Abstract
Taraxasterol is one of the important constituents of Taraxacum officinale L. (Compositae) with antioxidant potential. The present study was designed to evaluate and compare the antiurolithiatic effects of taraxasterol and potassium citrate in the ethylene glycol induced urolithiatic rat. Urolithiasis was induced by ammonium chloride and ethylene glycol in adult male rats. Taraxasterol (2, 4 and 8 mg/kg) and potassium citrate (2.5 g/kg) were treated for 33 days by gavage. Then, the animals were anesthetized and weighted and blood, urine, liver and kidney sampling were done. The kidney sections were prepared by hematoxylin & eosin staining. The liver and kidney coefficients, urine pH, calcium, magnesium, oxalate and citrate levels, serum albumin, calcium and magnesium levels, serum alanine aminotransferase, aspartate aminotransferase and lactate dehydrogenase activities, superoxide dismutase and glutathione peroxidase activities in serum, kidney and liver, number of calcium oxalate crystal deposits, score of crystal deposits, score of histopathological damages and score of inflammation in kidney sections were evaluated. The results showed that taraxasterol decreased liver and kidney coefficients (p < 0.001), serum calcium (p < 0.01) level, serum alanine aminotransferase (p < 0.001), aspartate aminotransferase (p < 0.001), lactate dehydrogenase (p < 0.05) activities, urine magnesium (p < 0.05) and oxalate (p < 0.001) levels, number of crystal deposits (p < 0.001), score of crystal deposits (p < 0.01), score of histopathological damages (p < 0.001) and score of inflammation (p < 0.01) in kidney sections, while increased urine pH (p < 0.01), calcium (p < 0.001) and citrate (p < 0.05), serum magnesium (p < 0.001) and albumin (p < 0.01) levels, superoxide dismutase and glutathione peroxidase in serum (p < 0.01), kidney (p < 0.05 and p < 0.001, respectively) and liver (p < 0.01 and p < 0.001, respectively) tissue homogenates in treated urolithiatic rats in comparison to the control urolithiatic rats. The effect of potassium citrate is the same as taraxasterol in treated urolithiatic rats. In conclusion, the effect of taraxasterol could be by improving liver function, changing serum and urine parameters, maintaining the antioxidant environment, reducing crystal deposition, excretion of small deposits from kidney and reducing the chance of them being retained in the urinary tract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is one of the most prevalent disease in the urinary system [1] and a multi-factorial disorder resulting from the combined influence of epidemiological, biochemical and genetic risk factors. It occurs both in men and women but the risk is generally higher in men. Urolithiasis is a worldwide problem with a high recurrence rate. Many patients will be affected by multiple stones throughout their lifetime, with estimated recurrence rates of 50% within 5–10 years and 75% within 20 years [2].
Urinary calculi may cause hydronephrosis, hemorrhage, obstruction, and infection in the urinary system. CaOx and calcium phosphate are composed 80% of urinary calculi [1]. CaOx stones account for the vast majority of calculi in primary and secondary forms of hyperoxaluria. In addition, hyperoxaluria can induce acute kidney injury as a result of widespread intra-renal CaOx deposition. Exposure to oxalate/CaOx crystals elicits a cascade of responses in renal epithelial cells that often leads to cell injury or death [3].
Crystal depositions may generate cellular biochemical events leading to the generation of both intracellular and intra-mitochondrial reactive oxygen species directly or indirectly by renal cell infiltration or/and renal cellular injuries which further deteriorate the morbidity in kidney stone disease [4].
Oxidative stress in renal cells is considered as the major cause of renal injury and inflammation, initiating a plethora of pathological disorders. Moreover, the abundance of reactive oxygen species may permanently damage macromolecules and interfere in redox-dependent signaling processes [4].
The surgical operation, lithotripsy and local calculus disruption using high-power laser are widely used to remove the calculi. However, these procedures are highly costly and recurrence is quite common by these procedures.
Natural products are a treasure of drugs and drug leads. Numerous studies have discussed the importance of compounds derived from plants in modern medicine. Taraxasterol—a pentacyclic-triterpene—is present in several plant genera including dandelion [Taraxasterol officinale L. (Compositae)]. Several studies have shown various pharmacological effects of taraxasterol, such as anti-inflammatory, anti-microbial [5], anti-tumor [6], anti-allergic [7], antioxidant and hepatoprotective activities [8, 9]. Till date, no scientific validation for the use of taraxasterol in urolithiasis has been established. On the other hand, it is shown that PC therapy decreases stone recurrence in patients with calcium nephrolithiasis [10]. So, the aim of the present study was to examine and compare antiurolithiatic effects of taraxasterol and PC on kidney stone in urolithiatic male rats.
Materials and methods
Chemicals and apparatus
Taraxasterol was obtained from Chem Face Co (Wuhan, Hubei, China), and its purity was 99.5% based on HPLC analysis. Reagents used for assays of GPx and SOD enzymes were commercial kits/products and reagents used for assays of blood parameters were purchased from Parsazmoon Co (Tehran, Iran). All other chemicals and reagents were analytical grade and procured from approved chemical suppliers.
Animals
Adult male Wistar albino rats (weighing 150–170 g) were obtained from Pasteur Institute (Karaj, Iran). They were maintained at 25 ± 2 °C, relative humidity of 45–55%, 12 h light/12 h dark cycle. The animals had free access to food and water ad libitum.
Urolithiasis induction
Urolithiasis was induced by adding AC (0.75%, w/v) for 3 days and EG (1%, v/v) for remaining 33 days in drinking water in all groups except group A (as control normal group) [11]. Thirty-six rats were randomly divided into six groups as follow:
Group A: Control normal group; without any treatment (n = 6).
Group B: Control urolithiatic group; received 0.5 ml saline as the vehicle by intragastric gavage simultaneously EG treatment (n = 6).
Groups C, D, and E: Experimental urolithiatic groups; received taraxasterol at doses 2, 4 and 8 mg/kg body weight by intragastric gavage simultaneously EG treatment (n = 6).
Group F: Control positive urolithiatic group; received PC at dose 2.5 g/kg body weight by intragastric gavage simultaneously with EG treatment (n = 6).
Sampling
On day 36, all animals were housed in individual metabolic cages, and 24 h urine samples were collected. The 24 h samples were stored at − 80 °C until urine analysis. Then, the animals were sacrificed for 12 h and weighted. The blood was collected from the heart under ketamine (75 mg/kg, body wt., i.p.) anesthesia. The blood samples centrifuged at 1500 g for 10 min after collection. The sera were stored in the − 80 °C until serum analysis. After removing kidney and liver, they were weighted.
Kidney and liver coefficients measurement
The liver and kidney coefficients were obtained from organ-to-body weight ratio.
Serum analysis
Serum calcium, magnesium and albumin levels and AST, ALT, SOD and GPx activities were evaluated by an autoanalyzer (Shimadzu CL-7200, Shimadzu, Kyoto, Japan).
Urine analysis
Urinary calcium and magnesium levels were measured using an autoanalyzer (Shimadzu CL-7200, Shimadzu, Kyoto, Japan). Urinary oxalate was measured by direct precipitation followed by titration. Urine citrate was measured by citrate acid enzyme kit. The pH of the urine was measured using the pH meter.
Preparation of tissue homogenate
The equal weight of liver and kidney tissues of all the groups were used for preparing tissue homogenate. The weighed frozen liver and kidney tissues were homogenized in a Glass-Teflon homogenizer with 50 mM phosphate buffer (pH 7.4) to obtain 1:9 (w/v) whole homogenate. The homogenates were then centrifuged at 11,000g for 15 min at 4 °C to discard any cell debris, and the supernatant was used for the measurement of SOD and GPx enzymes. Total protein contents were determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard [12].
Measurement of SOD activity
The kidney and liver supernatants were used to assay the SOD activity which is determined using xanthine and xanthine oxidase to generate superoxide radicals, which subsequently reacted with 2-(4-iodophenyl)3-(4-nitrophenyl)-5-triphenyltetrazolium chloride to form a red formazan dye. SOD activity was measured by the degree of inhibition of this reaction [13].
Measurement of GPx activity
The GPx assay was based on the oxidation of NADPH to NADP+, which is accompanied by a decrease in absorbance at 340 nm. The assay of GR activity was performed in the supernatant using BIOXYTECH GR-340TM Assay kit produced by OXIS International, Inc., Portland, USA. The method is based on the oxidation of NADPH to NADP + catalyzed by glutathione reductase. One molecule of NADPH is consumed for each molecule of reduced GSSG form. The reduction in GSSG is determined indirectly by the measurement of NADPH consumption, as demonstrated by a decrease in absorbance at 340 nm as a function of time. G6PDH activity was measured in 10% liver and kidney homogenates. The GPx activity was determined in supernatants using Sigma Diagnostic kit, Inc., St. Louis, USA. The method is based on spectrophotometric measurement of NADPH formation rate, which is proportional to the G6PDH activity [14].
Measurement of renal crystal deposition
After removing the kidney, they were weighted and then cleaned off extraneous tissue and rinsed in ice-cold saline. The right kidney was fixed rapidly with 10% neutralized formalin (pH 7.4). Paraffin-embedded kidney tissue samples were cut into 5 μm sections and stained (H & E). The sections were analyzed by polarized optical microscope to determine the number of calcium oxalate deposits per high-powered field at 10 random fields (×1000 magnification).
Score of renal crystal deposits were assessed as follows: no deposits = 0; crystal deposits in the papillary tip = 1; crystal deposits in the cortico-medullary junction = 2 and crystal deposits in the cortex = 3. If the crystals were found in the multiple regions, the scores were added together for total score [15].
Kidney pathological examination
Pathological damages of right kidney were semi-quantified using the following scoring system: invisible lesions = 0; tubular dilation = 1; tubular cell necrosis = 2 and tubular necrosis = 3. Additionally, inflammation was quantified using the following scoring system: no inflammation = 0; low inflammation = 1; moderate inflammation = 2; severe = 3 [15].
Statistical analysis
Data expressed as mean ± S.E.M. The data were analyzed using one-way analysis of variance followed by Tukey test and p < 0.05 considered as statistically significant.
Results
Effects of taraxasterol and PC on kidney and liver coefficients
The present results showed that oral administrations of AC 1% (3 days) and EG 0.75% (33 days) increased kidney and liver coefficients (p < 0.001 and p < 0.01, respectively) in group B in comparison to group A. On the other hand, administrations of the taraxasterol (2, 4 and 8 mg/kg, body wt., gavage) and PC (2.5 g/kg, body wt., gavage) decreased kidney and liver coefficients in groups C, D, E and F in comparison to group B (p < 0.001). Additionally, the effect of taraxasterol on kidney coefficient in group E is more potent than PC (Table 1).
Effects of taraxasterol and PC on serum parameters
The present results showed that oral administrations of AC and EG increased serum calcium (p < 0.001), ALT (p < 0.001) and AST (p < 0.001) levels, while decreased serum magnesium (p < 0.001), albumin (p < 0.01), SOD (p < 0.01) and GPx (p < 0.001) levels in group B. On the other hand, administration of the taraxasterol decreased serum calcium (p < 0.01), ALT (p < 0.001), AST (p < 0.001) and LDH (p < 0.05) levels, while increased serum magnesium (p < 0.001), albumin (p < 0.01), SOD (p < 0.01) and GPx (p < 0.01) levels in groups C, D and E. Additionally, treatment of PC decreased serum calcium (p < 0.001), ALT (p < 0.001) and AST (p < 0.001) levels, while increased magnesium (p < 0.001) and albumin (p < 0.001) levels in group F in comparison to group B (Table 1).
Effects of taraxasterol and PC on urine parameters
The present results showed that oral administrations of AC and EG decreased urine pH (p < 0.05) and calcium (p < 0.001) and citrate (p < 0.01) levels, while increased urine oxalate (p < 0.001) and magnesium (p < 0.05) levels in group B. Administrations of the taraxasterol and PC increased urine pH (p < 0.01 and p < 0.05, respectively) and calcium (p < 0.001 and p < 0.01, respectively) and citrate (p < 0.05), while decreased oxalate (p < 0.001) and magnesium (p < 0.05) levels in groups C, D, E and F compared to group B (Table 1).
Effects of taraxasterol and PC on SOD and GPx activities in tissue homogenates
The results showed that oral administrations of AC and EG decreased SOD and GPx activities in kidney tissue (p < 0.05 and p < 0.001, respectively) and liver (p < 0.05 and p < 0.001, respectively) tissue homogenates in group B. Administration of the taraxasterol but not PC increased the SOD and GPx activities in kidney (p < 0.05 and p < 0.001, respectively) and liver (p < 0.01 and p < 0.001, respectively) tissue homogenates in group E in comparison to group B (Table 1).
Effects of taraxasterol and PC on renal crystal deposits
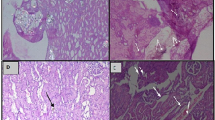
The present results showed that oral administrations of AC and EG increased the number of CaOx deposits in kidney sections (p < 0.001) and score of renal crystal deposits (p < 0.001) in group B compared to group (A) Taraxasterol decreased number of CaOx deposits (p < 0.001) in groups C, D and E and score of renal crystal deposits in group E (p < 0.01) compared to group (B) PC decreased number of CaOx deposits (p < 0.001) and score of renal crystal deposits (p < 0.001) in group F in comparison to group B. Effect of PC on score of renal crystal deposits is more potent than taraxasterol (p < 0.05) (Table 1; Fig. 1).
Effects of taraxasterol and PC on histopathological examination
The renal tissue in control urolithiatic rats was severely damaged. The histopathological damages including tubule dilation, tubular cell necrosis, tubular necrosis and enlarged renal tubules with crystal deposits in the lumen (Table 1; Fig. 2) and also inflammation were observed in urolithiatic rats (Table 1; Fig. 3). Treatment of taraxasterol and PC improved histopathological damages (p < 0.001), pathological scores (p < 0.001) and inflammation (p < 0.01 and p < 0.05, respectively) in groups C, D, E, and F compared to group B.
Discussion
Previous studies indicated that administrations of AC and EG to male rats resulted in the formation of renal calculi composed mainly of CaOx. Stone formation in EG fed is caused by hyperoxaluria, which causes increased renal retention and excretion of oxalate [16]. Renal CaOx deposition by AC and EG in rats is frequently used to mimic the urinary stone formation in humans. AC reported accelerating the lithiasis [17].
Nowadays, effective therapies to prevent urolithiasis are required. Unfortunately, despite considerable progress in medical therapy, there is no satisfactory drug to treat kidney stones.
In this regard, traditional herbal medicine can be a potent source of new antiurolithiatic remedies, because it is shown that plant extracts and their constituents have antiurolithiatic effects. They may effect on urine volume, urine pH, urinary oxalate and magnesium levels, serum uric acid, creatinine and urea levels, reactive oxygen species and inflammatory responses [8, 18, 19]. Taraxasterol is one of the effective constituents with various activities especially antioxidant and anti-inflammatory effects.
In the present study, the antiurolithiatic effect of taraxasterol was evaluated and compared to PC in EG-induced urolithiasis model. Taraxasterol and PC decreased kidney and liver coefficients in treated urolithiatic rats that may be because of suppressing renal inflammation processes and reducing the CaOx crystal deposits in treated urolithiatic rats.
In this study, the control urolithiatic group showed a significant decrease in urine pH and taraxasterol and PC increased urine pH significantly and made urine alkaline. The increase in pH may be responsible for dissolving the complexes of calcium and oxalate, which contributes to their significant antiurolithic activities. The present result is accordance with Nnemdi Ashibuogwu et al. (2016) study that showed the aqueous extract of Cola nitida seed increase urine pH in urolithiatic rats [20]. On the other hand, the type of stones formed in human can be predicted from the pH of the fasting urine and dissolution of calculi can be achieved by the alteration in urinary pH [23].
Treatments of taraxasterol and PC reduced serum calcium and urinary oxalate and magnesium concentrations, while increased serum magnesium and urinary calcium levels significantly in groups C, D, E, and F compared to group B.
The previous experiments showed that EG is metabolized to acidic metabolites such as oxalic acid, formic acid, hippuric acid and benzoic acids which cause metabolic acidosis and further produced defects in HCO3 reabsorption of the proximal tubule and then acidosis. The acidosis produces calcium leak in nephron associated with the absorption of calcium in gut and release of calcium in bone which leads to hypercalciuria and hypercalcemia [21]. Moreover, increased urinary calcium is an important factor favoring the nucleation and precipitation of CaOx or calcium phosphate and subsequent crystal growth in the urinary system [22]. Additionally, it is reported the administration of EG cause increased calcium levels in urine, serum and kidney homogenates, thereby promoting the formation of CaOx stones. In other words, the increase in calcium level in renal tissue might be due to the increased bioavailability of NO which in turn activates second messengers like cGMP which controls the increase in intracellular calcium levels. Literature indicates that NO donors have the capacity to control the intracellular rise in calcium levels [13, 23]. Furthermore, EG also increased oxalate production by elevating substrate availability for oxalate synthesizing enzymes like glycolic acid oxidase and lactate dehydrogenase, which catalyze the oxidation and reduction of glyoxalate into glycolate and oxalate [24]. Additionally, in previous studies, it is shown that Acorus calamus rhizome extract reduced calcium concentrations in the urine, serum, and kidney homogenate in urolithiatic rats and prevents the formation and aggregation of kidney stone by increasing the bioavailability of NO to sequester calcium through the cGMP pathway [25].
It is shown that normal urine contains many inorganic and organic inhibitors of crystallization. Magnesium is one of well-known urine inhibitors. Low levels of magnesium are also encountered in stone formers as well as in stone-forming rats. The magnesium level return to normal on drug treatment. Diets enriched with high magnesium have been found to protect against deposition of calcium oxalate in the kidneys. Magnesium produces complexes with oxalate and decrease the super-saturation of calcium oxalate by reducing the saturation of CaOx and as a result, reduces the growth and nucleation rate of CaOx crystals [26].
It has been reported that the main risk factor for recurrent CaOx calculus is hypocitraturia [27]. Urinary citrate can prevent CaOx crystal formation by inhibiting crystal nucleation and growth [28, 29]. Our results showed that urinary citrate level decreased in rat urolithiatic model. Taraxasterol increased the urinary citrate concentration in the treated groups compared with group B. Therefore, the hypercitraturic effect of taraxasterol might represent a potential mechanism for the antiurolithiatic action.
The present study also showed decreased serum albumin level in group B in comparison to group A and treatments of taraxasterol and PC significantly increased serum albumin level which indicates on the improvement of liver function. In agreement, it is reported that albumin would facilitate the elimination of CaOx crystals by inducing the formation of numerous crystals that would remain small enough to be easily eliminated, and among them, albumin would favor the formation of CaOx dihydrate, which is less associated with urinary stone buildup [30].
In this study, increased serum AST and ALT levels in group B were observed. This can be attributed to the damaged structural integrity to the cells causing the enzyme which is located in the cytoplasm to be released into the circulation. If the membrane of organelles such as mitochondria is damaged, soluble enzymes such as AST and ALT will also release. The release of these enzymes into the circulation will indicate both damaged plasma and organelle membranes. LDH is an oxalate synthesizing enzyme; its activity was increased on EG administration. It was released into the blood, serum, and urine and may be attributed to oxalate induced renal cellular damage. Renal damage is particularly confined to the proximal tubule, a part of the nephron closely involved in handling urinary oxalate [30]. In the present study, it is shown the decrease of serum AST and ALT levels by taraxasterol and PC in treated urolithiatic groups, significantly. Additionally, it is shown that antioxidant activity of taraxasterol is evaluated by measuring the levels of SOD and GPx in serum, kidney and liver which are used as biochemical markers in animals for evaluating of kidney and liver damages. In the present study, it is observed that treatment of taraxasterol in hyperoxaluric rat model elevated SOD and GPx levels of serum, kidney, and liver tissue homogenates in treated urolithiatic rats due to combat the enhanced cellular reactive oxygen species generated due to exposure of oxalate.
It is shown that exposure to oxalate/CaOx crystals leads to changes in renal epithelium which acts as nidus and enhances the retention of crystals within the kidneys and enhanced production of reactive oxygen species [31]. Renal tissue injury generates nidus by providing a site for retention of calcium oxalate crystals, which grows to stone by a cascade of events [32, 33].
Taraxasterol may interact with CaOx crystals and its ability to be a potent antioxidant may interrupt crystal growth at early stage preventing crystal deposition in renal tissue. Deregulated oxidative antioxidant balance had been reported in stone disease and taraxasterol treatment effectively ameliorates reactive oxygen species production by promoting antioxidants SOD and GPx.
Treatments of taraxasterol and PC decreased the number of CaOx crystal deposits in different parts of kidney sections, the score of crystal deposits, score of histopathological damages and score of inflammation in the kidney of treated groups, significantly.
Numerous reports highlight the crosstalk between enhanced reactive oxygen species and inflammation of the renal tissue. Generation of reactive oxygen species plays a major role in renal injury via inducing the renal expression of various macromolecules like MCP-1 and KIM-1 [34]. Experimentally induced CaOx crystal deposition in kidneys is also associated with localized inflammation as evidenced by infiltration of monocyte and macrophages to the site. Exposure of renal epithelial to CaOx crystals and high oxalate level in nephrons can damage epithelial cells, induce heterogeneous crystal nucleation and cause aggregation of crystals. Mulay et al. (2013) have reported that CaOx nephropathy is associated with IL-1β dependent inflammation of renal epithelium and kidney injury [35].
On the other hand, taraxasterol inhibited production of reactive oxygen species and TNF-α, IL-6, IL-1β, and MCP-1 production [36]. These inflammatory cytokines such as TNF-α, IL-6, and IL-1β could induce production of other inflammatory mediators and amply the inflammatory responses [37, 38].
Conclusion
In conclusion, the effect of taraxasterol could be by maintaining balance between stone promoters and inhibitors, improving liver function, changing serum and urine parameters including Ca, Mg, citrate and oxalate levels, maintaining the antioxidant environment, reducing CaOx deposits and excretion of small particles of CaOX from the kidney and the chance of them being retained in the urinary tract. Taraxasterol as a constituent of herbal extracts has shown a protective effect against the renal stone formation. However, the taraxasterol mechanism underlying this protective effect is not completely evaluated. Further studies should be conducted to better characterize the preventive effect of taraxasterol on kidney stone formation as well as to evaluate possible toxic effects associated with its long-time oral administration.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AC:
-
Ammonium chloride
- AST:
-
Aspartate aminotransferase
- CaOx:
-
Calcium oxalate
- EG:
-
Ethylene glycol
- GPx:
-
Glutathione peroxidase
- H & E:
-
Hematoxylin-Eosin
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin-1β
- i.p.:
-
Intraperitoneally
- KIM-1:
-
Kidney injury marker protein
- LDH:
-
Lactate dehydrogenase
- MCP-1:
-
Monocytes Chemo-attractant Protein-1
- NO:
-
Nitric oxide
- PCL:
-
Potassium citrate
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor
References
Hadjzadeh MA, Khoei A, Hadjzadeh Z, Parizady M (2007) Ethanolic extract of Nigella Sativa L. seeds on ethylene glycol-induced kidney calculi in rats. Urol J 4:86–90
Kambadakone AR, Eisner BH, Catalano OA, Sahani DV (2010) New and evolving concepts in the imaging and management of urolithiasis: urologists’perspective. Radiographics 30:603–623. https://doi.org/10.1148/rg.303095146
Jonassen JA, Cao LC, Honeyman T, Scheid CR (2003) Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr 13:55–72
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3:256–276. https://doi.org/10.3978/j.issn.2223-4683.2014.06.04
Zhang XM, Xiong HZ, Li LB (2012) Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW264.7 macrophages. J Ethnopharmacol 14:206–211. https://doi.org/10.1016/j.jep.2012.02.020
Jamshieed S, Das S, Sharma MP, Srivastava PS (2010) Difference in in vitro response and esculin content of Taraxacum officinale Weber. Physiol Mol Biol Plants 16:353–358. https://doi.org/10.1007/s12298-010-0038-2
Liu J, Xiong H, Cheng Y, Cui C, Zhang X, Xu L, Zhang X (2013) Effects of taraxasterol on ovalbumin-induced allergic asthma in mice. J Ethnopharmacol 148:787–793. https://doi.org/10.1016/j.jep.2013.05.006
Aggarwal D, Gautam D, Sharma M, Singla SK (2016) Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur J Pharmacol 791:611–621. https://doi.org/10.1016/j.ejphar.2016.10.002
You Y, Yoo S, Yoon HG, Park J, Lee YH, Kim S, Oh KT, Lee J, Cho HY, Jun W (2010) In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol 48:1632–1637. https://doi.org/10.1016/j.fct.2010.03.037
Krieger NS, Asplin JR, Frick KK, Granja I, Culbertson CD, Ng A, Grynpas MD, Bushinsky DA (2015) Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J Am Soc Nephrol 26:3001–3008. https://doi.org/10.1681/ASN.2014121223
Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48:1013–1018. https://doi.org/10.1016/j.fct.2010.01.011
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Sun Y, Oberley LW, Li Y (1988) Simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Vysakh A, Raji NR, Suma D, Jayesh K, Jyothis M, Latha MS (2017) Role of antioxidant defence, renal toxicity markers and inflammatory cascade in disease progression of acute pyelonephritis in experimental rat model Microb Pathog 31: pii: S0882–4010(16)30883-X. https://doi.org/10.1016/j.micpath.2017.05.047
Yamaguchi S, Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS (2005) Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int J Urol 12:290–298. https://doi.org/10.1111/j.1442-2042.2005.01038.x
Karadi RV, Gadge N, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 105:306–311. https://doi.org/10.1016/j.jep.2005.11.004
Atmani F, Slimani Y, Mimouni M, Hacht B (2003) Prophylaxis of calcium oxalate stones by Herniaria hirsute on experimentally induced nephrolithiasis in rats. BJU Int 92:137–140. https://doi.org/10.1046/j.1464-410X.2003.04289.x
Yuruk E, Tuken M, Sahin C, Kaptanagasi AO, Basak K, Aykan S, Muslumanoglu AY, Sarica K (2016) The protective effects of an herbal agent tutukon on ethylene glycol and zinc disk induced urolithiasis model in a rat model. Urolithiasis 44(6):501–507. https://doi.org/10.1007/s00240-016-0889-2
Shekha MS, Qadir AB, Ali HH, Selim XE (2014) Effect of fenugreek (Trigonella foenum-graecum) on ethylene glycol induced kidney stone in rats. Jordan J Biol Sci 7:257–260. https://doi.org/10.12816/0008248
Nnemdi Ashibuogwu M, Isaac Adeosun O, Ojo Akomolafe R, Olaniyi Sanni D, Sesan Olukiran O (2016) Diuretic activity and toxicity study of the aqueous extract of Cola nitida seed on markers of renal function and electrolytes in rats. J Complement Integr Med 13(4):393–404. https://doi.org/10.1515/jcim-2015-0115
Moochhala SH, Sayer JA, Carr G, Simmons NL (2008) Renal calcium stones: insights from the control of bone mineralization. Exp Physiol 93:43–49. https://doi.org/10.1113/expphysiol.2007.040790
Lemann J Jr, Worcester EM, Gray RW (1991) Hypercalciuria and stones. Am J Kidney Dis 17:386–391
Saha S, Verma RJ (2015) Antinephrolithiatic and antioxidative efficacy of Dolichos biflorus seeds in a lithiasic rat model. Pharm Biol 53:16–30. https://doi.org/10.3109/13880209.2014.909501
Soundararajan P, Mahesh R, Ramesh T, Begum VH (2006) Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44:981–986
Ghelani H, Chapala M, Jadav P (2016) Diuretic and antiurolithiatic activities of an ethanolic extract of Acorus calamus L. rhizome in experimental animal models. J Tradit Complement Med 6:431–436. https://doi.org/10.1016/j.jtcme.2015.12.004
Selvam R, Kalaiselvi P, Govindaraj A, Bala Murugan V, Sathish Kumar AS (2001) Effect of A. lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 43:89–93. https://doi.org/10.1006/phrs.2000.0745
Menon M, Mahle CJ (1983) Urinary citrate excretion in patients with renal calculi. J Urol 129:1158–1160
Xu H, Zisman AL, Coe FL, Worcester EM (2013) Kidney stones: An update on current pharmacological management and future directions. Expert Opin Pharmacother 14:435–447. https://doi.org/10.1517/14656566.2013.775250
Goldberg H, Grass L, Vogl R, Rapoport A, Oreopoulos DG (1989) Urine citrate and renal stone disease. CMAJ 141:217–221
Cerini C, Geider S, Dussol B, Hennequin C, Daudon M, Veesler S, Nitsche S, Boistelle R, Berthezene P, Dupuy P, Vazi A, Berland Y, Dagorn JC, Verdier JM (1999) Nucleation of calcium oxalate crystals by albumin: Involvement in the prevention of stone formation. Kidney Int 55:1776–1786. https://doi.org/10.1046/j.1523-1755.1999.00426.x
Veena CK, Josephine A, Preetha SP, Rajesh NG, Varalakshmi P (2008) Mitochondrial dysfunction in an animal model of hyperoxaluria: a prophylactic approach with fucoidan. Eur J Pharmacol 579:330–336. https://doi.org/10.1016/j.ejphar.2007.09.044
Verhulst A, Asselman M, Persy VP, Schepers MS, Helbert MF, Verkoelen CF, De Broe ME (2003) Crystal retention capacity of cells in the human nephron: involvement of CD44 and its ligands hyaluronic acid and osteopontin in the transition of a crystal binding- into a non-adherent epithelium. J Am Soc Nephrol 14:107–115. https://doi.org/10.1097/01.ASN.0000038686.17715.42
Verkoelen CF, Van Der Boom BG, Romijn JC (2000) Identification of hyaluronan as a crystal-binding molecule at the surface of migrating and proliferating MDCK cells. Kidney Int 58:1045–1054. https://doi.org/10.1046/j.1523-1755.2000.00262.x
Zuo J, Khan A, Glenton PA, Khan SR (2011) Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol Dial Transplant 26:1785–1796. https://doi.org/10.1093/ndt/gfr035
Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ (2013) Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J Clin Invest 123:236–246. https://doi.org/10.1172/JCI63679
Xueshibojie L, Duo Y, Tiejun W (2016) Taraxasterol inhibits cigarette smoke induced lung inflammation by inhibiting reactive oxygen species-induced TLR4 trafficking to lipid rafts. Eur J Pharmacol 789:301–307. https://doi.org/10.1016/j.ejphar.2016.07.047
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. https://doi.org/10.1038/nri3399
Likhitpanichkul M, Torre OM, Gruen J, Walter BA, Hecht AC, Iatridis JC (2016) Do mechanical strain and TNF-α interact to amplify pro-inflammatory cytokine production in human annulus fibrosus cells? J Biomech 49:1214–1220. https://doi.org/10.1016/j.jbiomech.2016.02.029
Acknowledgements
The authors would like to thank Deputy Research of the Science and Research Branch, Islamic Azad University, for support of the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures were conducted in accordance with the guidelines for the care and use of laboratory animals observed at the Science and Research Branch, Islamic Azad University and were in agreement with institutional guidelines for the care and use of laboratory animals (NIH, publication No. 85-23, revised 2010; European Communities Directive 86/609/EEC).
Rights and permissions
About this article
Cite this article
Yousefi Ghale-Salimi, M., Eidi, M., Ghaemi, N. et al. Antiurolithiatic effect of the taraxasterol on ethylene glycol induced kidney calculi in male rats. Urolithiasis 46, 419–428 (2018). https://doi.org/10.1007/s00240-017-1023-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-1023-9