underwent significant regeneration (p < 0.05) and malondialdehyde levels and the production of free radical moieties decreased. Pathological observation demonstrated that there was deformation due to renal tubular expansion in the control group, greater visible inflammatory cell infiltration into the interstitial spaces and partial destruction of the glomerular structures. α-lipoic acid improved the lesions to varying degrees. The extent of crystal deposition was lower in the preventive and therapeutic groups compared with the control group (p < 0.05).
Conclusion
The present study indicated that α-LA provides both preventive and therapeutic effects against the deposition of calcium oxalate crystals in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary stones are the third most common disorder of the urinary tract, affecting 1–5% of the population in Asia, 5–9% in Europe and 13% in North America. A recent study estimated that patients experience a high recurrence rate of 50% within 5–10 years and 75% within 20 years [1]. Patients with nephrolithiasis often suffer short-term symptoms such as acute renal colic, nausea, vomiting and hematuria, and long-term complications such as chronic urinary tract obstruction, hydronephrosis and renal damage [2]. Though the surgical procedures are widely used in removing of the renal stone, these therapies have obvious shortcoming. Thus, to ensure maximum efficiency of treatment and prevent urinary tract calculi, the study of nephrolithiasis treatments has become a current focus of attention [3].
As is well known, the urinary stones were mainly composed by four types (calcium oxalate, uric acid, calcium phosphate and struvite), of which CaOx accounts for the majority [4]. Therefore, to date, CaOx nephrolithiasis has received the most attention and has been reported in the greatest detail. The mechanism of oxalate stone formation is complex and remains unclear. The supersaturation of solutes combined with oxidative stress plays an important role during the process [5]. According to supersaturation theory, the formation of CaOx involves multiple processes, including supersaturation of hyperoxaluria, crystal nucleation, growth, aggregation, deposition within the renal tubules and migration to the renal papillary surfaces [6]. Renal epithelial cells are often impaired or undergo apoptosis because of exposure to CaOx crystals [7]. During this event, oxidative stress in the renal cells has been shown to be the principal reason for renal cell injury. A series of pathological disorders are activated including the persistent suppression of antioxidants, the release of multiple inflammatory cytokines and the permanent destruction of macromolecules by reactive oxygen species (ROS) [8]. Damage caused by ROS can be reduced by the use of antioxidants which can be found in a variety of natural substances [9]. It has been reported that antioxidants in herbs and remedies are safe and effective for urinary stones. Nevertheless, there are few medicines that can be used satisfactorily in clinical therapy, especially for the prevention or treatment of the recurrence of stones. Butterweck et al. [10] reported that it was unclear what ingredients were contained in many therapies, their mechanisms of action or their active components. Thus, an effective and safe medicine to treat and prevent the early formation of urolithiasis is urgently required.
α-Lipoic acid (α-LA, 1,2-dithiolane-3-pentanoic acid), a short-chain fatty acid, is a natural free radical scavenger found in many naturally occurring substances such as herbal remedies and animal-derived products [11]. On the one hand, it can repair injured cells by scavenging ROS and regenerating levels of other antioxidants. On the other hand, it easily dissolves in both water and fat. Therefore, it has a broad spectrum of antioxidant effect and has been proven in some clinical trials and animal experiments to be an effective antioxidant in diseases such as diabetes [12], nerve disorders [13] and liver disease [14]. Hence, α-LA has also been termed an “ideal antioxidant” or “universal antioxidant”. Furthermore, early stone formation in rats and humans is the same at the ultrastructural level. Renal calcium oxalate formatted by ethylene glycol in rats is frequently used to mimic deposition of the human renal calcium oxalate. During this process ammonium chloride reported to accelerate the crystal deposition [15]. Thus, the aim of this study was to investigate the effect of α-LA in the prevention and early treatment of ethylene glycol-induced CaOx deposition in rats. The mechanism of action was also preliminarily explored. With the potential characteristic of α-LA, our results may indicate whether α-LA can be used as a potential medicine for urolithiasis in future clinical practice.
Materials and methods
Chemical reagents and animals
α-LA (purity ≥ 98%, powder), ethylene glycol (purity ≥ 99.9%, liquid) and ammonium chloride (purity ≥ 98%, liquid) were obtained from Aladdin Bio-Chem Technology Co. (Shanghai, China). All other reagents were purchased from the Biological Engineering Research Institute Co. (Nanjing, China). All reagents were of analytical grade and procured by their supplier.
Sixty, 8-week-old, male, specific pathogen-free (SPF) Wistar rats weighing 200 ± 10 g were purchased from the Dossy Experimental Animals Co. (Chengdu, China). The animals were housed in a facility at Tianfu Life Science and Technology Park (Chengdu, China) and maintained as follows: (1) environmental temperature: 20–25 °C, (2) relative humidity: 50–55%, (3) light/dark cycle: 12 h/12 h, (4) clean water to drink, (5) rat chow provided in the standard laboratory feed.
Experimental design
The rats were randomly allocated into six groups of ten using a random number table, after a week of acclimatization. Groups were as follows (A to F): Group A served as the controlled group in which rats were maintained with regular food, drinking water and received 2 ml normal saline by gavage every day for 4 weeks; Group B acted as the intervention group in which rats received regular rat food, 1% glycol solution as drinking water and 2 ml 2% ammonium chloride per day by gavage for 4 weeks; Groups C and D were disease prevention groups in which both stone-inducing reagents (1% glycol solution as drinking water and 2 ml 2% ammonium chloride by gavage per day for 4 weeks) and different doses of α-LA as a supplement and placed in regular rat food (Group C: 0.1 mg/kg body weight/day/rat; Group D: 0.2 mg/kg body weight/day/rat) were administered. Groups E and F represented therapeutic groups in which total experimentation was a total of 8 weeks. Rats received regular rat food, 1% glycol solution as drinking water and 2 ml 2% ammonium chloride by gavage per day over the whole 8-week period. From the 4th week to the end of the study, rats in group E were given 0.1 mg/kg body weight/day/rat α-LA mixed with the rat food and 0.2 mg/kg body weight/day/rat in group F as a therapeutic medicine.
Urine/serum/renal homogenate analysis
The general condition of each rat was evaluated at the end of the study. Each rat was anesthetized and decapitated after collecting 24-h urine samples using the metabolic cage method. Urine samples were tested immediately for pH then a drop of concentrated hydrochloric acid was added prior to storage at 4 °C for future urinalysis. Calcium and magnesium levels in the urine were measured by automatic atomic absorption spectrometry. Urinary oxalate levels were analyzed according to method of Hodgkinson et al. [16].
Samples of retro-orbital sinus blood were collected which were then centrifuged at 3000 rpm/2000g for 15 min until the serum had fully separated. Blood urea nitrogen (BUN) was quantified and glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and malondialdehyde (MDA) activity as indicators of oxidative stress were measured.
The abdomen of each rat was opened and both kidneys were harvested. After the gross morphology of the kidneys was observed, both were placed into iced saline to wash away any blood and the left organ retained for pathological examination. The right kidney was placed in a tissue masher (15,000 rpm for 15 min) and ground to form a tissue homogenate which was then analyzed for GSH, CAT, SOD and MDA activity. using enzymatic activity kits (Biological Engineering Research Institute Co., Nanjing, China).
Kidney pathological examination and evaluation of crystal deposition
Kidney tissue was fixed in 10% neutral buffered formalin for 24 h then dehydrated in an increasing gradient of alcohol (75% for 4 h; 85% for 2 h; 90% for 2 h; 95% alcohol for 1 h; anhydrous for 20 min) then xylene (each for 10 min). After dehydration, the specimens were embedded in paraffin wax and then sectioned using a 5-μm microtome. Hematoxylin and eosin (HE) stain was used to observe renal architecture and Von Kossa stain for crystal deposition.
A light microscope (Nikon, Eclipse E100) was used to examine the slides. Three regions of the kidney (cortex, juxta-medulla and medulla) were photographed 100 times. Nine regions per slide and five slides per kidney were randomly observed to determine the quantity of CaOx crystal deposition. Crystal deposition in renal tubules was scored in accordance with the method of Lee et al. in which each region was scored 0–3 depending on the degree of crystal deposition (0 for no crystal, 1 for very little crystal, 2 for moderate levels of crystal and 3 for heavy crystallization [17].
Statistical analysis
One-way ANOVA was used to compare the means of multiple groups. A least squares difference (LSD)-t test was used to compare two groups using GraphPad Prism software for Windows, Version 6.01. p < 0.05 was considered statistically significant.
Results
Evaluation of the general condition of rats
None of the 60 rats in any of the six groups died over the duration of the experiment. Rats in group A were larger than at the start of the experiment; they were more active with strong limbs and their coat was white and shiny. They had eaten approximately 20–25 g/day and drank approximately 30–35 ml/day water during the final week of the experiment. In group B, the growth rate of the rats was significantly lower than in group A during the first week of the experiment, each rat eating approximately 10–15 g/day and drinking less than 20 ml. Rats were thinner, more apathetic, less active and with less body hair than group A at the end of the study. The general conditions of rats in groups C and D was similar to those in group A. The general condition of rats in groups E and F was similar to those in group B over the first 4 weeks of the experiment. However, the apparent intake of drinking water increased, and their mental condition improved significantly after the rats consumed α-LA. The weights of rats are presented in Table 1.
Urine/serum/renal homogenate analysis
Urine analysis
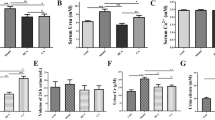
All urine specimens were yellowish, clear liquid with a pungent odor with no apparent differences in appearance or odor across groups. As shown in Table 1, the volume of urine and the pH in group A were significantly lower than in any other group (p < 0.05). Urinary calcium excretion was significantly higher in the prevention (C and D) and therapeutic (E and F) groups than in the control (p < 0.05). Furthermore, urinary magnesium excretion was higher than in other groups (p < 0.05).
Serum analysis
In serum specimens, there was no significant difference in BUN between groups (p > 0.05). Oxidative stress indicators in kidney homogenates were similar. GSH, SOD and CAT activity of the rats in the control were lowest (p < 0.05), while MDA was higher than in other groups (p < 0.05). In the prevention and therapeutic groups, GSH, SOD and CAT were higher than those in the control group and lower than the blank (p < 0.05), while the MDA was higher than the blank group and lower than control (p < 0.05). All these values were also dose dependent. The results are detailed in Table 2.
Renal homogenate analysis
As shown in Table 3, three antioxidant stress indicators (GSH, SOD, CAT) of the rats in the intervention group were significantly lower than those in the other groups (p < 0.05), while MDA was the highest in all groups (p < 0.05). In the prevention and therapeutic groups, GSH, SOD and CAT activity was higher than the intervention group and lower than the blank (p < 0.05), while MDA was higher than the controlled group and lower than the control. All results were dose dependent.
Pathological examination of kidney tissue and evaluation of crystal deposition
The kidneys of the controlled group were a normal shape, had a dark red color, smooth surface, compact with a smooth cross-section. In the intervention group, the color of the kidneys was yellowish-red with a surface that was irregular with tissue that was edematous and brittle. Additionally, in cross-section there was a gravel-like appearance. In the prevention and therapeutic groups, lesions (tissue edema and color change) were lighter than the intervention group. Cross-sections were not apparently different from normal kidneys.
Under a light microscope (100 times magnification), renal glomerular tubules in the controlled group were arranged densely and orderly. No apparent inflammatory cell infiltration was observed. In the intervention group, almost all renal tubules were dilated and deformed. Protein was observed in abundance in the renal tubules. A number of glomerular structures were destroyed by fibrous hyperplasia and inflammatory cells were observed in the stroma. Gray-brown crystals were observed in the majority of renal tubules as demonstrated by HE staining (Fig. 1), and black calcium deposits observed by Von Kossa staining (Fig. 2). Calcium oxalate crystals were observed in the majority of renal tubules which were partially connected. In the low-dose prevention and therapeutic groups, lesions were significantly lighter than in the intervention group, with no apparent inflammatory cell infiltration observed. Calcium oxalate crystals were deposited in a number of renal tubules.
In the high-dose prevention and therapeutic groups, kidney tissue was almost normal with intact glomerular structures. There was no edema or inflammatory cell infiltration observed in the specimens. Mildly dilated renal tubules and CaOx crystal deposits were observed in small regions. The difference in extent of Caox crystal deposition is shown in Fig. 3.
Effect of α-LA on oxalate in the kidney of experimental rats in each group: a controlled group, b intervention group, c low-dose prevention group, d high-dose prevention groups, e low-dose therapeutic groups, f high-dose therapeutic groups (bP < 0.05 vs Group B, cP < 0.05 vs Group C, dP < 0.05 vs Group D, e P < 0.05 vs Group E)
Discussion
The general condition of an animal is a marker of early disease progression [18]; thus these parameters were taken into consideration. In the present study, rats in the intervention group were in a worse condition than in other groups, most likely related to toxicity caused by the accumulation of large quantities of oxalic acid (mainly in the damaged organs, such as the kidneys and brain), which affected apathy, appetite, growth and development of the rats. The volume of urine also decreased due to a reduction in drinking water intake and nephrotoxicity of oxalic acid, which reduced the excretory function of the kidneys. However, the volume of urine in α-LA treated rats increased. Furthermore, α-LA reduced the symptoms of systemic poisoning in rats. Increased urine output can also reduce the concentration of oxalate in urine and increase the scouring effect on freshly deposited crystals in renal tubules [19].
The properties of urine are crucial in determining crystal deposition. Therefore, the study of urinary chemistry associated with the formation of minerals from stones will provide important information [20]. First, α-LA alkalized the urine to a certain extent. Boruczkowska et al. [21] found that alkaline urine decreased the supersaturation of CaOx and increased the protective components of the urine, such as citrate and potassium. Second, α-LA also changed the ionic strength of urine. The results demonstrate that α-LA increased magnesium excretion in urine and inhibited the excretion of calcium. The concentration of each ionic component in the urine is also an important factor affecting the formation of stones, of which calcium and magnesium are important factors. Increased urinary calcium is among the most important risk factor in the formation of CaOx stones. Approximately one-third of patients with calcium oxalate stones suffered from hypercalciuria [22]. In addition to the combination of ionic activity and other inhibitory factors, urinary calcium can also combine with oxalic acid ions in urine to form a large quantity of calcium oxalate, which greatly increases the saturation of calcium oxalate in urine, accelerating the formation of stones [23, 24]. Urinary magnesium is an additional important inhibitor in the stone formation process which can combine with oxalate ions to form magnesium oxalate which has a lower stability coefficient and reduces the supersaturation of oxalate ions [25]. In conclusion, α-LA changes the physical and chemical properties of urine to prevent and treat early CaOx crystals by increasing urine volume, magnesium levels, pH and reducing urinary calcium excretion.
CaOx crystals or large stones might lead to urinary obstruction which causes the accumulation of creatine, uric acid and BUN in serum [26]. However, no significant differences in serum BUN levels were observed among any group of rats. This might be related to the short duration of the study and strong compensatory function of the kidneys.
Pathology slides displayed tubule epithelial injury. A previous study reported that this type of injury is principally due to the toxicity caused by high concentrations of oxalic acid that result in increased inflammation and oxidative stress [27]. ROS mostly consist of free radicals or unpaired ions, in addition to their metabolites (including hydrogen peroxide, hydroxyl free oxygen, superoxide anions and nitric oxide radicals, etc.), and are important signals, in addition to being molecules with high chemical activity. They are primarily responsible for damage to carbohydrates, lipids, proteins and nucleotides [28, 29]. The body has a set of free oxygen scavenging enzymes, including GSH, SOD and CAT. GSH is responsible for decomposing hydrogen peroxide, whereas SOD scavenges superoxide anions and CAT decomposes hydrogen peroxide free radicals, the most reactive oxygen species. In physiological conditions, the oxidative stress reaction is in a state of dynamic equilibrium with the antioxidant system. As a product of free radical-mediated tissue damage, MDA is known to be a good indicator of the extent of the oxidative stress response [30]. It also has been shown in cell experiments that oxalic acid can induce increased ROS and oxidative stress that leads to renal epithelial cell damage. Furthermore, the principal site of peroxide-induced oxidative stress is within the mitochondria [28].
In the present experiments, GSH, SOD and CAT activity in the intervention group decreased significantly but MDA increased. Moreover, the shape of the tubules became distorted, in addition to leukocyte infiltration and greater quantities of CaOx crystals observed in the lumens of renal tubules. These observations may be closely related to oxygen-free radicals in the renal epithelial cells induced by oxalic acid. Nijveldt et al. [31] reported that cellular functions are inhibited by such radicals. Although the mechanism of the process is not entirely clear, lipid peroxidation is considered a key event. When cells are damaged, the adherence and retention of CaOx crystals occurs more easily [32, 33]. The retention of CaOx crystals further stimulates renal epithelial cells to produce related inflammatory factors [34]. The combination of these oxygen-free radicals and inflammatory factors initiates the destruction of renal tubular cells and induces apoptosis. However, the inflammatory response of the renal tubules in rats was significantly reduced when α-LA was administered prophylactically. The results indicate that antioxidant enzymes (GSH, SOD and CAT) in blood and kidney homogenates increased and the products of oxidative stress (MDA) declined in a dose-dependent fashion. Importantly, the quantity of CaOx crystals decreased suggesting that α-LA can protect renal tubular cells from CaOx crystal deposition through an anti-oxidative stress reaction in an oxalic acid environment, an effect that is positively dose-dependent. Furthermore, in the therapeutic group, the same outcomes were reached, indicating that damage due to oxalic acid is reversible if treated in a timely manner with α-LA. α-LA can improve the toxic resistance of the kidneys to oxalic acid, inhibit oxalic acid-induced oxidative stress and repair the antioxidant stress system. Thus, in the short term, α-LA has a therapeutic effect on damaged renal tubular epithelium that detaches adhered calcium oxalate crystals and prevents freshly formed free calcium oxalate crystals from being retained in the kidney.
In conclusion, these data confirm that α-LA provides particular preventive and therapeutic effects on early calcium oxalate crystal deposition in rats.
References
Bouatia M, Benramdane L, Idrissi MOB, Draoui M (2015) An epidemiological study on the composition of urinary stones in Morocco in relation to age and sex. Afr J Urol 21(3):194–197
Edrees B, Rasheed SA (1996) Urinary stone disease. BMJ Br Med J 312(7040):1219–1221
Chandrashekar KB, Fulop T, Juncos LA (2012) Medical management and prevention of nephrolithiasis. Am J Med 125(4):1–347
Liu Y, Chen Y, Liao B, Luo D, Zeng G (2018) Epidemiology of urolithiasis in Asia. Asian J Urol 5:274
Miller C, Kennington L, Cooney R, Kohjimoto Y, Cao LC, Honeyman T, Pullman J, Jonassen J, Scheid C (2000) Oxalate toxicity in renal epithelial cells: characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol 162(2):132–141
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367(9507):333–344
Jonassen JA, Lu-Cheng C, Thomas H, Scheid CR (2003) Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr 13(1):55
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3(3):256–276
Naghii MR (2014) Boron and antioxidants complex: a new concept for the treatment of kidney stones without rigorous pain. Endocr Regul 48(3):120–125
Butterweck V, Khan SR (2009) Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med 75(10):1095–1103
Moini H, Packer L, Saris NEL (2002) Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol 182(1):84–90
Song KH, Lee WJ, Koh JM, Kim HS, Youn JY, Park HS, Koh EH, Kim MS, Youn JH, Lee KU (2004) Alpha-lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Commun 326(1):197–202
Tasci I, Demir CF, Kuloglu T (2018) Effects of alpha lipoic acid on loss of myelin sheath of sciatic nerve in experimentally induced diabetic rats. Med Arch 72(3):178–181
Valdecantos MP, Pérez-Matute P, Prieto-Hontoria P, Moreno-Aliaga MJ, Martínez JA (2019) Impact of dietary lipoic acid supplementation on liver mitochondrial bioenergetics and oxidative status on normally fed Wistar rats. Int J Food Sci Nutr 70(7):1–11
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15(4):236–243
Hodgkinson A, Williams A (1972) An improved colorimetric procedure for urine oxalate. Clin Chim Acta 36(1):127–132
Lee YH, Huang WC, Chiang H, Chen MT, Huang JK, Chang LS (1992) Determinant role of testosterone in the pathogenesis of urolithiasis in rats. J Urol 147(4):1134–1138
Hunter JE, Butterworth J, Perkins ND, Bateson M, Richardson CA (2014) Using body temperature, food and water consumption as biomarkers of disease progression in mice with Eμ-myc lymphoma. Br J Cancer 110(4):928–934
Finlayson B (1974) Renal lithiasis in review. Urol Clin N Am 1(2):181–212
Kalyani D, Pawar AT, Chandrasekhar SB, Dighe SB, Goli D (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48(4):1013–1018
Boruczkowska A (1994) Effect of urine alkalization on excretion of renal citrate and degree of urine saturation with calcium oxalate in patients with calcium-oxalate urolithiasis and in healthy subjects. Pol Arch Med Wewn 91(2):77
Pak CY (1997) Nephrolithiasis. Curr Ther Endocrinol Metab 6(4):572
Chandhoke PS (2005) Metabolic abnormalities and the medical management of calcium oxalate nephrolithiasis. Miner Urol E Nefrol 57(1):9–16
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC (2003) Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63(5):1817–1823
Gvozdev NV, Petrova EV, Chernevich TG, Shustin OA, Rashkovich LN (2004) Atomic force microscopy of growth and dissolution of calcium oxalate monohydrate (COM) crystals. J Cryst Growth 261(4):539–548
Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G (2011) Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol 185(4):1304–1311
Scheid C, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P (1996) Oxalate toxicity in LLC-PK1 cells, a line of renal epithelial cells. J Urol 155(3):1112–1116
Thamilselvan S, Byer KJ, Hackett RL, Khan SR (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK 1 and MDCK cells. J Urol 164(1):224–229
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33(1):65–69
Slater TF (1984) Free-radical mechanisms in tissue injury. Biochem J 222(1):1–15
Nijveldt RJ, Nood E, Van HoornVan Boelens DEPG, Norren K, Van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74(4):418–425
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9
Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS (2001) Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int 59(2):637–644
Veena CK, Josephine A, Preetha SP, Rajesh NG, Varalakshmi P (1996) Mitochondrial dysfunction in an animal model of hyperoxaluria: a prophylactic approach with fucoidan. Eur J Pharmacol 579(1–3):330–336
Acknowledgements
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethics statement
This study granted an ethics approval from Ethics Committee of Sichuan University (reference number: 2018171A).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Bai, Y., Wang, J. et al. The preventive and therapeutic effects of α-lipoic acid on ethylene glycol-induced calcium oxalate deposition in rats. Int Urol Nephrol 52, 1227–1234 (2020). https://doi.org/10.1007/s11255-020-02423-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02423-z