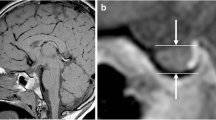
Abstract
Purpose
To verify and integrate the prevalence and phenotype of abnormalities in the sellar region in patients with growth hormone deficiency (GHD) using MRI data.
Methods
We searched PubMed and EMBASE up to December 14, 2020. The inclusion criteria were as follows: (1) pediatric patients diagnosed with nonacquired GHD and (2) detailed data sufficient to assess the proportion of sellar and parasellar abnormalities on brain MRI scans. Finally, thirty-two studies with 39,060 children (mean or median age, 3.4–14.1 years) were included. The number and type of MRI findings from all included studies were pooled by two authors. The heterogeneity across studies was evaluated with the Q test or the inconsistency index (I2) statistic. Subgroup analyses were performed according to the type of GHD (isolated GHD [IGHD] vs. multiple pituitary hormone deficiency [MPHD]), MRI magnet, geographical region, and cutoff serum growth hormone (GH) level.
Results
The pooled proportion of sellar and parasellar abnormalities was 58.0% (95% CI, 47.1–68.6%; I2, 98.2%). The MPHD group showed a higher proportion of sellar and parasellar abnormalities and pituitary stalk interruption syndrome than the IGHD group (91.4% vs. 40.1%, P<0.001; 65.3% vs. 20.1%, P<0.001). The patients in studies with low peak GH levels on stimulation tests were more associated with severe MR abnormalities (cutoff GH ≤ 5 μg/l vs. cutoff GH = 10 μg/l; 72.8 % vs. 38.0%; P<0.001).
Conclusion
The types and incidence of MRI abnormalities of the sellar region differ significantly between the IGHD and MPHD groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growth hormone deficiency (GHD) is a relatively common endocrine problem (1/3000~1/9000 in children [1]), which leads to short stature in childhood and could give rise to severe short stature in adulthood if there is no adequate supplemental hormone therapy.
GHD can be divided into acquired GHD and nonacquired (congenital) GHD according to its etiology. A clinical history of head trauma, central nervous system (CNS) infection, tumor, and cerebral irradiation are relatively common causes of acquired GHD. Though most of these causes of acquired GHD are easy to detect in imaging studies, the structural abnormalities in the vicinity of the pituitary gland of some nonacquired GHD cases may not be clearly revealed.
After the first study of pituitary stalk transection (interruption) using MRI was reported in 1987 [2], MRI has been regarded as the most superior and essential imaging modality for evaluating sellar and parasellar structural abnormalities in patients with GHD. In addition, in the initial evaluation of patients with GHD, it is important to use MRI to identify midline anomalies that may accompany nonacquired GHD, tumors, and other infiltrative diseases that may cause pituitary hormone abnormalities.
Furthermore, MRI provides important information for predicting prognosis and determining the need for screening in patients with nonacquired GHD. According to previous studies, isolated GHD (IGHD) patients with pituitary abnormalities are known to have a higher rate of progression to multiple pituitary hormone deficiency (MPHD) at follow-up than IGHD patients without pituitary abnormalities [3]. In addition, it has been reported that patients with IGHD who had pituitary abnormalities had better GH replacement treatment results than IGHD patients who did not have these abnormalities [4,5,6].
There have been many studies using MRI for nonacquired GHD. However, most of the studies were conducted in relatively small study populations, and the reported incidence of sellar and parasellar structural MRI abnormalities between studies has varied greatly [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. For example, the incidence of isolated pituitary hypoplasia in IGHD patients ranges from as little as 4% [8] to as much as 50% [23]. The largest recent population study was conducted by Magnie et al., published in 2013, on 15,043 children with nonacquired GHD in KIGS (Pfizer International Growth Database) from 1987 to 2011 [24]. This study was a highly valuable study that confirmed that there is an important association between the pituitary structure and function in a large study population of various countries. However, as a limitation of the study, the exact number of patients in the two groups, IGHD and MPHD, was not revealed, so the proportion of MRI abnormalities between the two groups was not clearly identified; furthermore, it was difficult to clarify the criteria for pituitary hypoplasia from the KIGS data, which was the most common MRI abnormal finding. Moreover, the influences of other variables, such as the imaging protocol, geographical region, and the cutoff serum GH level for the diagnosis of GHD, were not evaluated. These areas are worth further investigation in a large study population. From this point of view, we performed a systematic review and meta-analysis to verify and integrate the MRI phenotype of patients with GHD.
Materials and methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [25].
Search strategy and selection criteria
PubMed and EMBASE databases were searched up to December 14, 2020, using the following search terms: pituitary AND ((growth hormone deficiency) OR (GH deficiency)) AND ((MRI) OR (MR imaging) OR (magnetic resonance imaging)) AND ((idiopathic) OR (nonacquired) OR (acquired) OR (secondary) OR (congenital) OR (pathologies) OR (structural abnormalities) OR (organic)) AND (children OR pediatric OR paediatric). The literature search was restricted to articles published in English. The literature search was performed by one reviewer (J.S.H.).
The inclusion criteria were as follows: (1) pediatric patients (≤ 18 years old) diagnosed with nonacquired GHD [26] and (2) detailed data sufficient to assess the proportion of sellar and parasellar abnormalities on brain MRI scans. The exclusion criteria were as follows: (1) acquired GHD (craniopharyngioma, microadenoma, history of head trauma, cranial irradiation, or CNS infection) and complex syndromes [24, 26, 27]; (2) conference abstracts, review articles, letters, erratum, articles in press, case reports, or books; (3) adult patients; (4) insufficient data for evaluating outcomes; and (5) overlapping study populations and data. Studies with larger study populations were chosen in cases of overlapping patients and data.
Data extraction and quality assessment
Data were extracted by two reviewers (J.S.H. and S.W.J.) using a standardized extraction form and disagreement was settled by consensus.
-
1.
Study characteristics: authors, publication year, study period, institution, country, and study design
-
2.
Patient characteristics: number of patients, age, sex, mean or median age, diagnostic method of GHD, and type of GHD (IGHD vs. MPHD)
-
3.
MRI characteristics: MR imaging magnet, matrix size, field of view (FOV), slice thickness, contrast enhancement, and imaging criteria of sellar and parasellar abnormalities
-
4.
MRI findings: proportion of total and specific sellar and parasellar abnormalities (isolated anterior pituitary hypoplasia (IAPH), empty sella, isolated stalk abnormality, isolated ectopic posterior pituitary (EPP), pituitary stalk interruption syndrome (PSIS), congenital mass), proportion of midline brain anomalies, and proportion of CNS abnormalities outside the pituitary area. PSIS was defined as triad of thin or interrupted pituitary stalk, aplasia or hypoplasia of the anterior pituitary, and absent or EPP [2, 28].
Two independent reviewers (J.S.H. and S.W.J., with 8 and 10 years of radiology experience, respectively) assessed the quality of each included study using the Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS 2.0) with consensus [29]. The RoBANS tool is configured to evaluate the risk of bias in a total of 8 domains, including comparability of participants, selection of participants, confounding variables, intervention (exposure) measurement, blinding of outcome assessment, outcome evaluation, incomplete outcome data, and selective outcome reporting. The “comparability of participants” domain was not applicable in most of the included studies and was excluded in the quality assessment. The risk of bias of all domains was classified into three categories: “low risk,” “high risk,” and “unclear risk.” Quality assessment results were presented using Revman 5.0 version (Cochrane Community, Oxford, UK).
Statistical analysis
The primary outcome of this study was the pooled proportion of sellar and parasellar abnormalities, midline brain anomalies, and CNS abnormalities outside the pituitary area in patients with GHD. The statistical heterogeneity across studies was evaluated with the Q test or the inconsistency index (I2) statistic, and a P-value < 0.1 in the Q test and I2 ≥ 50% were considered to indicate significant heterogeneity [30]. Hartung-Knapp adjustment for the random-effects model for the meta-analysis of single proportions was performed [31]. Individual study weights were evaluated with the inverse variance method with the arcsine transformation [32]. In addition, we further investigated subgroup analyses according to the type of GHD (IGHD vs. MPHD), MRI magnet, geographical region, and cutoff serum GH level for the diagnosis of GHD (cutoff GH ≤ 5 μg/l vs. cutoff GH=10 μg/l) to explore the potential sources of heterogeneity. P values < 0.05 were regarded as statistically significant [33]. In subgroup analysis according to cutoff serum GH level, sellar abnormalities were additionally divided into two groups by severity of the MR abnormalities (IAPH vs. isolated EPP or PSIS or any two out of the three abnormalities of anterior pituitary hypoplasia, stalk abnormality, and EPP) [3, 34]. Begg’s test was used to evaluate publication bias [35]. A funnel plot was visually assessed and P < 0.05 of Begg’s test results represented the presence of publication bias. All statistical analyses were performed by an experienced professional statistician (J.S.L.) using the “meta” package in R software version 4.0.2 (R Foundation for Statistical Computing).
Results
In total, 885 articles were initially identified by a systematic search. After a full-text review of 75 articles, 32 studies with 39,060 children were ultimately included (see the flow diagram in Fig. 1) [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22, 24, 34, 36,37,38,39,40,41,42,43,44,45,46,47,48,49].
Characteristics of included studies and quality assessment
The study and patient characteristics are described in Table 1. In brief, 12 studies were retrospective, and one study was prospective in design. The number of patients ranged from 18 to 15,043, with mean or median ages of 3.4 to 14.1 years. The diagnosis of GHD was based on two pharmacologic stimulation tests in most studies (supplementary material 1). Regarding peak GH levels of pharmacologic stimulation tests, 17 studies defined GHD when the peak GH was < 10 μg/l. In 6 studies, cutoffs for the peak GH level were ≤ 5 μg/l [12, 16, 22, 39, 40, 44]. Twenty-four studies included both IGHD and MPHD patients. Two studies [8, 14] and one study [40] included only IGHD and only MPHD patients, respectively.
The imaging methods are described in Table 2. MR imaging was conducted by either 0.5T, 1T, 1.5T, or 3T magnets. In studies that reported imaging slice thickness, most studies used slice thicknesses of ≤ 3 mm, except for a few studies [15, 17]. In studies with available FOV data, varying FOVs were used from 11 to 23 cm. In 16 studies, contrast-enhanced MRI scans was performed. In 13 studies, use of contrast-enhanced MRI scans could not be assessed due to a lack of information. Imaging criteria for each sellar abnormality are provided in supplementary material 2.
The quality of the included studies was generally acceptable, but the risk of bias caused by incomplete outcome data was relatively high (31.3%) (supplementary material 3). This is because there were studies with a relatively large proportion (> 20%) of GHD patients who did not undergo MRI. In the two domains of blinding of outcome assessment and outcome evaluation, the proportion of unclear risk of bias was high (59.4% and 34.4%, respectively). The reasons for unclear risk of bias were that the report did not specify whether blinding was performed regarding clinical information for MRI reviewers or the report did not specify the criteria for abnormal sellar and parasellar MRI findings, respectively.
Sellar and parasellar abnormalities
The proportion of sellar and parasellar abnormalities was acquired in 32 studies, composed of 17,540 patients, and ranged from 8.3 to 100% [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22, 24, 34, 36,37,38,39,40,41,42,43,44,45,46,47,48,49]. The pooled proportion of sellar and parasellar abnormalities was 58.0% (95% CI, 47.1–68.6%) (Fig. 2). Significant heterogeneity was noted in the Q test (P<0.001) and Higgins I2 (98.2%). No publication bias existed (P=0.94 by Begg’s test), which was illustrated in the funnel plot analysis (supplementary material 4). The pooled estimates of each sellar and parasellar abnormality are summarized in Table 3. The most common type of abnormality was PSIS (34.5%; 95% CI, 20.8–49.6%). The etiologies of sellar and parasellar mass were hypothalamic hamartoma, arachnoid cyst, pituitary cyst, and Rathke cyst [24, 48].
Forest plot of proportions for total sellar and parasellar abnormalities on brain MRI scans in patients diagnosed with growth hormone deficiency. The green box represents the point estimate, and its area represents the weight given to the study. A horizontal line indicates the 95% CI, and diamonds represent the overall pooled proportions
Midline brain anomaly and CNS abnormalities outside the pituitary area
The proportion of midline brain anomalies was assessed in 29 studies, composed of 16,947 patients, and ranged from 0 to 45.7%. The pooled proportion of midline brain anomalies was 1.1% (95% CI, 0.1–3.0%) (Fig. 3). Significant heterogeneity was noted in the Q test (P<0.001) and Higgins I2 (89.3%). Publication bias was detected by Begg’s funnel plot analysis (P = 0.037) (supplementary material 5a). The adjusted pooled proportion of midline brain anomalies was 2.6% (95% CI, 0.9–5.1%) after applying a trim-and-fill method (supplementary material 5b). The etiologies of midline brain anomalies were hypoplasia of the optic nerve or optic chiasm, septo-optic dysplasia, corpus callosum dysgenesis, abnormality of the septum pellucidum, solitary central maxillary incisor syndrome, midline palate cleft syndrome, GHD with anophthalmia, nasal pyriform aperture stenosis, medially deviated carotid artery, complex anomaly of the hypothalamo-hypophyseal tract, sphenoidal meningocele, vermian agenesis, and craniopharyngeal canal in ten studies [11, 13, 17, 20, 24, 36, 44, 46, 48, 49]. The pooled proportion of CNS abnormalities outside the pituitary area was 0.4% (95% CI, 0.0–1.2%) (supplementary material 6a). Significant heterogeneity was noted in the Q test (P<0.001) and Higgins I2 (82.1%). Publication bias existed based on Begg’s funnel plot analysis (P = 0.024) (supplementary material 6b), and the adjusted pooled proportion of CNS abnormalities outside the pituitary area was 1.2% (95% CI, 0.4–2.3%) after applying the trim-and-fill method.
Forest plot of proportions for midline brain anomalies on brain MRI scans in patients diagnosed with growth hormone deficiency. The green box represents the point estimate, and its area represents the weight given to the study. A horizontal line indicates the 95% CI, and diamonds represent the overall pooled proportions
Subgroup analysis
The results of subgroup analyses by type of GHD, geographical region, MRI magnet, and cutoff serum GH level for the diagnosis of GHD are summarized in Tables 4 and 5 and supplementary material 7–10. Patients with MPHD showed a higher proportion of sellar and parasellar abnormalities than those with IGHD (91.4% vs. 40.1%, P<0.001). In the analysis of specific sellar and parasellar abnormalities, the pooled proportion of PSIS was higher in patients with MPHD than in those with IGHD (65.3% vs. 20.1%, P<0.001). The type of GHD did not influence heterogeneity in other specific sellar and parasellar abnormalities or midline brain anomalies (P≥0.103). Geographical region, MRI magnet, and cutoff GH level for the diagnosis of GHD were not significant factors of heterogeneity regarding sellar and parasellar abnormalities (P=0.668, 0.228, and 0.101, respectively). When sellar abnormalities were divided into two groups by severity (isolated anterior PH vs. severe MR abnormalities [isolated EPP or PSIS or any two out of the three abnormalities of anterior pituitary hypoplasia, stalk abnormality, and EPP]), the pooled proportion of severe MR abnormalities was significantly higher in patients with studies with cutoff GH values ≤ 5 μg/l compared to those with a cutoff value of GH=10 μg/l for provocation tests (72.8% vs. 38.0%; P < 0.001) (supplementary material 11).
Discussion
In our systematic review and meta-analysis, the pooled proportion of sellar and parasellar abnormalities was 58.0% in children diagnosed with GHD, and significant heterogeneity was noted among the studies. Approximately 1.1% (2.6% after adjustment) of patients with GHD showed midline brain anomalies. Patients with MPHD showed a higher proportion of sellar and parasellar abnormalities compared with those with IGHD (91.4% vs. 40.1%). In the analysis of each sellar and parasellar abnormality, the pooled proportion of PSIS was higher in patients with MPHD than in those with IGHD (65.3% vs. 20.1%). Other variables of geographical region, MRI magnet, and cutoff GH level for the diagnosis of GHD did not influence heterogeneity.
The consensus guideline published by the Growth Hormone Research Society recommended performing brain imaging for all children diagnosed with GHD [50]. Despite the increasing use of brain MRI for children with GHD, the real-world incidence of abnormal pituitary anatomy in this population is not well known due to variability in the diagnostic criteria, the lack of a united reference standard of pituitary size, and different opinions on the role of MRI in the diagnosis of GHD [51, 52]. To our knowledge, this is the first systematic review and meta-analysis to report the proportion of sellar and parasellar abnormalities on MRI, and the pooled estimate was 58.0% in children with GHD.
Previous studies have identified a relationship between pituitary abnormality phenotype and endocrine profile in children with GHD; a patient with normal MRI findings or anterior pituitary hypoplasia is more likely to have IGHD, whereas PSIS is associated with MPHD [51]. In children with MPHD, normal pituitary anatomy is uncommon [51]. This study also confirmed via meta-analysis that the pooled proportion of sellar and parasellar abnormalities was as high as 91.4% in the MPHD group and was significantly higher than that in the IGHD group (40.1%). Moreover, we performed a meta-analysis to confirm the pituitary phenotype-endocrine profile correlation. The pooled estimate of PSIS incidence was significantly higher in patients with MPHD (65.3%). In addition, patients with MPHD showed a higher proportion of EPP and complex sellar abnormalities (i.e., any two out of the three abnormalities of anterior pituitary hypoplasia, stalk abnormality, and EPP); conversely, patients with IGHD showed a higher proportion of IAPH, although the differences were of borderline significance (P=0.109, 0.103).
The anterior and posterior pituitary, the hypothalamus, the optic nerves, and the forebrain have a common origin—the anterior neural plate—in the developing embryo [53]. Midline developmental anomalies are regarded as a spectrum disorder with variable clinical phenotypes from mild to severe forms [54]. In this study, various midline brain anomalies were identified in children with GHD and the pooled proportion was 1.1% (2.6% after adjustment). There was no significant difference in the pooled estimates between the IGHD and MPHD groups. This result might be limited because only a few included studies (n=10) reported cases with concomitant midline brain anomalies and there might be a possibility of underestimation of the incidence. Although the prevalence seems rare, all radiologists and researchers involved in the diagnosis and management of children with GHD should have good knowledge of the association between the pituitary gland and other midline brain defects. The interpretation of brain MRI data should be performed by neuroimaging experts who are familiar with midline brain anomalies.
Previous studies have demonstrated conflicting results regarding the association between the severity of GHD and brain MRI abnormalities. According to Acharya et al. [55] and Frindik et al. [52], severe GHD with a low peak GH level on stimulation tests was more associated with pituitary abnormalities on MRI, compared to mild GHD. In contrast, a recent paper by Alba et al. demonstrated that the severity of GHD did not predict pituitary abnormalities, and they concluded that brain MRI should be performed in all children with GHD regardless of the severity of the deficiency [56]. In our subgroup analysis, the pooled proportion of sellar and parasellar abnormalities was not significantly different between subgroups defined by the cutoff GH level on stimulation tests. However, patients in studies with low peak GH levels on stimulation tests were more associated with severe MR abnormalities than those in studies with peak GH levels of 10 μg/l. This result could be limited, because we categorized the studies by a cutoff GH level of “10 μg/l” rather than using a constraint of “5 to 10 μg/l” due to insufficient data, and patients in the subgroup could have a wide range of peak GH levels on stimulation tests.
There are limitations in our study. First, the diagnostic criteria of abnormal MRI were variable among the included studies, and the prevalence of pituitary abnormalities in each study could be influenced by the different criteria. In most of the included studies, the diagnosis of pituitary hypoplasia was based on pituitary height. However, the diagnostic criteria used for pituitary hypoplasia were not consistent, evaluating height based on the normal population (below 2 standard deviations) or using an absolute value (e.g., 2 mm or 3 mm). Worldwide normative morphological data using artificial intelligence algorithms or volumetric measurements using three-dimensional MRI might improve the quality of pituitary assessment in future studies [57]. Second, gene defects are increasingly recognized as factors of abnormal pituitary development; however, we could not perform subgroup analysis due to insufficient data. Third, the diagnostic method of GHD by the GH stimulation test was not uniform in the included studies. Therefore, we conducted subgroup analysis according to the peak GH cutoff values.
In conclusion, this systematic review and meta-analysis demonstrated that sellar and parasellar abnormalities were common in children diagnosed with GHD. The patients with GHD rarely had concomitant midline brain anomalies. Patients with MPHD showed more frequent and more complex sellar and parasellar abnormalities than patients with IGHD. In addition, this study confirmed a significant correlation between the frequency of severe MR abnormalities and low cutoff serum GH levels on stimulation tests in a large population of nonacquired GHD. The observed anatomic abnormality-endocrine profile correlation supports that brain MRI is essential to identify congenital structural abnormalities of the sellar and parasellar regions in children with nonacquired GHD.
Data availability
Not applicable
Code availability
Not applicable
Abbreviations
- GH:
-
Growth hormone
- GHD:
-
Growth hormone deficiency
- CNS:
-
Central nervous system
- IGHD:
-
Isolated growth hormone deficiency
- MPHD:
-
Multiple pituitary hormone deficiency
- IAPH:
-
Isolated anterior pituitary hypoplasia
- EPP:
-
Isolated ectopic posterior pituitary
- PSIS:
-
Pituitary stalk interruption syndrome
References
Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M (1994) Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr 125(1):29–35. https://doi.org/10.1016/s0022-3476(94)70117-2
Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T et al (1987) Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology 165(2):487–489. https://doi.org/10.1148/radiology.165.2.3659371
Jagtap VS, Acharya SV, Sarathi V, Lila AR, Budyal SR, Kasaliwal R, Sankhe SS, Bandgar TR, Menon PS, Shah NS (2012) Ectopic posterior pituitary and stalk abnormality predicts severity and coexisting hormone deficiencies in patients with congenital growth hormone deficiency. Pituitary 15(2):243–250. https://doi.org/10.1007/s11102-011-0321-4
Coutant R, Rouleau S, Despert F, Magontier N, Loisel D, Limal JM (2001) Growth and adult height in GH-treated children with nonacquired GH deficiency and idiopathic short stature: the influence of pituitary magnetic resonance imaging findings. J Clin Endocrinol Metab 86(10):4649–4654. https://doi.org/10.1210/jcem.86.10.7962
Deal C, Hasselmann C, Pfäffle RW, Zimmermann AG, Quigley CA, Child CJ, Shavrikova EP, Cutler GB Jr, Blum WF (2013) Associations between pituitary imaging abnormalities and clinical and biochemical phenotypes in children with congenital growth hormone deficiency: data from an international observational study. Horm Res Paediatr 79(5):283–292. https://doi.org/10.1159/000350829
Khadilkar VV, Prasad HK, Ekbote VH, Rustagi VT, Singh J, Chiplonkar SA, Khadilkar AV (2015) Response of Indian growth hormone deficient children to growth hormone therapy: association with pituitary size. Indian J Pediatr 82(5):404–409. https://doi.org/10.1007/s12098-014-1412-9
Abrahams JJ, Trefelner E, Boulware SD (1991) Idiopathic growth hormone deficiency: MR findings in 35 patients. Am J Neuroradiol 12(1):155–160
Ariza Jiménez AB, Martínez Aedo Ollero MJ, López Siguero JP (2020) Differences between patients with isolated GH deficiency based on findings in brain magnetic resonance imaging. Endocrinol Diabetes Nutr 67(2):78–88. https://doi.org/10.1016/j.endinu.2019.09.001
Bressani N, di Natale B, Pellini C, Triulzi F, Scotti G, Chiumello G (1990) Evidence of morphological and functional abnormalities in the hypothalamus of growth-hormone-deficient children: a combined magnetic resonance imaging and endocrine study. Horm Res 34(5-6):189–192. https://doi.org/10.1159/000181823
Dutta P, Bhansali A, Singh P, Rajput R, Bhadada S (2010) Clinico-radiological correlation in childhood hypopituitarism. Indian Pediatr 47(7):615–618. https://doi.org/10.1007/s13312-010-0135-x
Hamilton J, Blaser S, Daneman D (1998) MR imaging in idiopathic growth hormone deficiency. AJNR Am J Neuroradiol 19(9):1609–1615
Hanew K, Tachibana K, Yokoya S, Fujieda K, Tanaka T, Igarashi Y, Shimatsu A, Tanaka H, Tanizawa T, Teramoto A, Nishi Y, Hasegawa Y, Hizuka N, Hirano T, Fujita K (2006) Clinical characteristics, etiologies and pathophysiology of patients with severe short stature with severe GH deficiency: questionnaire study on the data registered with the foundation for growth science, Japan. Endocr J 53(2):259–265. https://doi.org/10.1507/endocrj.53.259
Kalina MA, Kalina-Faska B, Gruszczyńska K, Baron J, Małecka-Tendera E (2012) Usefulness of magnetic resonance findings of the hypothalamic-pituitary region in the management of short children with growth hormone deficiency: evidence from a longitudinal study. Childs Nerv Syst 28(1):121–127. https://doi.org/10.1007/s00381-011-1594-7
Kara Ö, Esen I, Tepe D, Gülleroğlu NB, Tayfun M (2018) Relevance of pituitary gland magnetic resonance imaging results with clinical and laboratory findings in growth hormone deficiency. Med Sci Monit 24:9473–9478. https://doi.org/10.12659/msm.911977
Liotta A, Maggio C, Giuffrè M, Carta M, Manfrè L (1999) Sequential contrast-enhanced magnetic resonance imaging in the diagnosis of growth hormone deficiencies. J Endocrinol Investig 22(10):740–746. https://doi.org/10.1007/bf03343638
Mészáros F, Vergesslich K, Riedl S, Häusler G, Frisch H (2000) Posterior pituitary ectopy in children with idiopathic growth hormone deficiency. J Pediatr Endocrinol Metab : JPEM 13(6):629–635. https://doi.org/10.1515/jpem.2000.13.6.629
Nagel BH, Palmbach M, Petersen D, Ranke MB (1997) Magnetic resonance images of 91 children with different causes of short stature: pituitary size reflects growth hormone secretion. Eur J Pediatr 156(10):758–763. https://doi.org/10.1007/s004310050707
Secco A, di Iorgi N, Napoli F, Calandra E, Ghezzi M, Frassinetti C, Parodi S, Casini MR, Lorini R, Loche S, Maghnie M (2009) The glucagon test in the diagnosis of growth hormone deficiency in children with short stature younger than 6 years. J Clin Endocrinol Metab 94(11):4251–4257. https://doi.org/10.1210/jc.2009-0779
Stawerska R, Lewiński A, Smyczyńska J, Hilczer M, Kowalska E, Kaniewska D, Karasek M (2007) Circadian pattern of prolactin secretion in children with growth hormone deficiency and congenital organic lesions in the hypothalamic-pituitary region. Neuro Endocrinol Lett 28(6):765–774
Tsai SL, Laffan E, Lawrence S (2012) A retrospective review of pituitary MRI findings in children on growth hormone therapy. Pediatr Radiol 42(7):799–804. https://doi.org/10.1007/s00247-012-2349-7
Vannelli S, Avataneo T, Benso L, Potenzoni F, Cirillo S, Mostert M, Bona G (1993) Magnetic resonance and the diagnosis of short stature of hypothalamic-hypophyseal origin. Acta Paediatr (Oslo, Norway : 1992) 82(2):155–161. https://doi.org/10.1111/j.1651-2227.1993.tb12629.x
Zimmermann A, Schenk JP, Grigorescu Sido P, Pfaffle R, Lazea C, Zimmermann T, Heinrich U, Weber MM, Bettendorf M (2007) MRI findings and genotype analysis in patients with childhood onset growth hormone deficiency--correlation with severity of hypopituitarism. J Pediatr Endocrinol Metab : JPEM 20(5):587–596. https://doi.org/10.1515/jpem.2007.20.5.587
Marwaha R, Menon PS, Jena A, Pant C, Sethi AK, Sapra ML (1992) Hypothalamo-pituitary axis by magnetic resonance imaging in isolated growth hormone deficiency patients born by normal delivery. J Clin Endocrinol Metab 74(3):654–659. https://doi.org/10.1210/jcem.74.3.1740501
Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB (2013) Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol 168(2):211–217. https://doi.org/10.1530/eje-12-0801
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Quigley CA, Ranke MB (2015) International Classification of Pediatric Endocrine Diagnoses (ICPED), ICPED Consortium. www.icped.org. Accessed September 6th 2020
Nwosu BU, Lee MM (2008) Evaluation of short and tall stature in children. Am Fam Physician 78(5):597–604
Simmons GE, Suchnicki JE, Rak KM, Damiano TR (1992) MR imaging of the pituitary stalk: size, shape, and enhancement pattern. AJR Am J Roentgenol 159(2):375–377. https://doi.org/10.2214/ajr.159.2.1632360
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ (2013) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66(4):408–414. https://doi.org/10.1016/j.jclinepi.2012.09.016
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
IntHout J, Ioannidis JP, Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 14:25. https://doi.org/10.1186/1471-2288-14-25
Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G (2019) Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 10(3):476–483. https://doi.org/10.1002/jrsm.1348
Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14(2):134–143. https://doi.org/10.1007/s11121-013-0377-7
Tillmann V, Tang VW, Price DA, Hughes DG, Wright NB, Clayton PE (2000) Magnetic resonance imaging of the hypothalamic-pituitary axis in the diagnosis of growth hormone deficiency. J Pediatr Endocrinol Metab : JPEM 13(9):1577–1583. https://doi.org/10.1515/jpem.2000.13.9.1577
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Arends NJ, VdL W, Robben SG, Hokken-Koelega AC (2002) MRI findings of the pituitary gland in short children born small for gestational age (SGA) in comparison with growth hormone-deficient (GHD) children and children with normal stature. Clin Endocrinol 57(6):719–724. https://doi.org/10.1046/j.1365-2265.2002.01605.x
Argyropoulou M, Perignon F, Brauner R, Brunelle F (1992) Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr 120(6):886–891. https://doi.org/10.1016/s0022-3476(05)81955-9
Bordallo MA, Tellerman LD, Bosignoli R, Oliveira FF, Gazolla FM, Madeira IR, Zanier JF, Henriques JL (2004) Neuroradiological investigation in patients with idiopathic growth hormone deficiency. J Pediatr 80(3):223–228
Kemp SF, Alter CA, Dana K, Baptista J, Blethen SL (2002) Use of magnetic resonance imaging in short stature: data from National Cooperative Growth Study (NCGS) Substudy 8. J Pediatr Endocrinol Metab : JPEM 15(Suppl 2):675–679. https://doi.org/10.1515/jpem.2002.15.s2.675
Li G, Shao P, Sun X, Wang Q, Zhang L (2010) Magnetic resonance imaging and pituitary function in children with panhypopituitarism. Horm Res Paediatr 73(3):205–209. https://doi.org/10.1159/000284363
Longui CA, Rocha AJ, Menezes DM, Leite FM, Calliari LE, Kochi C, Monte O (2004) Fast acquisition sagittal T1 magnetic resonance imaging (FAST1-MRI): a new imaging approach for the diagnosis of growth hormone deficiency. J Pediatr Endocrinol Metab : JPEM 17(8):1111–1114. https://doi.org/10.1515/jpem.2004.17.8.1111
Maghnie M, Strigazzi C, Tinelli C, Autelli M, Cisternino M, Loche S, Severi F (1999) Growth hormone (GH) deficiency (GHD) of childhood onset: reassessment of GH status and evaluation of the predictive criteria for permanent GHD in young adults. J Clin Endocrinol Metab 84(4):1324–1328. https://doi.org/10.1210/jcem.84.4.5614
Naderi F, Eslami SR, Mirak SA, Khak M, Amiri J, Beyrami B, Shekarchi B, Poureisa M (2015) Effect of growth hormone deficiency on brain MRI findings among children with growth restrictions. J Pediatr Endocrinol Metab : JPEM 28(1-2):117–123. https://doi.org/10.1515/jpem-2013-0294
Otto AP, França MM, Correa FA, Costalonga EF, Leite CC, Mendonca BB, Arnhold IJ, Carvalho LR, Jorge AA (2015) Frequent development of combined pituitary hormone deficiency in patients initially diagnosed as isolated growth hormone deficiency: a long term follow-up of patients from a single center. Pituitary 18(4):561–567. https://doi.org/10.1007/s11102-014-0610-9
Pampanini V, Pedicelli S, Gubinelli J, Scirè G, Cappa M, Boscherini B, Cianfarani S (2015) Brain Magnetic Resonance Imaging as First-Line Investigation for Growth Hormone Deficiency Diagnosis in Early Childhood. Horm Res Paediatr 84(5):323–330. https://doi.org/10.1159/000439590
Pinto G, Adan L, Souberbielle JC, Thalassinos C, Brunelle F, Brauner R (1999) Idiopathic growth hormone deficiency: presentation, diagnostic and treatment during childhood. Ann Endocrinol 60(3):224–231
van Iersel L, van Santen HM, Zandwijken GRJ, Zwaveling-Soonawala N, Hokken-Koelega ACS, van Trotsenburg ASP (2018) Low FT4 Concentrations around the Start of Recombinant Human Growth Hormone Treatment: Predictor of Congenital Structural Hypothalamic-Pituitary Abnormalities? Horm Res Paediatr 89(2):98–107. https://doi.org/10.1159/000486033
Walvoord EC, Rosenman MB, Eugster EA (2004) Prevalence of adrenocorticotropin deficiency in children with idiopathic growth hormone deficiency. J Clin Endocrinol Metab 89(10):5030–5034. https://doi.org/10.1210/jc.2004-0364
Zenaty D, Garel C, Limoni C, Czernichow P, Léger J (2003) Presence of magnetic resonance imaging abnormalities of the hypothalamic-pituitary axis is a significant determinant of the first 3 years growth response to human growth hormone treatment in prepubertal children with nonacquired growth hormone deficiency. Clin Endocrinol 58(5):647–652
Growth Hormone Research Society (2000) Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab 85(11):3990–3993. https://doi.org/10.1210/jcem.85.11.6984
Di Iorgi N, Allegri AE, Napoli F, Bertelli E, Olivieri I, Rossi A, Maghnie M (2012) The use of neuroimaging for assessing disorders of pituitary development. Clin Endocrinol 76(2):161–176. https://doi.org/10.1111/j.1365-2265.2011.04238.x
Frindik JP (2001) Pituitary Morphologic Anomalies and Magnetic Resonance Imaging in Pediatric Growth Hormone Deficiency. Endocrinologist 11(4):289–295
Schlosser G (2006) Induction and specification of cranial placodes. Dev Biol 294(2):303–351. https://doi.org/10.1016/j.ydbio.2006.03.009
McCabe MJ, Alatzoglou KS, Dattani MT (2011) Septo-optic dysplasia and other midline defects: the role of transcription factors: HESX1 and beyond. Best Pract Res Clin Endocrinol Metab 25(1):115–124. https://doi.org/10.1016/j.beem.2010.06.008
Acharya SV, Gopal RA, Lila A, Sanghvi DS, Menon PS, Bandgar TR, Shah NS (2011) Phenotype and radiological correlation in patients with growth hormone deficiency. Indian J Pediatr 78(1):49–54. https://doi.org/10.1007/s12098-010-0211-1
Alba P, Tsai S, Mitre N (2020) The Severity of Growth Hormone Deficiency Does Not Predict the Presence or Absence of Brain Magnetic Resonance Imaging Abnormalities - A Retrospective Review. Eur Endocrinol 16(1):60–64. https://doi.org/10.17925/ee.2020.16.1.60
Marziali S, Gaudiello F, Bozzao A, Scirè G, Ferone E, Colangelo V, Simonetti A, Boscherini B, Floris R, Simonetti G (2004) Evaluation of anterior pituitary gland volume in childhood using three-dimensional MRI. Pediatr Radiol 34(7):547–551. https://doi.org/10.1007/s00247-004-1208-6
Acknowledgments
We thank Ji Sung Lee (Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) for the statistical assistance.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data
Consent to participate
Statement of informed consent was not applicable since the manuscript does not contain any patient data
Consent for publication
All of the authors gave consent for publication of the article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, J., Jo, S.W., Kwon, E.B. et al. Prevalence of brain MRI findings in children with nonacquired growth hormone deficiency: a systematic review and meta-analysis. Neuroradiology 63, 1121–1133 (2021). https://doi.org/10.1007/s00234-021-02665-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02665-3