Abstract
Background: Three-dimensional MRI (3D-MRI) is a reliable tool for the evaluation of anatomical volumes. Volumetric measurement of the normal anterior pituitary gland in childhood has been performed in the past by 2D-MRI calculations, but has inherent inaccuracies. Objective: To obtain accurate normal anterior pituitary gland volume in childhood using 3D-MRI coronal sections. Materials and methods: The anterior pituitary gland was measured using coronal T1-weighted 3D-gradient-echo sequences (section thickness 0.75 mm). The study group was composed of 95 prepubertal children (age range 2 months–10 years) with clinically normal pituitary function and no pituitary or brain abnormalities. Results: A measurement error of 0.2–0.4% was assessed by using a phantom study. Volumetric evaluation of the anterior pituitary gland showed progressive growth of the gland from a mean 131±24 mm3 at 2–12 months, to 249±25 mm3 at 1–4 years and 271±29 mm3 at 5–10 years. Conclusions: These data may be useful for paediatricians in the evaluation of patients with neuroendocrine diseases, in particular growth hormone deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paediatric pituitary gland disorders such as transection of the pituitary stalk, midline malformations or tumours can be easily assessed by traditional MRI. In patients with growth-hormone deficiency (GHD), MRI has revealed morphological abnormalities such as pituitary hypoplasia, pituitary stalk agenesis and ectopia of the posterior pituitary (PPE). GHD caused by gland hypoplasia, however, can be difficult to detect on traditional MRI. Comparison of the affected gland size with a database of physiological volumes could, therefore, help with the diagnosis [1, 2].
Previous studies have measured the size of the adenohypophysis by indirect methods, e.g. calculating MRI bidimensional values of the gland and reconstructing the volume using mathematical formulae [3–7]. Other authors have considered the gland height measured by MRI to be a reliable means of evaluation of gland size [6]. However, morphological variability of the pituitary gland can reduce the reliability of these methods. Furthermore, the available data have been mainly obtained in the adult population.
More recent 3D-MRI techniques have offered a more reliable method of studying anatomical volumes. However, the normal volumetric development of the pituitary gland in prepubertal children has not been studied in sufficient detail [8, 9].Our study aimed at providing reliable data for normal paediatric pituitary gland volume. These data could be used by paediatricians to improve their diagnostic approach in the evaluation of GHD.
Material and methods
Pilot study
Validation of the volumetric study was achieved by calculating the volume of two phantoms (150 and 300 mm3) made of a cylinder of wax, submerged in a gel contained in a test tube (Fig. 1). The same coronal T1-weighted (T1-W) 3D-gradient-echo (3D-GE) sequence used was subsequently applied in the study of the adenohypophysis (Fig. 2). Two experienced neuroradiologists each independently performed a measurement on three different days to take account of possible volumetric changes determined by variability in the electromagnetic field. Pearson’s correlation coefficient was used to assess interobserver agreement.
Population
The study group comprised 95 children (46 boys; 49 girls; age range 2 months–10 years) with neither clinical pituitary dysfunction nor brain abnormalities (Table 1). Patients were divided into three groups based on age: group I, 2–12 months; group II, 1–4 years; group III, 5–10 years. Children with a history of preterm delivery or breech birth were excluded from the study. All children were prepubertal. Informed consent was obtained for all patients.
MRI study
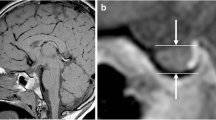
A 1.5-T unit (Gyroscan, Philips Medical Systems, Eindhoven, The Netherlands), with 23-mT gradient intensity and a dedicated head coil was used. Thin-section volumetric studies were obtained using coronal T1-W 3D-GE sequences of the sellar region without the use of contrast agents. Image axis was oriented along the pituitary stalk and scan time was about 3 min (TR/TE 30/4.2 ms, flip angle 30°, slices 30, FOV 110 mm, RFOV (%) 100.00, slice thickness 0.75 mm, matrix 512×512). For every slice, the perimeter of the gland was delineated and the area (total pixel area) was measured using the system software. The neurohypophysis, hyperintense on T1-W images, set the limit of the measurement (Fig. 3). Values of each area were added and the result multiplied by the slice thickness (0.75 mm). All volume measurements were performed independently by the two neuroradiologists who had measured the phantoms. Pearson’s correlation coefficient was used to assess interobserver agreement.
Statistical methods
Normal distribution of the examined variable in the three groups was assessed by means of the Kolmogorov–Smirnov test (P>0.20). Once the distribution was stated as normal in all three groups, data were analysed by means of one-way ANOVA with a ‘between’ factor AGE with three levels (level 1: 0–12 months; level 2: 1–4 years; level 3: 5–10 years). The significance of single differences was assessed by means of ‘post hoc’ analysis with Tukey test for unequal sample sizes. Accepted significance level was P<0.05.
Results
Pilot study
The phantom studies demonstrated the accuracy of volumetric assessment. Measurement error for the 150 mm3 and the 300 mm3 phantom was 0.4% and 0.2% respectively. Pearson’s interobserver correlation coefficient was 0.8.
Clinical study
Values obtained for the three groups examined are summarized in Table 2. Gradual and progressive growth of the anterior pituitary gland was observed. No significant volumetric differences were noted between the two sexes (Fig. 4). Pearson’s interobserver correlation coefficient was 0.75.
Discussion
Volumetric studies of the pituitary gland have been initially performed evaluating the sella by conventional radiographs and later by CT. However, these studies did not provide correct information regarding the volume of the gland [10–12].
More recent 2D-MRI studies have either measured the height of the pituitary gland or evaluated its volume by using mathematical formulae, such as that of the ellipsoid (volume=1/2×length×width×height) [13–18]. However, in normal and pathological conditions, the pituitary gland is not always an ellipsoid. This is more so in children when the morphology of the gland is in continuous evolution.
The methodology we used is presently the most accurate imaging technique available to measure anatomical volumes. Any bias that different operators could have in the evaluation of the gland and the limits related to the extrapolation of the gland volume from bidimensional data are overcome by the volumetric acquisition of the image.
Our study focused on data prior to puberty. This is when GHD-related short stature is most often diagnosed. Moreover, this is when correct therapy is most successful at resolving the deficit.
We found statistically significant differences when comparing the three age groups. However, the significance was greater when comparing the first and second or first and third group. The gland changes during childhood are greatest within the first 4 years of life [14]. The smaller increase of pituitary growth between 5 and 10 years of age is the cause of a lower significance in the comparison of the second and third group of patients.
A previous study on pituitary gland volume is at variance with our data, especially when evaluating the second group of patients (1–4 years). We surmise that this difference could be due to the fact that the study was based on a Japanese population that normally has an average height lower than the corresponding population of Western countries. The measurement error in the phantom study was higher than the present study. Moreover, Takano et al. [19] used sagittal 3D-MRI sequences that are more subject to flow artefacts from the cavernous sinus.
Clinical manifestations of pituitary dysfunction consist of hypo- or hyperfunction of the gland. Clinically comparable aspects can be caused by primitive pituitary gland lesions or by organic lesions of the hypothalamus. They can also be secondary to interruption of the hypothalamic-pituitary axis or due to neurosecretory dysfunction.
Short stature is a common reason for referral to a paediatrician. If GHD is suspected, it can be part of a syndrome of multiple pituitary gland hormone deficiency (MPHD) or of an isolated deficit of GH (IGHD) [20–22]. A detailed history, thorough physical examination, meticulous height measurements over time, together with GH test and MRI studies, are the key to the diagnosis. In fact, MRI studies have often demonstrated morphological anomalies of the pituitary gland in these conditions [23]. Furthermore, a retrospective study by Maghnie et al. [1] has demonstrated that a high number of children with IGHD and normal or reduced dimensions of the pituitary gland show normalization of GH secretion after completion of specific therapy; GHD is permanent in those patients with pituitary hypoplasia, agenesis of the pituitary stalk or PPE. Data for physiological anterior pituitary gland volume in prepubertal children could, therefore, be useful in assisting paediatricians in the diagnostic process.
References
Maghnie M, Strigazzi C, Tinelli C, et al (1999) Growth hormone deficiency (GHD) of childhood onset: reassessment of GH status and evaluation of the predictive criteria for permanent GHD in young adults. J Clin Endocrinol Metab 84:1324–1328
Growth Hormone Research Society (GRS) (1998) Consensus guidelines for the diagnosis and treatment of adult with growth hormone deficiency: summary statement of the Growth Hormone Research Society workshop on adult growth hormone deficiency. J Clin Endocrinol Metab 83: 379–381
Maghnie M, Triulzi F, Larizza D, et al (1990) Hypothalamic-pituitary dwarfism: comparison between MR imaging and CT findings. Pediatr Radiol 20:229–235
Triulzi F, Scotti G, Di Natale B, et al (1994) Evidence of a congenital midline brain anomaly in pituitary dwarfs: a magnetic resonance imaging study in 101 patients. Pediatrics 93:409–416
Kornreich L, Horev G, Lazar L et al (1998) MR findings in growth hormone deficiency: correlation with severity of hypopituitarism. AJNR 19:1495–1499
Argyropoulou M, Perignon F, Brunelle F, et al (1991) Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol 21:247–249
Murray RA, Maheshwari HG, Russel EJ, et al (2000) Pituitary hypoplasia in patients with a mutation in the growth hormone-releasing hormone receptor gene. AJNR 21:685–689
Hamilton J, Blaser S, Daneman D (1998) MR imaging in idiopathic growth hormone deficiency. AJNR 19:1609–1615
Nagel B, Palmbach M, Petersen D, et al (1997) Magnetic resonance images of 91 children with different causes of short stature: pituitary size reflects growth hormone secretion. Eur J Pediatr 156:758–763
Peyster RG, Hoover ED, Viscarello RR, et al (1983) CT appearance of the adolescent and preadolescent pituitary gland. AJNR 4:411–414
Mc Lachlan MS, Williams ED, Fortt RW, et al (1968) Estimation of pituitary gland dimension from radiographs of the sella turcica. A post mortem study. Br J Radiol 41:323–330
Pikulev LA, Gerasimov SM, Cheremisin VM, et al (1970) Relationship between the volume of the hypophysis and the volume of the sella turcica (radioanatomic study). Arkh Anat Gistol Embryol 59:98–104
Sharafuddin MJ, Luisiri A, Garibaldi LR, et al (1994) MR imaging diagnosis of central precocious puberty: importance of changes in the shape and size of pituitary gland. AJR 162:1167–1173
Tsunoda A, Okuda O, Sato K (1997) MR height of the pituitary gland as a function of age and sex: especially physiological hypertrophy in adolescence and in climacterium. AJNR 18:551–554
Lurie SN, Doraiswamy PM, Husain MM, et al (1990) In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab 71:505–508
Wiener SN, Rzeszotarski MS, Droege RT, et al (1985) Measurement of pituitary gland height with MR imaging. AJNR 6:717–722
Doraiswamy PM, Potts JM, Axelson DA, et al (1992) MR assessment of pituitary gland morphology in healthy volunteers: age- and gender-related differences. AJNR 13:1295–1299
Oliveira HA, Salvatori R, Krauss MP, et al (2003) Magnetic resonance imaging study of pituitary morphology in subjects homozygous and heterozygous for a null mutation of the GHRH receptor gene. Eur J Endocrinol 148:427–432
Takano K, Utsunomiya H, Ono H, et al (1999) Normal development of the pituitary gland: assessment with three-dimensional MR volumetry. AJNR 20:312–315
Kucharczyk W (2000) Etiology of congenital growth hormone deficiency. AJNR 21:1000
Netchine I, Talon P, Dastot F, et al (1998) Extensive phenotypic analysis of a family with growth hormone (GH) deficiency caused by a mutation in the GH-realising hormone receptor gene. J Clin Endocrinol Metab 83:432–436
Maghnie M, Triulzi F, Larizza D, et al (1991) Hypothalamic-pituitary dysfunction in growth hormone-deficient patients with pituitary abnormalities. J Clin Endocrinol Metab 73:79–83
Hellstrom A, Wiklund LM, Svensson E, et al (1998) Midline brain lesions in children with hormone insufficiency indicate early prenatal damage. Acta Paediatr 87:528–536
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marziali, S., Gaudiello, F., Bozzao, A. et al. Evaluation of anterior pituitary gland volume in childhood using three-dimensional MRI. Pediatr Radiol 34, 547–551 (2004). https://doi.org/10.1007/s00247-004-1208-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-004-1208-6